

After having, in the previous document, expounded the traction-lifting many-veined (tabanoid) functional type of wings, we will now investigate how the venation of these wings stand to each other in terms of their formal derivation from the dipterous order-prototype and from each other. [All Figures of wings from HENNIG 1954, unless otherwise stated].
The most primitive (i.e. original) venation of wings of the tabanoid type we find in Rhagio scolopaceus (Rhagionidae). It can be formally derived from the dipterous venational prototype by having the veins R3 and 2A completely reduced. See next Figure (two images).
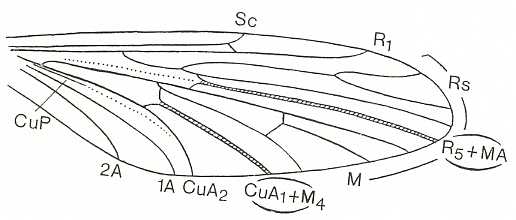
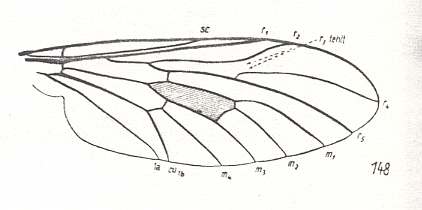
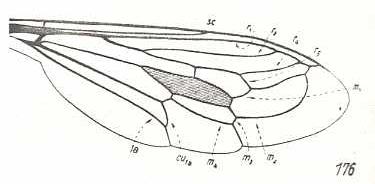
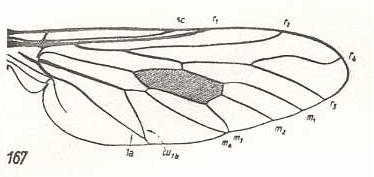
Figure 1 : Dipterous venational prototype (top) and the wing-venation of Rhagio scolopaceus (Rhagionidae) (bottom).
If we go around the margin of the wing we find in the prototype the veins :
SC - R1 - R2 - R3 - R4 - R5 - M1 - M2 - M3 - M4 - CuA - 1A -2A,
and in the wing of Rhagio the veins :
SC - R1 - R2 - R4 - R5 - M1 - M2 - M3 - M4 - CuA -1A.
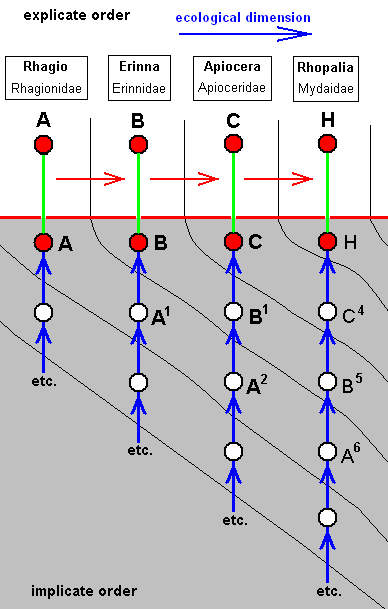
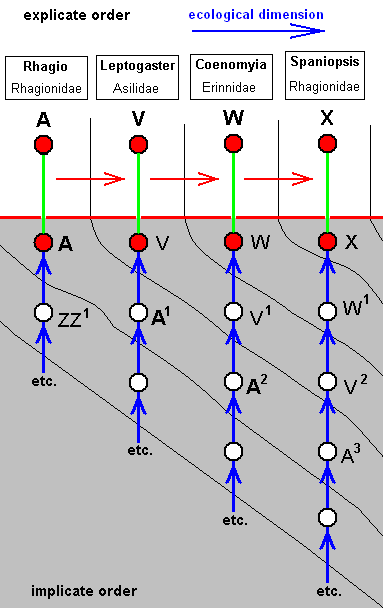
Derivational lines connecting wing-venations of the tabanoid functional type
From the wing-venation of the just depicted Rhagio a number of derivational lines (or sequences) depart, ending up in derived venations among which there are quite a few that seem to be derivational end-points.
Let us give a number of these lines. The derivational direction must be read top-bottom.

|
Rhagio scolopaceus, Rhagionidae. Subcosta (SC) well developed. Radial Sector (RS) 3-branched. R3 absent. Discoidal cell present. The cross-vein ta [= r-m] present in its original condition. M1, M2, M3, and M4 present. tb1 [= base of M4] present. tb2 [= m-cu] present. CuA [labelled as cu1b in the Figure] present. 1A [in the Figure labelled as 1a] present, reaching wing-margin near end of CuA. No further anal veins present. |
|
Erinna atra, Erinnidae. Fork of R4+5 shortened. CuA and 1A with common end-point at margin. |
|
Apiocera bigoti, Apioceridae. RS still 3-branched. R2 and R4 run into R1. M3 and M4 with common end-stalk. CuA and 1A with common end-stalk. There is, in this wing, a tendency to make the hind-marginal area free of veins, by either having them curved up or having them coalesced at, and, subsequently, retreated from, the wing's hind-margin. |
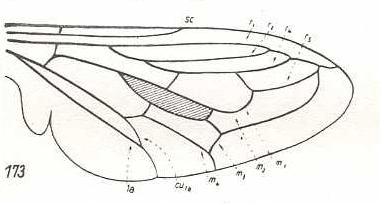
|
Megascelus nigricornis, Apioceridae. R2, R4, and R5 run into R1. M1 and M2 run into R5 M3 and M4 with common end-stalk. CuA and 1A with long common end-stalk. Wing broadened. |
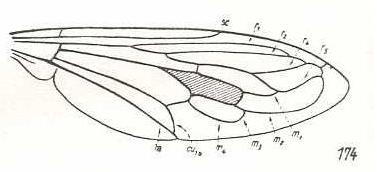
|
Neorhaphiomidas hardyi, Apioceridae. R2, R4, and R5 run into R1. M1 and M2 run into R5 M3 and M4 lost their common end-stalk. CuA and 1A with very short common end-stalk. Wing narrowed. Although the final stage of the process of freeing the hind-margin of veins is completed in Megascelus (previous form), the present form (Neorhaphiomidas) is not simply a variant of the previous form, but must be the end-stage of a slightly different, but largely parallel, process of freeing the hind-margin of veins. (this is because of the specialization-crossing between the two wings with respect to the stalks of respectively M4 + M3, and CuA and 1A). In fact the venation of Neorhaphiomidas can, like that of Megascelus, be derived from that of Apiocera. |
The two diagrams of morphoklines following from this derivational line are then :
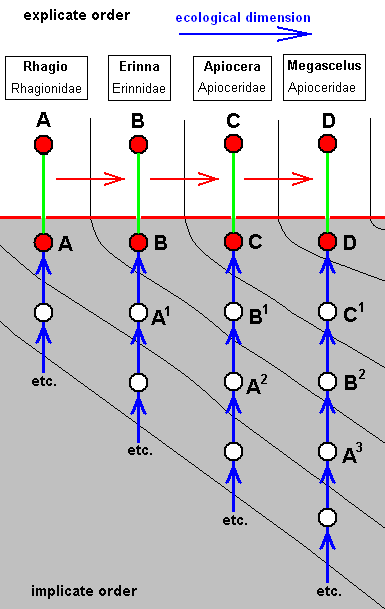
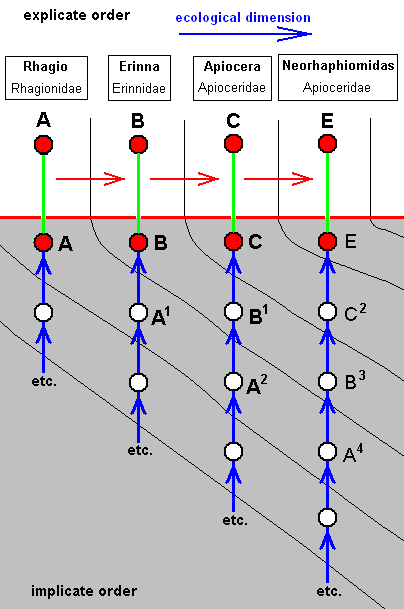
As expressed by these diagrams, all five species, viz., Rhagio scolopaceus, Erinna atra, Apiocera bigoti, Megascelus nigricornis, and Neorhaphiomidas hardyi, have, in the Implicate Order, developed independently of each other. But the empirically determinable venational derivational line shows us in what way the transformations may have taken place in the noëtic history of each of these species.
In its noëtic history the venation of Megascelus (D) (left diagram) has originated not from that of Apiocera, i.e. not from that of C, but from that of C1, whose venation is similar, but not identical, to that of C.
On the other hand, the venation of Neorhaphiomidas (E) (right diagram) has originated also not from that of C, but from that of C2, which is similar to that of C, but less so than C1 is.
In the following three lines the first three members are the same as in the previous line. Therefore, the first two of them are not depicted again. For these see first derivational line .
Second derivational line :

|
Apiocera bigoti, Apioceridae. R2 and R4 run into R1. M3 and M4 with common end-stalk. Base of M4 absent. CuA and 1A with common end-stalk.
|
|
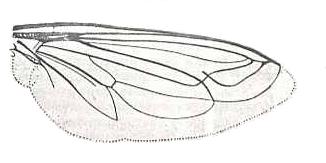
Dolichogaster nigricornis, Mydaidae. R2, R4 and R5 run into R1. M1 and M2 run into R5. M3 and M4 with common end-stalk. CuA and 1A with longer common end-stalk. |
|
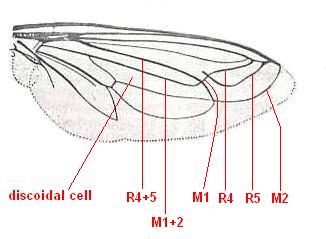
Eremomydas bek, Mydaidae. (After ROHDENDORF, 1951) R2, R4, and R5, run into R1. M1 absent. M2 curves up and ends up at the anterior margin of the wing-apex not far from end of R1. M3 and M4 form one smooth curved vein, more or less parallel to the posterior wing-margin. Their common end-stalk lost. CuA and 1A come together well before the wing-margin. Their common end-stalk is lost. Basal half of the wing fairly broad. A better interpretation of the venation of this form might be : |
|
Eremomydas bek, Mydaidae. Alternative venational interpretation. R2, R4, and R5, run into R1. M1 and M2 curve up, where M1 ends up in R5, while M2 ends up at the anterior margin of the wing-apex not far from end of R1. This interpretation then implies that the cross-vein ta [= r-m] has vanished. |
The corresponding diagram of morphoklines then is :
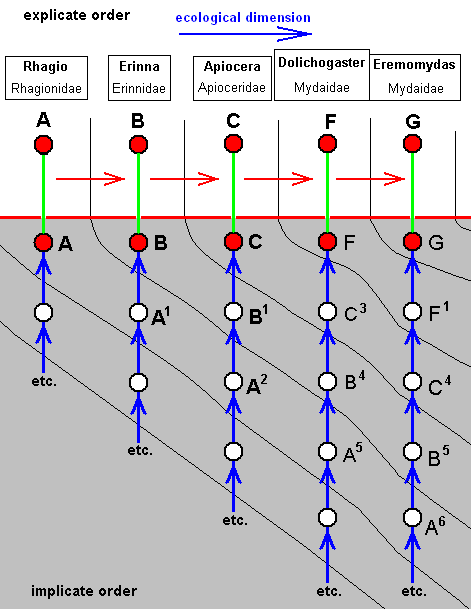
Again, the derivational line indicates how the respective venations might have evolved from each other in the individual noëtic histories of the species :
The species F has not, in the Implicate Order, originated from C, but from C3, which venationally is more or less similar to C.
The species G has not, in the Implicate Order, originated from F, but from F1, which venationally is similar to F.
Third derivational line :
(For the first and second members of this line see first and second member of first derivational line )

|
Apiocera bigoti, Apioceridae. R2 and R4 run into R1. M3 and M4 with common end-stalk. Base of M4 absent. CuA and 1A with common end-stalk. |
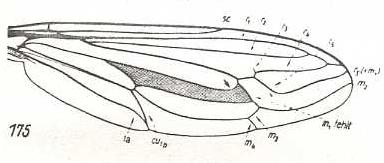
|
Rhopalia paulseni, Mydaidae. R2, and R4 run into R1. M1 absent. M2 long, curving upwards. M3 and M4 with common end-stalk. M4 and discoidal cell lengthened. Base of M4 present, but very short. CuA and 1A with common end-stalk. |
The corresponding diagram of morphoklines is :
Fourth derivational line :
(For the first and second members of this line see first and second member of first derivational line )

|
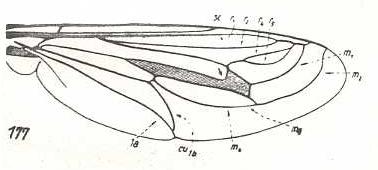
Apiocera bigoti, Apioceridae. R2 and R4 run into R1. M3 and M4 with common end-stalk. Base of M4 absent. CuA and 1A with common end-stalk. |
|
Mitrodetus dentitarsis, Mydaidae. R2, R4, R5, and M1 run into R1. R4 and R5 converge and form a common end-stalk that ends up in R1. M2 long, curving upwards parallel to M1. M3 and M4 have lost their common end-stalk. M4 and discoidal cell lengthened. Base of M4 present, but very short. CuA and 1A with common end-stalk. |
The corresponding diagram of morphoklines is :

Fifth derivational line :

|
Rhagio scolopaceus, Rhagionidae. R3 absent. |
|
Thereva nobilitata, Therevidae. Fork of R4+5 shortened. End-sections of M3 and M4 coming close together. CuA and 1A with common short end-stalk. So the wing-venation of this species is well derivable from that of Rhagio |
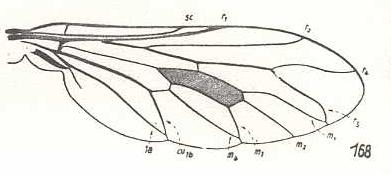
|
Chrysanthemia chrysanthemi, Therevidae. R5 and M1 with a common short end-stalk. M3 and M4 with common short end-stalk. CuA and 1A with common short end-stalk. So the venation of this species is well derivable from that of Thereva. And with this we have now a strict derivational series : Rhagio ==> Thereva ==> Chrysanthemia. |
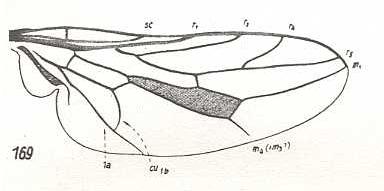
|
Omphrale fenestralis, Omphralidae. R4 and R5 curved upwards. M2 and M3 absent. M4 originating from discoidal cell, not reaching wing-margin. CuA and 1A with long common end-stalk. The venation of this species, although more or less similar to that of Chrysanthemia, cannot be derived from it, because M1 and R5 are separated again from each other. So the noëtic history of this species is independent of that of the previous one, but nevertheless morphologically related to it. |
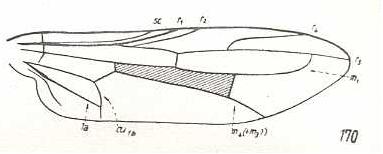
|
Pseudatrichia chilensis, Omphralidae. M1 ending up in R5 well before apex. M2 and M3 absent. M4 originating from discoidal cell, reaching wing-margin. CuA and 1A with shorter common end-stalk. Wing elongate. The venation of this species is not strictly derivable from that of Omphrale because of the different shape of the wing (letting us uncertain as to the direction of the derivation), and because of the slightly more primitive state of M4 and of the CuA-1A fork. So the venation of Pseudatrichia actually originates from a venation that is not the same but nevertheless very similar to that of Omphrale. |
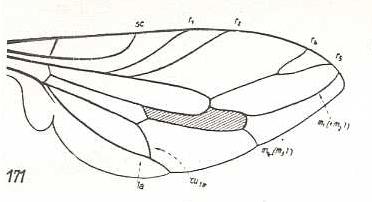
|
Apiocera maritima, Apioceridae. SC, R1, and R2, strongly curved upwards. Fork of R4+5 very short. M1 ending just above wing-apex. M2 and M3 absent. M4 originating from discoidal cell, reaching wing-margin. CuA and 1A with a moderately long end-stalk. Wing broadened. With respect to Pseudatrichia there exists a specialization-crossing : Apiocera represents a derived condition with respect to the curvings of SC, R1, and R2, while Pseudatrichia is in a derived condition as a result of the course of M1. |
The corresponding diagram of morphoklines is :

The venation of species K (Chrysanthemia chrysanthemi) (and with it the species) does not originate from that of J, but originated (in the Implicate Order) from that of J1, which is similar (but not identical) to that of J.
The venation of species L (Omphrale fenestralis), as we see it in the Explicate Order, is far from strictly derivable from that of Chrysanthemia as [this non-derivability is] expressed by the separate column of its noëtic history in the present diagram, but is nevertheless morphologically related to that of Chrysanthemia, expressed by the black line connecting the two in the diagram. This line says that the noëtic precursor L1 of Omphrale has some morphological traits also present in K, that is in Chrysanthemia.
Also the noëtic history of the species M (Pseudatrichia chilensis) is independent from the previous ones in the sense of : M has not originated from L, but from M1 which has some moprphological traits in common with L.
The same applies to the species N (Apiocera maritima). It has an independent noëtic history. It moreover belongs to a family different from that of the previous two species (indicated by the color of the columns).
Sixth derivational line :

|
Rhagio scolopaceus, Rhagionidae. R3 absent. |
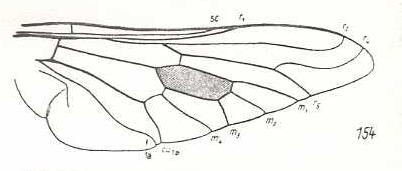
|
Pelecorrhynchus personatus, Tabanidae. Fork of R4+5 shortened. R4 markedly curves upwards (parallel to R2), ending up at the wing-margin before wing-tip. The latter markedly pointed. M1, M2, M3, M4, CuA, and 1A still in a primitive condition. Basal half of wing broadened. Anal lobe and alula strongly developed. |
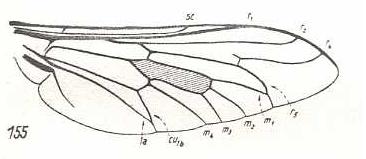
|
Tabanus bromius, Tabanidae. CuA and 1A have a common end-stalk. |
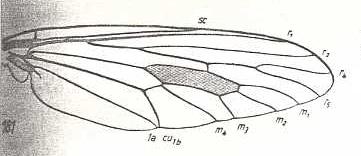
|
Pangonia maculata, Tabanidae. R5 and M1 curving towards each other, having a common end-stalk. So also CuA and 1A. |
The corresponding diagram of morphoklines is :

Seventh derivational line :

|
Rhagio scolopaceus, Rhagionidae. R3 absent. |
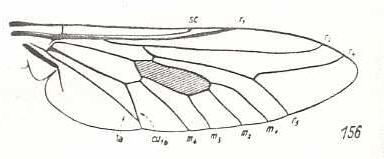
|
Heteropogon scoparius, Asilidae. Fork of R4+5 shortened. M1, M2, M3, M4, CuA, and 1A still in a primitive condition. CuA and 1A coming very close to each other. |
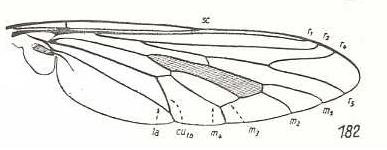
|
Bathypogon brachypterus, Asilidae. Fork of R4+5 widened. M3 and M4 coming together, forming a common end-stalk. Also CuA and 1A forming a common end-stalk. |
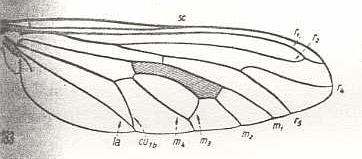
|
Laphria marginata, Asilidae. R2 ends up in R1. M3 and M4 coming together, forming a common end-stalk. Also CuA and 1A forming a common end-stalk. |
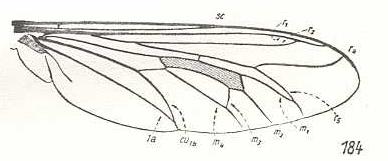
|
Dasythrix grisea, Asilidae. R2 ends up in R1 well before the latter's end. R5 and M1 coming together, forming a common end-stalk. So also M3 and M4, and CuA and 1A. R5+M1, M2, and M3+M4 hardly reach the wing-margin. The venation is in the process of making the wing's hind-margin clear of veins. |
The corresponding diagram of morphoklines is :

Eighth derivational line :

|
Rhagio scolopaceus, Rhagionidae. R3 absent. |
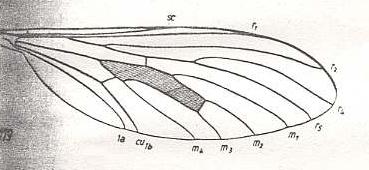
|
Leptogaster cylindrica, Asilidae. Basal section of M4 suppressed. CuA and 1A running parallel to each other. |
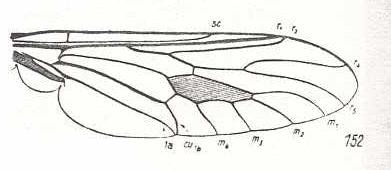
|
Coenomyia ferruginea, Erinnidae. Basal section of M4 suppressed. CuA and 1A almost meeting each other at the wing-margin. Wing elongate. |
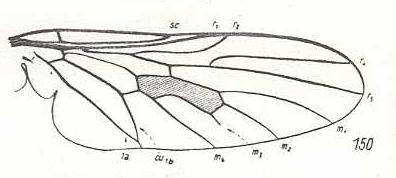
|
Spaniopsis clelandi, Rhagionidae. M3 reduced. Only its basal section survived. CuA and 1A having a short common stalk. |
The corresponding diagram of morphoklines is :
Ninth derivational line :

|
Rhagio scolopaceus, Rhagionidae. R3 absent. M1, M2, M3, M4, CuA, and 1A all present in their original configuration. R5, M1, M2, M3, M4, and of course CuA and 1A, all curved obliquely toward the wing's hind-margin. |
Because of R3, there is a specialization-crossing between Rhagio scolopaceus (R3 absent) and Hirmoneura bellula (R3 present), but not between Hirmoneura obscura (10th line) (R3 absent) and Rh. scolopaceus in this respect.
The wing-venation of the present species (and also of the ones that follow) is very idiosyncratic, and therefore not really derivable from that of Rhagio [in fact, apart from R3, only formally so]. More precisely, we should hold that the venation of Hirmoneura bellula is not d i r e c t l y derivable from that of Rhagio [let alone the species as a whole!], but only indirectly so, meaning that although being members of the same linear series of true venational derivations they lie far apart from each other in that series. The transition from Rhagionid venation to Nemestrinid venation involves a wholesale rearrangement of the entire venation. |
Hirmoneura bellula, Nemestrinidae. R3 present as a short vein running from R4 to R2. The cross-vein r-m (ta) almost suppressed by the anterior part of the discoidal cell (which part is M1+2). The veins SC, R1, R2, R4, R5, M1, and M2 running closely parallel to each other to the apical part of the wing. Also M4 runs in the same direction. [The course of all the mentioned veins is therefore strongly d e r i v e d with respect to the course of these veins in Rhagio]. Extra cross-veins are present between R5 and M1, and between M1 and M2. M3 present as a very short vein between M2 and M4. The veins M3 and M4 form a common end-stalk ending at the posterior margin of the wing. CuA and 1A end up close together at the wing-margin. Wing more or less elongate. A pseudo longitudinal vein has developed (seen in most, if not all, Nemestrinidae) running from the base of RS obliquely to the hind-margin of the wing. This vein is composed of parts of other veins : base of RS, R4+5, apical section of M1+2, basal part of M2, basal part of M3, and common stalk of M3 and M4. As in all Nemestrinidae the basiala is large, provided with a strong phragm. |
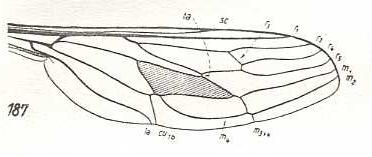
|
Symmictoides simplex, Nemestrinidae. R3 still present as a short vein running from R4 to R2. The cross-vein r-m (ta) almost suppressed by the anterior part of the discoidal cell (which part is M1+2). SC shortened. The veins R1, R2, R4, R5, M1, and M2 running closely parallel to each other (but not so neatly as in Hirmoneura bellula) to the apical part of the wing. Extra cross-veins absent. Basal section of M3 suppressed by M4. Still M3 and M4 end up with a common stalk. Basal section of M4 (although not a cross-vein, it is indicated as tb in the drawing of Hirmoneura bellula), as it normally connects with the cross-vein m-cu, is suppressed. CuA and 1A end up close together at the wing-margin. |
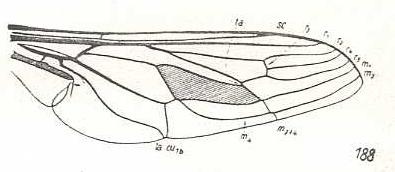
|
Rhynchocephalus fasciatus, Nemestrinidae. R3 still present as a short vein running from R4 to R2. Basal half of the wing somewhat broadened. Anal lobe and Alula stgrongly developed. |
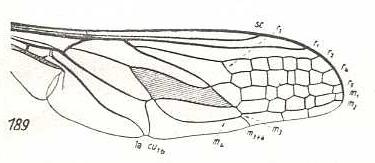
|
Nemestrinus aegyptianus, Nemestrinidae. R3 still present as a short vein running from R4 to R2. The veins R2, R4, R5, M1, and M2 still present as longitudial veins heading towards the apex of the wing. Between them many cross-veins are developed. |
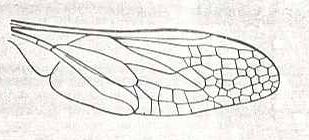
|
Nemestrellus abdominalis, Nemestrinidae. (After ZACK, in ROHDENDORF, 1951) R3 apparently still present as a cross-vein like veinlet running from R4 to R2. The veins R2, R4, R5, M1, and M2 still present as longitudial veins heading towards the apex of the wing. Their more or less zigzagged course tends to mask them as true longitudinal veins. As a further stage of the development of a more dense venation in the apical part of the wing, cross-veins are in the present form (Nemestrellus) also present between R1 and R2. Further are they developed between the discoidal cell and M4, and between the latter and the posterior wing-margin. |
The corresponding diagram of morphoklines is :
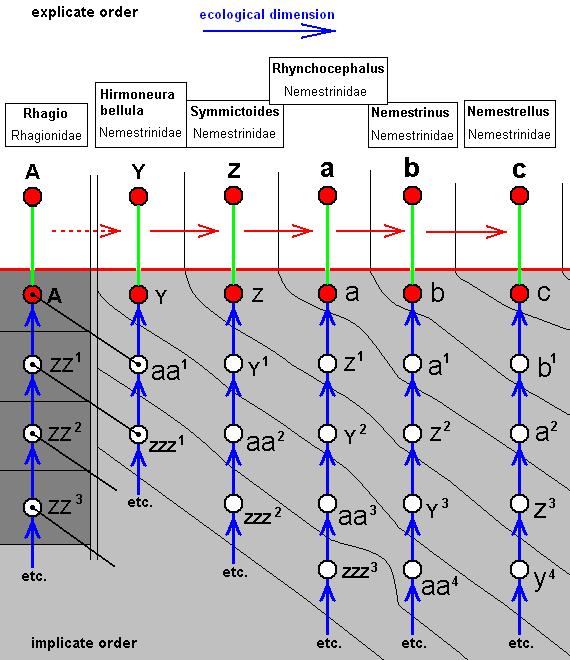
The venation of Y (Hirmoneura bellula, Nemestrinidae) has not directly originated from that of A (Rhagio, Rhagionidae), but at most only indirectly so. Indeed, the overall formal plan of the venation of A is still preserved in that of Y. So although Y originates not from A but from aa1, the latter has some similarity with A, as indicated by the black line connecting them.
Tenth derivational line :
The next three derivational lines have in their beginning the same four members (and in the same order), that is to say, the last member of this common part is the wing-venation of Rhynchocephalus fasciatus (Nemestrinidae), and we let begin the lines with this species :

|
Rhynchocephalus fasciatus, Nemestrinidae. R3 still present as a short vein running from R4 to R2. Basal half of the wing somewhat broadened. |
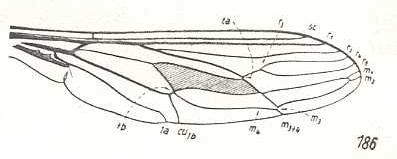
|
Hirmoneura obscura, Nemestrinidae. R3 entirely absent. The venation of the present species highly similar to that of Rhynchocephalus, but the basal part of the wing less broadened. |
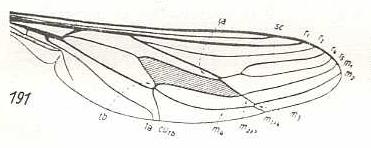
|
Trichophthalma barbarossa, Nemestrinidae. R3 still entirely absent. Wing-venation almost identical to that of Hirmoneura obscura. |
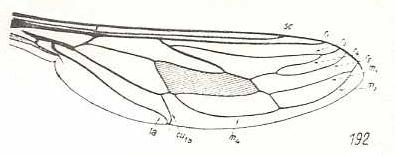
|
Stenopteromyia bolivari, Nemestrinidae. R3 still entirely absent. R4 runs into R2. M1 and M2 with a common end-stalk. Common stalk of M3 and M4 absent. CuA and 1A at their ends very close together. As in all the above forms, the phragm is very conspicuous. |
The corresponding diagram of morphoklines is :
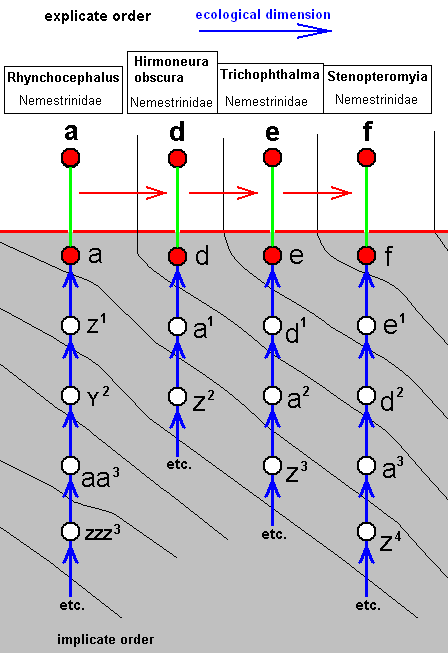
Species/strategy d has not, as we will suppose, originated from species/strategy a (while its venation can strictly be derived from that of a ), but has originated from species/strategy a1, which is similar to a.
The same is the case with the other species/strategies.
Eleventh derivational line :

|
Rhynchocephalus fasciatus, Nemestrinidae. R3 present as a short vein running from R4 to R2. Basal half of the wing somewhat broadened. |
|
Neorhynchocephalus tauscheri, Nemestrinidae. R3 still present as a short vein between R4 and R2. Cross-vein ta [= r-m] suppressed. Common end-stalk of M3 and M4 absent. The cross-vein m-cu and the base of M4 suppressed. |
|
Fallenia fasciata, Nemestrinidae. R3 still present. R4 and R5 converge to each other and coalesce. Their common end-stalk ends up in the anterior margin of the wing-apex. M1 runs into the conmon end-stalk of R4 and R5. M2 ends up at the anterior margin of the wing-apex. The common end-stalk of M3 and M4 is absent. CuA and 1A have a common short end-stalk. |
The corresponding diagram of morphoklines is :
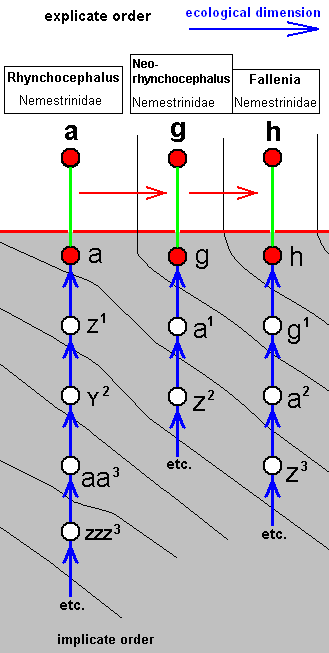
As in most other cases, although the wing-venation of Neorhynchocephalus can perfectly be drived from that of Rhynchocephalus, and that of Fallenia from that of Neorhynchocephalus, we do not expect such derivability of the whole species [i.e. including characters other than wing-venation], especially because these three species belong to three different genera. So, to take an example, g has not originated from a, but from a1, which is similar to a.
Twelfth derivational line :

|
Rhynchocephalus fasciatus, Nemestrinidae. R3 present as a short vein running from R4 to R2. Basal half of the wing somewhat broadened. |
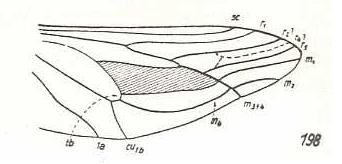
|
Atriadops spec., Nemestrinidae. R3 absent (or preserved as a short furrow). R4 absent (or preserved as a fold or furrow). R5 present. It or R4+5 originates from the discoidal cell. M1 and M2 present, running to the posterior margin of the wing-apex. Common end-stalk of M3 and M4 present. Base of M4 and the cross-vein m-cu present. CuA and 1A far away from each other, not converging. These last two features demonstrate the presence of specialization-crossing. |
For this derivational line and the next one we will give one single diagram of morphoklines.
Thirteenth derivational line :
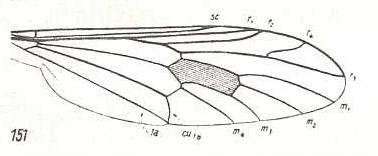
The thirteenth derivational line is not really a series of successive derivations, but only one venation being derived from a highly similar one. It is the venation of Nycterimorpha speiseri that is derived from that of Nycterimyia horni (both belonging to the family Nemestrinidae. see next two Figures). Not only the venation, but also the shape of the wings are peculiar in these species. Because the venation of these species has been changed so much with respect to the original nemestrinid venation [It is, by the way, theoretically better here not to speak of the venation having been "changed", but of the venation being just d i f f e r e n t] the determination of the identity of the veins may contain uncertainties. And the morphological gap existing between these forms, on the one hand, and the prototypic venation of any dipterous family, on the other, is so wide that we cannot let the venation of either Nycterimorpha or Nycterimyia be preceded by any other venation formally lying between them and the venation of Rhagio scolopaceus (Rhagionidae) which is the venational prototype of the group of families here under discussion (and which, at the same time, is very close to the venational order-prototype of Diptera). So although the venation of Nycterimorpha can easily be derived from that of Nycterimyia, the latter is morphologically more or less independent (it represents a "new chapter" in nemestrinid venation).
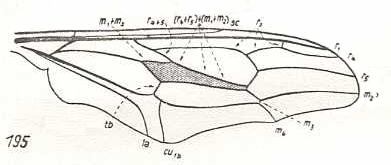
|
Nycterimyia horni, Nemestrinidae. R2 ending up in R1 well before the latter's end. R4+5, after having separated from R2+3, joins with M1+2 (and thus the vein [R4+5]+[M1+2] forming the anterior border of the discoidal cell), then, at the end of the cell runs upwards before splitting up in R4 and R5. While R5 proceeds to the wing apex, R4 curves back and joins with R2. And when, after a while, the latter connects with R1, R4 continues its course to the anterior margin of the wing-apex. M1 is lost. M2 is present and proceeds to the wing-apex, after it has given off M3 which directly joins with M4 forming their common end-stalk. Base of M4 clearly present (in the drawing indicated as tb). The cross-vein m-cu suppressed. CuA and 1A parallel, not converging to each other. In the basiala the phragm (as in all nemestrinids) is strongly developed. We have now in fact derived this venation from the venational order-prototype of Diptera, or from that of Rhagio for that matter (see Figure 1, above ). |
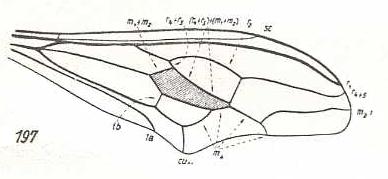
|
Nycterimorpha speiseri, Nemestrinidae. R1 very long. It bends down to meet the apical wing-margin. R2 only present until it meets with R4. The rest of it has vanished. R4 either totally vanished or coalesced with R5. M1 lost. Basal section of M3 suppressed. Common end-stalk of M3 and M4 present. M4, after having split off from M3+4, and thus coming off from the discoidal cell (this basal section of it being indicated in the drawing by tb), joins with CuA for some distance (and thus suppressing the cross-vein m-cu), but instead of now going to the posterior wing-margin (and meeting M3 again, as in Nycterimyia) M4 curves up towards the discoidal cell, joining with the latter's posterior border (which is in fact M3), and finally joins with M2. End sections of CuA and 1A relatively far apart from each other. While the overall (peculiar) shape of this wing is the same as that of Nycterimyia, its proximal half has narrowed. |
The corresponding diagram of morphoklines for derivational lines 13 and 12 is :
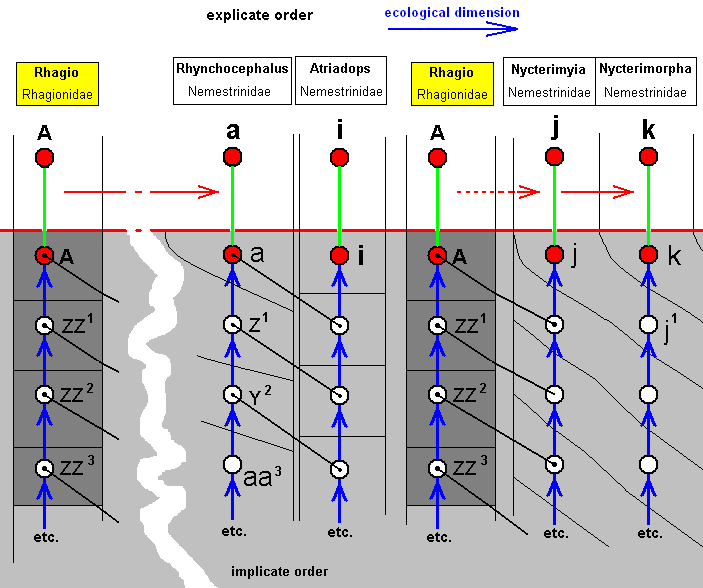
The venation in Rhynchocephalus can, via some intermadiates, be derived from that of Rhagio, see ABOVE . The venation in Nycterimyia also can eventually be derived from that of Rhagio. Although, in the Implicate Order one and the same noëtic history of a given organismic species exists only as one single line (in noëtic space no pattern can be exactly repeated into identical 'individuals'), we, in our diagram, introduce the noëtic history of Rhagio scolopaceus twice, just for convenience.
The venation of Atriadops shows about the same venational configuration as that of Rhynchocephalus, but cannot be derived from it because of specialization-crossing. Therefore, the species i (Atriadops spec.) and the species a (Rhynchocephalus fasciatus) noëtically developed independently of each other, while both possessing some common elements.
The venation of Nycterimyia can, indirectly, be derived from that of Rhagio, but it has a peculiar wing shape, rendering it (together with next species) a bit isolated from other Nemestrinids. The venation of the mentioned next species, namely that of the genus Nycterimorpha may be derived from that of Nycterimyia resulting in : The species/strategy k, although not directly originating from the species/strategy j, has originated from the strategy j1 of which the venation is similar to that of j.
Fourteenth derivational line :
The next two derivational seqences, concering the family Bombyliidae (bee flies), should, within this family, either begin with Exoprosopa stupida or with Thyridanthrax afer. If we take Exoprosopa to start the line within Bombyliidae, then we cannot let it be preceded by Rhagio scolopaceus because the latter has R3 lost while the former still preserves a section of it. If we, on the other hand, take Thyridanthrax to be the first member, it can follow up on Rhagio, but cannot give rise to the many other bombyliid wings which have preserved R3. So the two derivational lines, situated entirely within the family Bombyliidae, both begin with the venation of Exoprosopa.
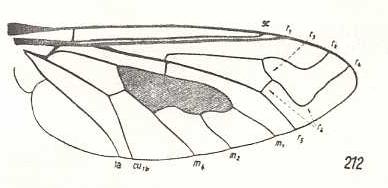
|
Exoprosopa stupida, Bombyliidae. R2 markedly curved up towards the anterior margin of the wing-apex, far away from the end-point of R1. R3 present, branching off from R2+3, and ending up in R4. The latter also markedly curving up to the anterior margin of the wing-apex. R5 directly heading towards the posterior wing-margin. Of the Medial system M3 is absent. Basal part of M4 suppressed. The cross-vein m-cu present. CuA and 1A only slightly converging. Phragma well developed. We have now in fact derived this venation, not from that of Rhagio, but from that of the order-prototype of Diptera (see Figure 1 (upper image) ). |
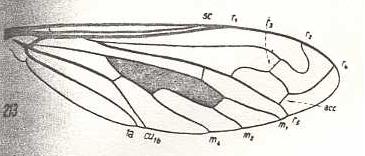
|
Ligyra lugubris, Bombyliidae. R2 strongly undulates, such that its end-section runs backwards. It gives off R3 which ends up in R4. The latter runs more or less parallel with R2. Between R4 and R5 an accessory cross-vein (acc). |
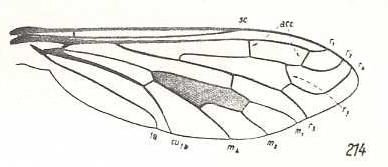
|
Henica longirostris, Bombyliidae. R3 clearly present, branching off from R2+3. R2 slightly curving up to the anterior margin of the wing-apex. Between R1 and R2(+3) two accessory cross-veins (acc). R4 curving up parallel to R2. |
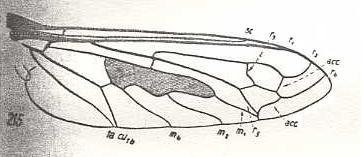
|
Hyperalonia bombyliformis, Bombyliidae. R3 clearly present, branching off from R2+3. R2 slightly curving up to the anterior margin of the wing-apex. Between R2 and R4 one accessory cross-vein (acc). Between R4 and R5 one accessory cross-vein. R4 curving up more or less parallel to R2. R5 approaching M1 and joining up with the last mentioned cross-vein. It then goes, merged with M1, to the posterior wing-margin. M3 missing. |
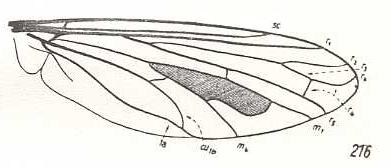
|
Toxophora maculata, Bombyliidae. R3 clearly present, branching off from R2+3. Basal section of R4 looks like a cross-vein between R4 and R5. R5 and M1 not converging. M2 and M3 missing. CuA and 1A have a common end-stalk. Specialization-crossing with Hyperalonia because of R5 and M1 not having a common end-stalk. |
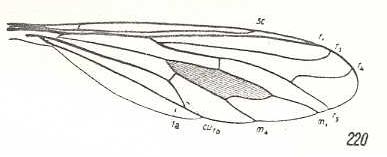
|
Systropus excisus, Bombyliidae. R3 completely absent. Wing markedly narrowed at its base. The wing is elongate. |
The corresponding diagram of morphoklines is :
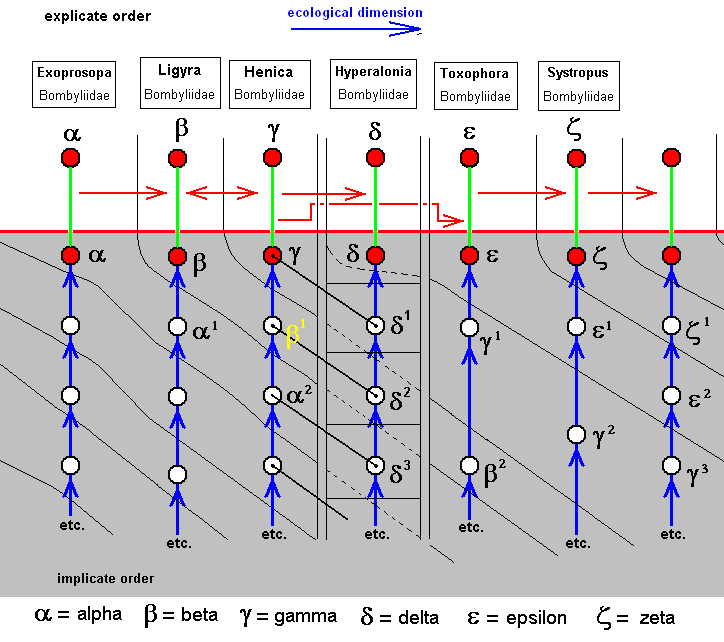
To signify the species/strategies we here use Greek letters (for no more special reason than having at our disposal yet more symbols). Some explanatory examples :
Beta does not originate from Alpha, but from Alpha-1, wich is similar to Alpha.
Delta does not originate from Gamma, but from Delta-1, which shares some common traits with Gamma.
Epsilon does not originate from Delta, neither from Gamma, but from Gamma-1, which is similar to Gamma.
Zeta does not originate from Epsilon, but from Epsilon-1, which is similar to Epsilon.
The last column is added in order to see how this sequence might continue.
The wing-venations of the species Beta and Gamma can be derived from each other both ways.
The venational derivational sequence is interrupted at Delta after having it included. Then, from the species next to Delta, the sequence is picked up again, i.e. the [wing-venation of the] species Epsilon can be derived from [that of] the species Gamma (but in fact the species Epsilon as a whole has originated from the species/strategy Gamma-1).
Fifteenth derivational line :

|
Exoprosopa stupida, Bombyliidae. R2 markedly curved up towards the anterior margin of the wing-apex, far away from the end-point of R1. R3 present, branching off from R2+3 and ending up in R4. The latter also markedly curving up to the anterior margin of the wing-apex. R5 directly heading towards the posterior wing-margin. Of the Medial system M3 is absent. Basal part of M4 suppressed. The cross-vein m-cu present. CuA and 1A only slightly converging. Phragma well developed. We have now in fact derived this venation, not from that of Rhagio, but from that of the order-prototype of Diptera (see Figure 1 (upper image) ). |
|
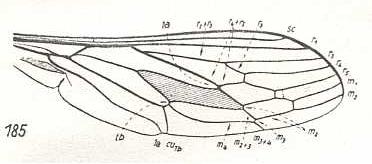
Argyramoeba oedipus, Bombyliidae. R3 partly vanished (the 'remnant allegedly left by it' may be a new formation : re-activation of part of the old 'bed' of R3). M3 may be present as a very short remnant. CuA and 1A converging. |
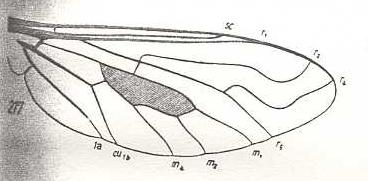
|
Thyridanthrax afer, Bombyliidae. R3 totally absent. M3 totally absent. |
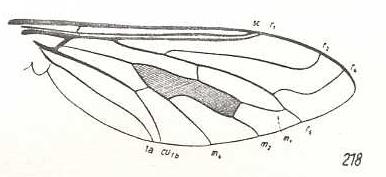
|
Callistoma fascipennis, Bombyliidae. R3 still absent. So also M3. R5 and M1 have a common end-stalk. |
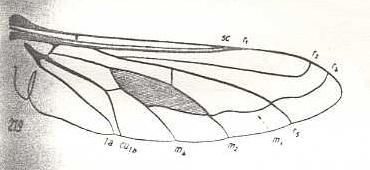
|
Heterostylum robustum, Bombyliidae. R3 still absent. So also M3. M1 and R4 lining up, as if one vein. Wing elongate. |
The corresponding diagram of morphoklines is :
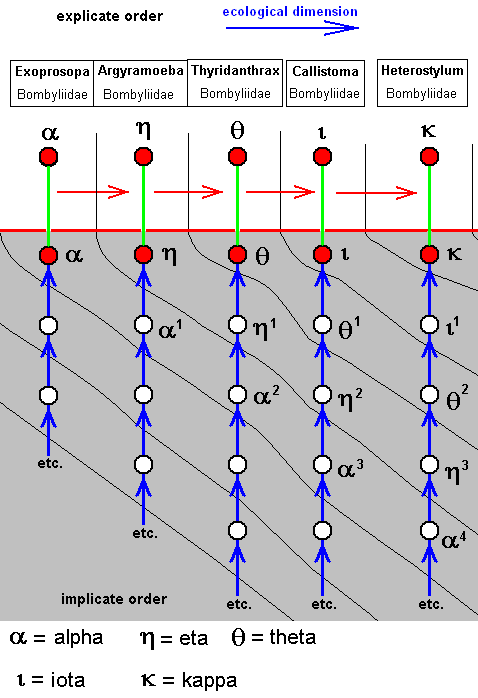
Such as : The species Eta has not originated from the species Alpha, but from Alpha-1 which is similar to Alpha.
Sixteenth derivational line :

|
Rhagio scolopaceus, Rhagionidae. R3 absent. M1, M2, M3, and M4, present. CuA an 1A converging. |
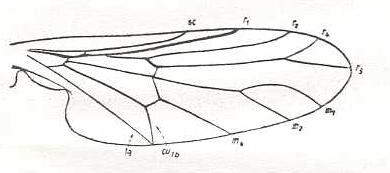
|
Hilarimorpha singularis, Hilarimorphidae (being close to Rhagionidae or Empididae, or even to Bombyliidae). R3 absent. Fork of R4+5 very short. M3 absent. Discoidal cell absent (i.e. intermedial cross-vein tp absent). CuA and 1A having a common end-point on the posterior wing-margin. |
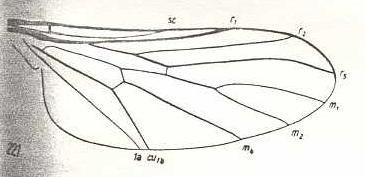
|
Cyrtosia meridionalis, Bombyliidae. Fork of R4+5 vanished. The curving of the remaining vein shows it to be R5. Discoidal cell still absent. M3 still absent. Cua and 1A converging. |
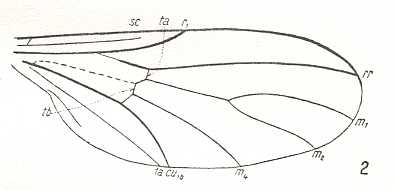
|
Empidideicus spec., Bombyliidae. (After EDWARDS, 1926, in HENNIG, 1954) SC slightly reduced (its end-section vanished). R2 absent, meaning that now RS is unbranched. M3 and Discoidal cell still absent. Main trunk of M turned pale. |
The corresponding diagram of morphoklines is :
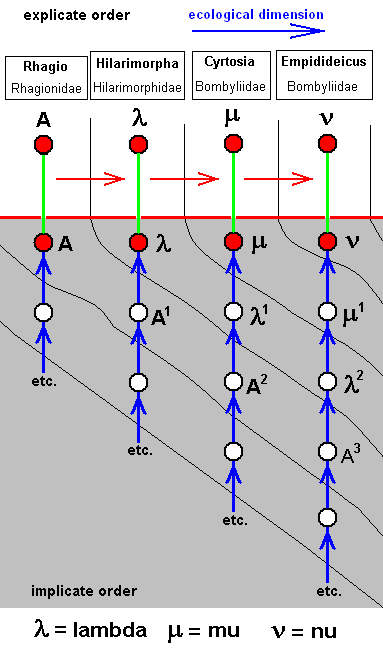
Such as : The species Lambda has not originated from the species A, but from A-1 which is similar to A.
Seventeenth derivational line :

|
Rhagio scolopaceus, Rhagionidae. R3 absent. M1, M2, M3, and M4, present. CuA an 1A converging. |

|
Hilarimorpha singularis, Hilarimorphidae (being close to Rhagionidae or Empididae, or even to Bombyliidae). R3 absent. Fork of R4+5 very short. M3 absent. Discoidal cell absent (i.e. intermedial cross-vein tp absent) [The next Figure clarifies the way the discoidal was lost]. CuA and 1A having a common end-point on the posterior wing-margin. |
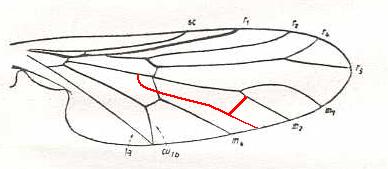
|
Again Hilarimorpha singularis. It is shown how the discoidal cell was lost in this and other forms. |

|
Cyrtosia meridionalis, Bombyliidae. Fork of R4+5 vanished. The curving of the remaining vein shows it to be R5. Discoidal cell still absent. M3 still absent. Cua and 1A converging. |
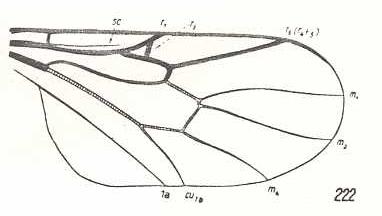
|
Glabellula nobilis palaestinensis, Bombyliidae. (After ENGEL, 1933, in HENNIG, 1954) It is perhaps important to note that this wing is only 1 mm long (and is placed by ROHDENDORF, 1951, in the Lifting Costalized (stratiomyoid) functional wing-type). SC partly reduced. R1 thick and short. R2 very short, ending up in R1. R5 (or the coalescence of R4 and R5) present as a thick vein. M3 absent. Discoidal cell absent. The 'cubital fork', consisting of CuA, the cross-vein m-cu and M4, and also the base of M4 have shifted distad. CuA and 1A almost parallel. |
The corresponding diagram of morphoklines is :
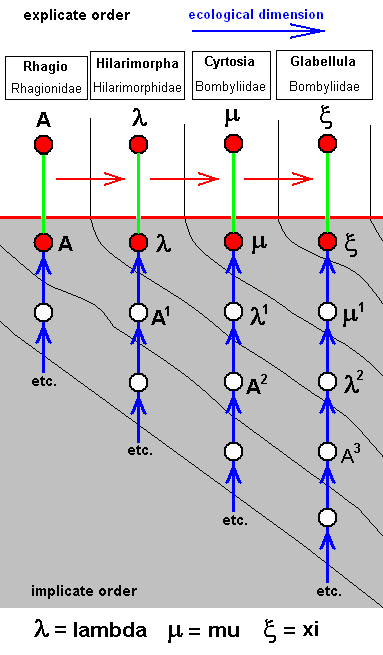
Such as : The species Mu has not originated from the species Lambda, but from Lambda-1 which is similar to Lambda.
The last three forms of the above sequence are in fact hard to derive from venations of primitive brachycera-orthorrapha such as that of Rhagio. And, moreover, the wings of these forms do certainly not belong to the tabanoid functional type. They are, morphologically and derivationally, more or less independent [This is, however, true of many other venations as well. We express this in our diagrams of morphoklines by having their noëtic histories semi-independent.]
The following three forms of tabanoid wings are more clearly derivationally independent :
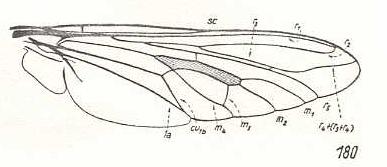
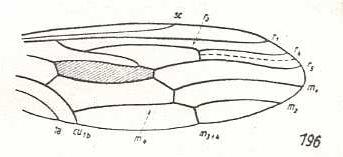
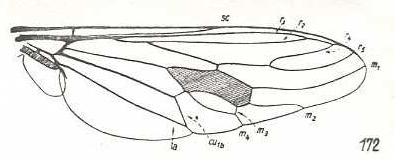
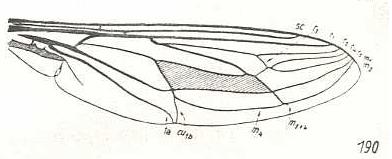
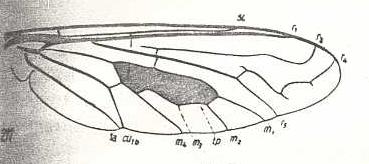
Figure 3: Wing of Cyclopsidea spec., Nemestrinidae.
R4 and R5 present if we interpret the whole crossvein-like connection between two Radial veins as being the base of R4. In that case R2 and R3 are absent, and R2+3 present. Also R4+5 is present partly merging with the anterior border of the discoidal cell (M1+2). If, on the other hand, we interpret that connection as part of a T-vein and the fold (dashed line) as R3, then, R2, R3, and R5 are present, whereas of R4 only its basal section is preserved. M4 shifted distad along CuA.
(After HARDY, 1946, in HENNIG, 1954)
The above seventeen derivational lines (sequences) may be summarized in the following diagram :
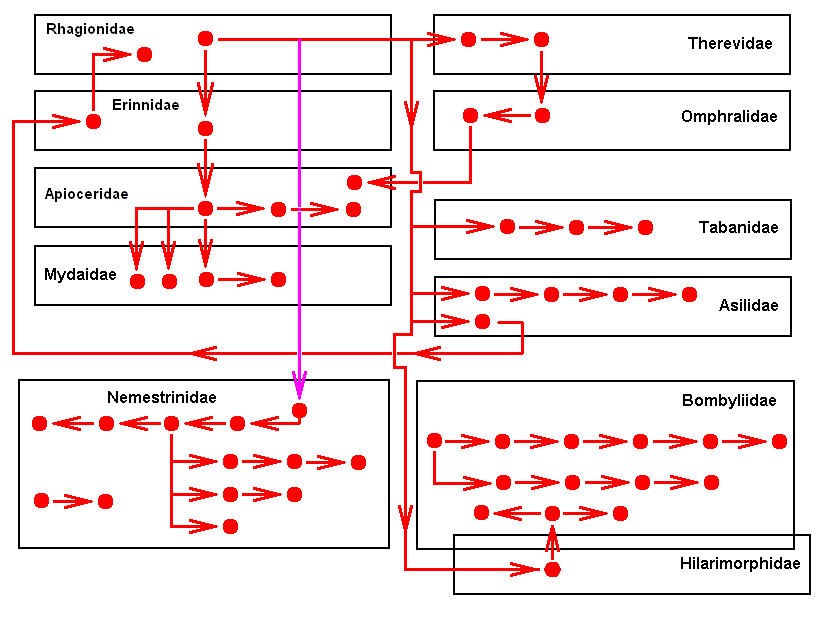
We clearly see that the derivational structure is not hierarchical (i.e. like an ancestral tree) but reticular, albeit without (precisely) closed loops. It is not hierarchical because of the existence (in Bombyliidae and in Nemestrinidae) of derivationally isolated sequences (and even of (three) points (Promachus leoninus Lw., Asilidae. Cyclopsidea spec., Nemestrinidae. Trichopsidea spec., Nemestrinidae. See Figures 2, 3, and 4 above ))
The points, and the first members of these isolated sequences apparently cannot be derived from a wing of some primitive representative of the Brachycera-orthorrapha, but can be derived from the dipterous order-prototype, although the derivation is rather long, in the sense that one or more intermediate forms are required. Because we have not investigated all existing (recent ot fossil) Brachycera-orthorrapha, such intermediate forms may well exist or have existed. And indeed, we assume them so.
If we say that in principle all dipterous venations can be derived from the order-prototype, it means that they can be derived from one and the same type of venation, but that does not necessarily imply that all dipterous venations have evolved from the venation of one single ancestral species.
Having concluded the derivational aspects of tabanoid wings, we will, in the next document, discuss the next functional wing-type in Diptera, namely the Lifting Costalized Stratiomyoid Type.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for further study of Organic Evolution, Part LXXII.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII
Back to Evolutionary Part LVII
Back to Evolutionary Part LVIII
Back to Evolutionary Part LXII
Back to Evolutionary Part LXIII
Back to Evolutionary Part LXIV
Back to Evolutionary Part LXVI
Back to Evolutionary Part LXVII
Back to Evolutionary Part LXVIII
Back to Evolutionary Part LXIX
Back to Evolutionary Part LXXa
Back to Evolutionary Part LXXb
Back to Evolutionary Part LXXI