

Here we have the phase, characterized by that particular evolutionary step in which for the first time the basic feature of vespine life appeared -- concealing the prey. Paralyzation of the prey preceded this stage, but up until [the evolutionary appearance of the ability and habit of] paralyzation there is no preliminary preparation for getting the prey concealed, as is also absent in the terebrants. On the other hand, the very pursuit of displacing the paralyzed prey is now seen even when a prey is looked for which is, without being forcibly displaced, already living in a concealed way -- in the soil, in various burrows and hiding places. Further, as before corresponding to the previous course of development, for every wasp-larva only one prey is prepared, sufficient as to its size to the satiation of it. In connection with this state of affairs the prey is not yet transported by air (heaviness of the prey), but dragged along a portage, or more, by separate jumps and short flutters, sometimes even over the surface of water. Here the wasps themselves, with a few exceptions, move, like the bethyloids, backwards, holding the prey only with their mandibles.

Thus, Scolia hirta Schr. applies to its prey, the larva of Cetonia (a chafer-like beetle), only one 'blow' and always on the same place -- at the underside in the middle between the pro- and mesothorax, consequently precisely under the ventral ganglionic mass. The sting remains in the wound for a certain time, and, judging from the movement of the abdomen of the scoliid, it attempts to sting the ganglion itself or at least to bath it with venom. The effect directly sets in, and the prey becomes totally immobile, excluding really only the antennae and mouthparts, which now and then may weakly move (FABRE, 1906).
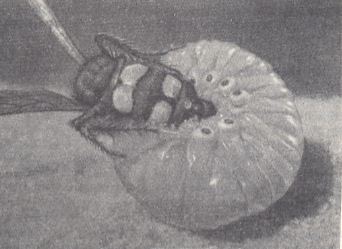
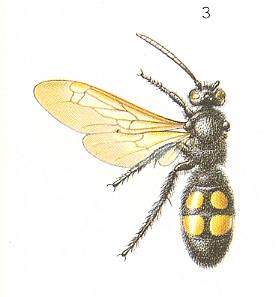
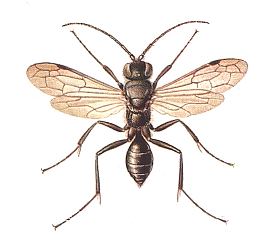
Figure 3 : A female Scolia flavifrons F. paralyzes a larva of a rhinoceros beetle.
(After MALYSHEV, 1966)
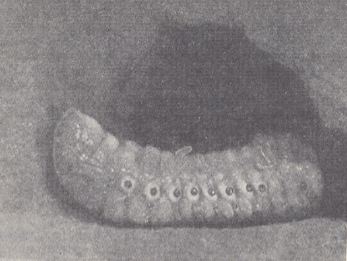
Figure 4 : Paralyzed larva of a rhinoceros beetle with an egg of Scolia flavifrons deposited on it, in the cavity of the cell.
(After MALYSHEV, 1966)
One thing especially catches the eye, namely the selfcontrol of the wasp, not letting its sting go through until in the course of the tenacious struggle the wasp is in a position to direct the sting at a determined place on the thorax of the prey, where indeed the ganglionic mass is present. The wasp simply being in contact with the delicate body of the prey, the larva of the rhinoceros beetle, on all sides accessible to stinging, has no effect until the right place is [at last] found. It becomes clear that the effort of the wasp is to apply the venom not simply somewhere in the blood, but direct it right into the center of activity of the prey, into the central nervous system. These are already entirely not those methods anymore as observed in terebrants.
About this, see NIELSEN, 1935. According to the special investigations of NIELSEN the venom of the wasp Ammophila campestris Jur. contains a specific neurotoxine, causing severe degenerative changes in the ganglion cells, of which the nuclei, as a result of paralyzation of the prey, show "diffuse dissolution or a dust-like disintegration".
But today we see that FABRE was right.
The detailed study of the behavior of the digger wasp Notogonia pompiliformis Pz. (Liris nigra v. d. L.), carried out by SEINER, 1957, 1958, removes all doubts in this respect. The small, 9-12 mm long Notogonia, systematically stands close to the above mentioned Larrinae, and also hunts for crickets (Gryllidae). The paralyzation itself of its prey was studied from various angles, especially as to the traces that remain on the integument of the prey as a result of each sting, [further] as to the angle under which the sting is lowered at the point of stinging, and with it also the effect of each sting. It was found that in the paralyzation of the cricket 4 ganglia of its ventral nervous chain were hit. Of them 3 thoracic and 1 suboesophagal. In usual normal conditions the number and consecutivity of the stings during the whole operation did not vary much. The traces of stinging, having appeared on the prey's body a few hours after the operation, were clearly localized at 6 determined points, where every time the sting penetrates the prey in a corresponding direction, constant for these points, independently of the wholly different position in which at the moment of operation the wasp itself might be. These points also were the zones of access of the sting to the nerve nodes, which [conclusion] is confirmed by the immediate effect visible at each act of stinging.
The paralyzing acts of wasps, the ability of them to hit the prey, acting with the venom-bearing sting toward the central nervous system, is no myth, as thought also WHEELER, 1923, but a fact, deserving close study. It is especially clear and nicely expressed precisely in those cases in which only one prey is hit, sufficiently large for the wasp larva to feed on.
It was when for the preservation of their larvae the ancestors of the wasps began to hide their preys at distant places when the paralyzing act of them gained new significance -- depriving the prey of the possibility to resist transport. In fact, transporting a prey that is fairly larger than the attacker -- larger, in order to completely raise the not less large parasite -- in cases where the prey is able to resist, if it were only passively so (it could be stuck in the grass, soil, etc.), is a task that the wasp can hardly accomplish. This difficulty is in a significant degree removed when the prey is paralyzed.
It is interesting that already among the bethyloid wasps the small black Epyris extraneus Brid. (of the Epirinae, family Bethylidae), similar in its look to Tiphia, shows a striving for displacing its prey. For this wasp, see next Figure.
This epyrine attacks the larva of the mealbeetle (Tenebrionidae) -- which is remarkably larger than the wasp itself -- and paralyzes it almost to complete immobility. Then it grabs the prey, apparently at its antenna, and drags it, while moving backwards, into one or another crack in the soil. There it makes some deepening around the beetle larva and adheres one egg to the prey [that egg] in a strictly determined position : on the ventral side of the first abdominal segment in a longitudinal direction such that the head-end of the egg is directed to the head of the prey. The hatched epyrine larva first lies along the prey's abdomen (see next Figure), but later assumes a vertical position, making a right angle with the body of the prey. Having finished feeding, the larva leaves the remains of the prey and makes a cocoon.
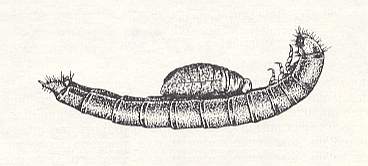
Figure 6 : Young larva of Epyris extraneus (Bethylidae) on the larva of the mealbeetle Gonocephalum. (After WILLIAMS, 1919, in MALYSHEV, 1966)
Just a simple variant of concealing [the prey] consists in the fact that the prey remains after paralyzation in that same hiding place where it lived before, only that it is pushed-in deeper. This is observed, for instance, in Methoca [= Methocha] ichneumon[o]ides Latr. (usually placed into the family Thynnidae).
The family Thynnidae is derived from lower Scoliidae by HANDLIRSCH, 1906-1908.
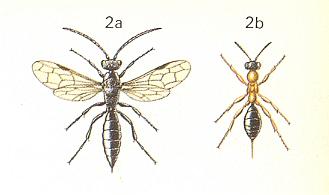
Looking like an ant, the wingless female of this wasp (the males are winged) attacks the predatory larva of the tiger beetle Cicindela, living in a vertical burrow. See next Figure.
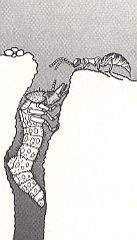
Figure 8 : A female Methocha (Tiphiidae) approaching the burrow of its prey, the larva of a tiger beetle. Methocha is a tiphiid wasp, although the females are wingless and superficially antlike. (After WILLIAMS, 1919, in EVANS and EBERHARD, 1973)
The struggle often takes place at the very entrance of the burrow. The Methocha allows the tiger-beetle larva to grab it with its jaws around the waist, after which it bends, paralyzes the prey by a sting at the throat, and then drags it down into the burrow, where it lays an egg onto it. Similar habits also have the South American wasps Pterombus of the family Tiphiidae, which also hunt tiger-beetle larvae. In contrast to Methocha they attempt to drag the, in the depth of the burrow paralized, larva closer to the exit.

Figure 9 : A spider Trochosa terricola Thor., paralyzed by a spider wasp (Pompilidae), lies at an open place while the wasp is building a burrow.
(Photograph by Byremura, in MALYSHEV, 1966)
For some examples of Pompilidae (spider wasps), see next Figures.
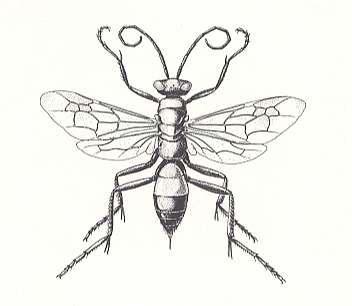
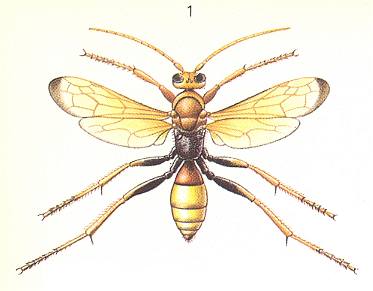
Figure 10 : A spider wasp, Calicurgus (Pompilidae). The unusually long legs help to distinguish these wasps, all of which prey upon spiders.
(After EVANS and EBERHARD, 1973)
Thus, in the present Phase of development the wasps concealed their prey just in its own hiding place, or in an accidental place [a deepening or the like, that happened to be there already], or even in a special one, as in Scoliidae and many Pompilidae. But the most interesting moment [feature, that appears] here is another : the very striving to drag the paralyzed prey to where it was not before. Even when we admit that this is essentially a mere continuation of the struggle, its appearance after paralyzing is remarkable. Anyway, similar unexpected matters [appearing] in the further evolution of the maternal instincts of wasps are encountered ever so often, and from time to time also in a more clear form.
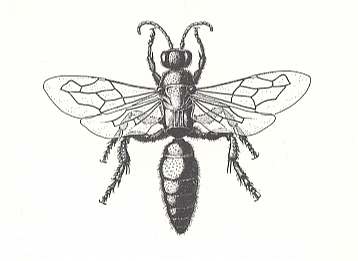
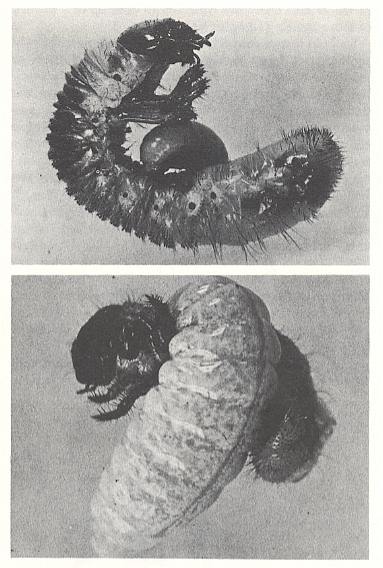
Figure 13 : The larva of Tiphia (Tiphiidae) feeding on a grub of the Japanese beetle. Top image, a young larva that has fed for only a day or two. Lower image, a nearly mature larva that has almost consumed the grub.
(U.S. Department of Agriculture, in EVANS and EBERHARD, 1973)
In a similar way also Myzine andrei Ferton (Tiphiidae) -- after having with one sting paralyzed the mealbeetle larva (Tentyria sp., Tenebrionidae) attempting to save itself by running away -- digs itself down into the sand at the same place, dragging behind it its prey with its jaws. So we see that the digging of a burrow takes place here at the same place where before the prey was conquered. Concrete division of these actions [from one another] is not yet present here. They follow up each other immediately. In the majority of the spider wasps (Pompilidae), however, these actions are already well separated from each other.
A certain indication as to the building activity in semi-wasps (Chrysididae) cannot be reckoned homologous, the more so by the fact that these forms come into contact with their preys only through the wall of the cell or cocoon, and not directly so.
The first thing with which the originated truly building instinct was strengthened was the nest plug, not yet distinct from a cap of the cell. Not any other special wall was present in the original cell, the nest itself being unicellular.
We saw that the scoliid wasps with their short but powerful legs, specialized, like the tiphiids, in finding and paralyzing subterranean larvae of beetles, especially chafer-like ones, which they also began to place each one of them into a special chamber -- into the unicellular nest. In all this, as told, they merely managed the preys, hit by them, into a deeper layer of soil, with it protecting them also from repeated attack by their kins. Showing in this way the basic activity in isolated (from the external environment) conditions, the scoliid wasps, as if rooted in their own habits, did not evolve beyond the present Primary-vespine Phase.
The second mentioned group of wasps -- the Pompilidae or spider wasps -- fell into different conditions. Possessing long legs (especially their hind ones) and unusual dexterity, they specialized in hunting spiders. The hunt of the pompilids, were, evidently, more dangerous than that of scoliids, and often the hunter came under the threat of death. Eventually the hunting instincts of the spider wasps had reached a remarkable perfection, as judged from the hunt of, for example, Pompilus ciliatus Lep. (Pompilus rytiphorus Kohl.) after the karakurt [must be a sort of (dangerous) spider] -- ( Here probably belongs also the wasp "kambaz", also hunting the karakurt in the steppes of Central Asia. Interesting data on the habits of the "kambaz" are present in our literature, but, unfortunately, without a precise identification of the wasp itself (MARIKOVSKY, 1947)), -- Cryptocheilus (Calicurgus) annulatus F. (see for this genus Figure 10 and 11a, above), and Pompilus (Anoplius) samariensis Pall. hunting for the tarantula spider, further, Pepsis femoratus Spin. going after the bird-eating spider, and Psammochares plantus (Fok.) after the ladder spider.
How high a degree of tuning there is of the hunting methods of the Pompilids with the habits of their preys, can be seen in an example of the hunt of the pompilid Episyron tripunctatus Dahlb. after all of the known cross spiders. Having spotted the cross spider that, at the center of its web, waits for its prey, the pompilid moves toward it, going over the sticky threads of the web with such an ease as does the spider itself. Having smelled the approaching enemy, the spider slips down along a web thread, along which the wasp follows it, overtaking it. The prey is paralyzed by one stinging act at the oral region -- at the point, that is, where the venom directly penetrates into the adjoining nerve centers and infects them. In all this, the wasp acts as if it really knew the location of these centers.
Observations, done in California on six species of gigantic pompilids of the genera Pepsis and Hemipepsis, showed that all of them hunt for tarantulas. Upon engagement of the wasp with its horrible prey, on both sides preparing movements set in, but the precise methods of engagement may be different. Not seldom the wasp, having bent its absomen and having it stretched forward as far as possible, is able to paralyze the prey, without a need for a cumbersome grip. The wasp can sting the tarantula into the delicate membrane at the base of the leg or between the coxa ['hip'] and trochanter [small part connecting the coxa with the femur (= thigh)], or at the region of the mouth, but all close to the ganglionic mass, or directly into it. It is held to be usual for the wasps to look for a ready cavity, serving as a nest, before hunting a tarantula. In the cell the paralyzed tarantula is placed on its side with its head pointing to the exit. The lightly bent egg, having a length of about 5 mm, is adhered to the abdomen of the prey with its posterior somewhat thickened end.
When the instinct of paralyzing the prey had reached in this way a high degree of development, the Scoliidae as well as the Pompilidae acquired the possibility to hunt for a very large, and consequently, very heavy prey. This state of affairs brough with it that now the wasp's task was not only to defeat the prey, but also to drag a heavy weight to the place where the nest will be. We may think that precisely in connection with this situation we have the fact that precisely the representatives of the mentioned genera Scolia, Pepsis, Salius belong to the largest Hymenoptera.
Spider wasps (Pompilidae) not only paralyze, but sometimes also mutilate the prey. In some cases this is just a mere chewing of the legs without inflicting a wound, making it easier for the wasp to drag the prey through the narrow channel of the nest (Pompilus scelestus Cr.). In other cases, though, a part of the legs, although not obligingly so, is completely amputated (Pompilus fuscipennis Lep.) according to PECKHAM, 1905.
In all this, as to the general methods of guaranteeing offspring, the spider wasps in their great majority remained essentially in this same Primary-vespine Phase as also the Scoliidae :
They hunt, paralyze the prey, conceal it into an unicellular nest, deposit an egg onto the prey, and then close the nest.
Also Pompilus (Anoplius) viaticus Latr. independently prepares a cavity for [storing] the just paralyzed prey, a spider of the family Lycosidae (see Figure 9, above). See also next Figure.
In order to contemplate more clearly the changes having taken place in the further transformation of the instincts of wasps, let us signify the various actions of the primary maternal behavior of the type represented by Pompilus (Anoplius) viaticus by different symbols :
Primary-vespine (pompiloid) phase (= present phase of development) [Earlier we have called this phase "First Vespoid (pompiloid) Phase", which name can be held to be equivalent]
A = Hunt :
a1 = Finding a prey.
a2 = Attacking the prey and paralyzing it.
a3 = Bring it to a certain place for it to stay temporarily.
B = Constructing or adapting a nest (= definite quarters of the prey) :
b1 = Finding an appropriate place for the nest.
b2 = Burrowing of the nest, adapting it, etc., usually in connection with visiting the prey.
C = Transporting the prey to, into, and in the nest.
c1 = Placing the prey before the nest.
c2 = Inspecting the nest.
c3 = Bringing the prey into the nest.
D = Laying of the egg.
E = Closure of the nest (from the outside).
e1 = Closure of the true cell.
e2 = Closure of the nest itself.
Each one of these acts itself, of course, represents a complex action, which might be subdivided into a number of more simple ones. But usually such subdivision demands a substantial knowledge of the way of life, not only of the species concerned, but also of the forms that are related to it. For our purposes it is, for the time being, sufficient to acknowledge that the primitive maternal activety of the wasp consists of the above enumerated acts. With the help of the given symbols (indexes) we now characterize the work of the wasp that executes the original (i.e. as a starting point) order of actions as we see it in the present phase (the first vespoid [pompiloid] phase), for instance in Pompilus viaticus Latr.
Which can more compactly be written as :
Because normally the wasp takes care for several young, the complete maternal activity is of course polyserial :
or
So this is the original order of the basic instinctive actions of the maternal wasp in her providing for her offspring [= guaranteeing offspring].
Compare the supplement of the author [Malyshev] to the article of ADLERZ (1916) concering the living conditions and instincts of the spider wasp Pompilus viaticus Latr.
The spider wasps basically went along the line of a small complexification of their activities, namely along the line of temporary concealment of the prey as long as they are busy with preparing a burrow for it. Only some of them, although only a few, evolved, in contrast to scoliid wasps, still further than the present phase, and made of their own the methods of the higher wasps. On the contrary, in the next group -- in the digger wasps (Sphecoidea) the described methods, representing the original type, are the exception [So in this group most of them have evolved further in this respect].
Speaking now of the digger wasps Sphecoidea, we must first of all mention the hunting of representatives of the family Ampulicidae after cockroaches. See next Figure.
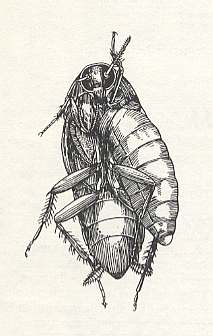
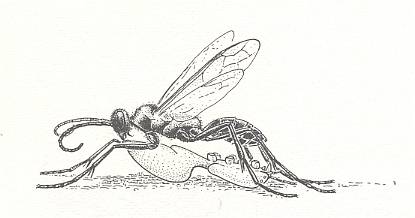
Figure 15 : Larva of Ampulex caniculatus Say. (Ampulicidae) feeding on a paralyzed woodland cockroach. (After WILLIAMS, 1929, in MALYSHEV, 1966)
Thus, according to observations of HINGSTON, 1925, near Bagdad (Iraq), Ampulex assimilis Kohl. hunts for the females of the there common cockroach Schelfordella tartara Sauss., living on trunks of the date palm and in various cracks. Having grabbed the cockroach, the wasp stings it, lasting half a minute, in the anterior part of the thorax from below. The effect of paralyzing is not complete : The limbs of the cockroach move, and it can stand on its feet. The sting is sometimes repeated yet another time at the same place. After that, the wasp conceals the prey somewhere at a proper place on the trunk of the palm and there lays an egg onto one of the femora of the cockroach. Here on the femur the larva starts feeding.
It is interesting in this respect to mention that the all out rare Rhopalosoma poeyi Cress., of which the systematic position is not clarified, develops as an ectoparasite on the wood cricket Orochares saltator Uhler. With respect to all this, we should reckon in that wood crickets also deposit eggs in a mass, namely in shoots of plants.
Let us now consider the habits of the wasp Sphex lobatus F. (Sphecidae) hunting for the enormous cricket Brachytrypes portentosis Licht. in India (HINGSTON, 1929). Having chased the cricket out of its burrow, the wasp grabs it at its wings by its jaws, bends, and applies 2-3 quick and shallow stings into the thorax, so light [these stings are] that they hardly penetrate through the integument of the prey. These preliminary superficial stings a bit weaken, as HINGSTON assumes, the prey, and eases for the wasp to apply the decisive paralyzing 'blow' into the throat of the cricket, tenacious and long lasting, penetrating the ganglion. The paralyzation of the cricket now sets in, immediately and completely, but temporarily. So after 10-15 minutes the cricket little by little recovers. Evidently, the wasp injects some anesthesic liquid, hitting the nerve tissue, not inflaming and not causing in it any damage. The venom is quickly sucked up by the blood [and thus diluted], and recovery starts. The wasp drags the paralyzed prey into its [that is, the cricket's] hiding place and deposits an egg onto the thorax between the forelegs.
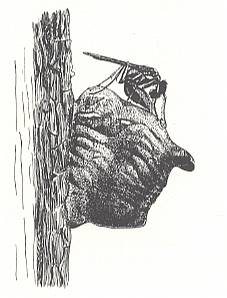
A little more complex are the habits of the Langedoc digger wasp Sphex occitanicus Lep., hunting for the heavy katydid Ephippigera ephippiger F. After having grabbed the prey at its thoracic shield, the sphex stings it into the thorax from below. After this, a sting follows at another place, into the throat. The result is that the paralyzation is constant, but locally : the insect is fully alive, but can neither stand up nor move around. See next Figure.

Figure 16 : Sphex occitanicus Lep. (Sphecidae) drags the paralyzed katydid Ephippigera to its burrow, constructed [by the wasp] after the hunt.
(After BLANCHARD, 1868, in MALYSHEV, 1966)
After this, the sphex digs a burrow, drags to it the katydid and deposits an egg beneath one of its thick hindlegs (FABRE, 1906). According to FABRE the weight of the prey being too heavy, is the reason that the sphex digs a burrow after the hunt. However, the reason must rather be found in the fact that the sphex merely acts in an order that is established during the course of evolution, in which course none of its ancestors built a place for the prey before the hunt. It is observed that, after having paralyzed the prey, this wasp performs a new operation : having stretched out the neck articulation of the katydid at the dorsal side, the wasp rubs [if my rendition of the Russian verb form "mnjet is correct], with its jaws, while not, however, causing any wound, that particular place where the head ganglion [brain] is. After this, the prey entirely looses mobility and is not able in the slightest way to resist during tranport.
Such an operation above the head ganglion of the prey was subsequently called "malaxation", although with this term one sometimes means, but without sufficient foundation, also other supplementary operations. NIELSEN supposes that by means of malaxation, being a special massage of the tissues, the penetration of the venom from the thoracic ganglia of the prey to the upper throat ganglion is quickened, where also a severe degeneration of the cells sets in ( NIELSEN, 1935).
Here still has to be mentioned that Sphex subfascatus Dahlb., according to observations of FERTON, 1901, 1923, on the island of Corsica [France], paralyzes the female of the Italian locust Calliptamus italicus L. Having licked up drops of liquid oozing from the prey's mouth, the sphex digs a burrow where it hides its prey. The licking-up of nutritive saps from the prey's mouth evidently serves as an essential source of feeding for the wasp, especially on the dry upland plain at the end of the season, when there are only very few flowers. Then the sphex paralyzes also other locusts, licking also droplets of nutritive liquid from them, but then abandons them, not using them as nutritive provisions for their larvae.
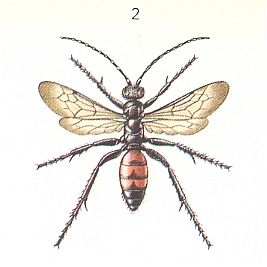
Psammophila hirsuta Scop. ["Psammophila" is almost certainly synonymous here with "Ammophila"], being closely related to the Sphex's, also, according to FABRE, construct a nest after the hunt, but it already hunts for the caterpillar of the winter moth. See next Figure.
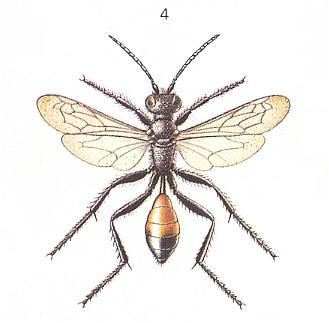
It paralyzes its prey with nine consecutive stings from below in each of the nine segments, beginning with the anterior one. This complex operation it sometimes wraps up with "malaxation" -- a special compression at the region of the upper throat ganglion. The wasp here uses its jaws but leaves no external wound whatsoever. The beatings by the jaws are violent, methodically, with interruptions, and are repeated many times over, until the jaws of the prey become immobile. In one observed case Psammophila hirsuta, after pressing at the region of the occiput, executed the same operation at the majority of segments of the caterpillar. Similar habits have been observed also in other species of Psammophila. Generally, also in the present phase of development, real tearing up the prey with the jaws does not take place. Moreover, the wasps themselves, just as the scoliids, the spider wasps (pompilids), and others, eagerly visit flowers accessible to them, where they feed.
At this present stage, at which we have arrived, following the course of the development of the maternal instinct in the Hymenoptera, a great obstacle was formed to further evolution of their hunting instincts as well as of their building instincts. This obstacle is the consecutive order in the work, which [order] was created by the very course of evolution which we just had considered. It expresses itself by the fact that preparation of provisions [hunt, catch, and paralyzation of the prey] came first, followed by the construction of the cavity [accommodation] for the prey. Such an original consecutive order inevitably had to evoke a whole series of difficulties. First of all, the prey had to be, in these conditions, from the very beginning sufficiently large in order to nourish [up till the end] the wasp larva. Therefore, in transportation great effort of the wasp was demanded, taking, moreover much time, because in all this it almost or wholly could not use its wings. Then, for the transport itself as well as for the normal feeding of the larva, it was demanded that the prey would be well-paralyzed. And therefore it was necessary that such a prey had the proper organization of the nervous system and a definite relationship with the venom of the paralyzator. All this significantly limited the selection of prey, and so created a further difficulty to the wasps. Finally, and perhaps most important, the found and paralyzed prey necessarily had to be left [temporarily] alone without watching it during the period that the wasp selects a [proper] place for the nest and builds an accommodation. In such conditions, as direct observations point to, the obtained prey -- even if it was already partly transported to the building site -- not seldom became the possession of someone else, and the efforts of the builder having become in this way in vain.
On such robbery of a paralyzed prey even some wasps had specialized. Thus, for instance, the female of Ceropales maculatus F. (Pompilidae), sneaking toward a spider, temporarily left alone by a[nother] pompilid beside its nest, quickly deposits an egg into the lung split of the prey. Having built the burrow, the pompilid brings in it the spider and deposits an egg on its abdomen next to the respiratory opening of the spider, into which the Ceropales-egg had been put. The newly-born larva of the latter turns to the pompilid-egg and destroys it, and then feeds on the spider prepared in the cell. To this danger especially was subjected the majority of the spider wasps (Pompilidae), and in them were [evolutionarily] worked out various protective devices. However, the corresponding efforts of these wasps [evolutionarily programmed in these wasps] (temporarily hiding of the prey amidst plants, regular inspection of them, and other such precautions] were not hitting the kernel of the matter and turned out all in all time-consuming and insufficient. No need to say that in such circumstances to construct a more complex and more reliable nest [as we see it in the higher wasps] was for this whole group of wasps not possible.
With all this we conclude our exposition of the Primary-vespine (pompiloid) Phase of hymenopterous evolution.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XLIX.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII