

The delay [= laying eggs at a later time in the season] of laying eggs into the galls of archaic saw-flies had different consequences for those terebrant-inquilines in which, in contrast to the Trigonalidae, did not take place a degeneration of the ovipositor, but a progressive development of it. The possession of a fairly developed borer provided these primitive terebrants with the possibility to lay their eggs onto more or less fully-grown larvae of saw-flies that freely lie in the cavity of galls caused by them. After having destroyed [and consumed] the larva of the saw-fly, the ectoparasitic larva of the inquiline first could continue its [evolutionarily] original feeding on the walls of the gall. Similar cases of mixed feeding, although seldom and in somewhat different conditions, is observed also today. Thus, according to a statement of NIELSEN (1915), noted earlier, Eurytoma salicis Thoms., after having first destroyed [and consumed] the larva of the saw-fly Euura (Cryptocampus) angustus Htg., which lives in a gall on green shoots of willow, continues feeding on the walls of the gall. In the same way, as was reported, Eurytoma inquilinum R.-Kor. attacks the larva of Isosoma rossicum R.-Kor. and sucks it out, and then gnaws on the walls of its gall. And also Syntomaspis eurytomae Puz.-Mal. first destroys the larva of the Eurytoma and then feeds on the remains of the kernel of the plum.
Subsequently, when laying of eggs onto completely-grown larvae of the preys [evolutionarily] began, the supplementary feeding on the walls of the galls lost its significance to the terebrants. Now their instinct began to specialize on the preys themselves, already independently of the fact whether they produce galls or whether they simply live inside plants. To the attack of the original terebrants of the present group became subjected here endophytic larvae of various insects, living in similar ecological conditions, first of all, one must assume, larvae of stem saw-flies and horn-tails, and then also beetles (especially Buprestidae and Cerambicidae) living inside plants.
It is clear that in the indicated conditions in which preys are infected, preys, that live not far beneath the surface of stems, leaves, and the like, to the terebrants there was no need for whatever special morphological adaptations. But subsequently, in search for new preys a part of the terebrants began to infect preys that live far beneath the surface, in the thickness of wood. See next Figure.
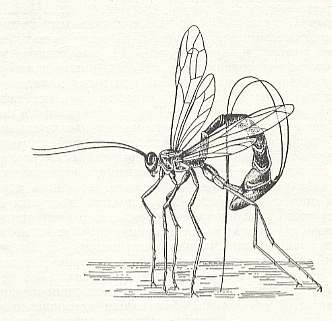
Figure 1 : The ichneumonoid terebrant Thalessa lunator F. drives its ovipositor into the thickness of wood, infected with a larva of a horn-tail.
(After RILEY, 1888, in MALYSHEV, 1966)
In these special conditions, in order to directly infect the prey-larva, a corresponding development of the ovipositor was demanded. In some such terebrants it indeed enjoyed an extraordinary development, eventually reaching a maximal size. Thus, for instance, in one representative of the Pimplinae from Peru it reaches a length of 15 cm, being 7.5 times longer than the [rest of the] body of this terebrant, and also one braconid from Colombia (Iphiaulax sp.) possesses an ovipositor 14 times longer than its body. It is not known for what exactly, and in what way, such an extraordinary device is used. The author already earlier had the opportunity to tell that such a specialization of the organism, directed to be able to reach deeply concealed host-larvae, here has arrived at its very limit, and has, we must think, led these terebrants to a dead end [meaning that, because retrogade evolution of complete whole structures is (probably rightly) assumed to be impossible ('forbidden'), from the state the terebrant is in now, no subsequent (evolutionary) derivation is possible anymore]. After the object to which egg-laying was directed generally had become the endophytically living prey, still one step forward had to be made : this object, at least to terebrants in which the ovipositor has not undergone a strong development, became the very prey, [having become so] independently of the nature of the place where it lives. And as soon as the instinct of the insect had freed itself from the necessity of a narrow (limited) selection of preys, concealed in the plant substrate, it began to specialize on preys that live out in the open, unconcealed -- on the surface of shoots, leaves, and the like. In the beginning, one might think, these were preys that were still closely related to those that lived in plants, such as, for instance, larvae of saw-flies living [now] in the open. Subsequently in the overwhelming majority of forms this connection was completely lost, and now also those preys became to be subjected to attacks that were in no way related to the original forms anymore, such as caterpillars of butterflies and even their pupae.

[For this and other aquatic terebrants, see Part XXXVI of the present Series, "Extract from Wesenberg-Lund" ]

As an indicative example of laying the eggs onto (or next to) precisely that larval state of the host at the expense of which the terebrant also develops [that is, no delayed development] may serve the behavior of the peculiar chalcid Leucospis. Here before us we have a case of a terebrant, [evolutionarily] earlier having been an egg-eater, but now having already lost the connection with the egg stage of the host, that became a solitary parasite of a large prey, still carrying the characteristic traces of structural reduction of the egg-eaters. According to the substantial investigations of FABRE (1855), the female Leucospis (L. gigas F.) with the ovipositor bores through the mineral walls of a cell of a mason bee (Chalicodoma muraria F. (next Figure) and Ch. pyrenaica Lep.) and suspends its egg next to the fully-grown Chalicodoma-larva that already had twined round a cocoon. Having emerged from the egg, the mobile Leucospis-larva, looking like primitive planidium, restlessly crawls around and destroys the other eggs of its relatives in the case when they happen to be in the same cocoon. Having completed all this, it moults and transforms into the usual worm-like terebrant larva, and then starts to swallow the prey.

May be, to this phase of development we might, as to their behavior, also reckon the mysterious Pelecinidae, which will be dealt with later.
A part of the terebrants, while emerging from the egg, laid onto a prey that has concluded its development, was able to penetrate under the host's muscular skin and, having adapted there to the new physiological conditions, avoided in this way the direct influence of the external environment. Subsequently, such terebrant species began to lay eggs directly into the body of the prey, where also they had almost all of their [individual] development (until pupation) or all of their development.
Earlier it was already stated that the endoparasitism of the terebrants had originated in different phases of their evolution -- in the egg-eating (endo-oophagous), in the delayed-parasitic, and in the passively-parasitic phase. Now we see yet another way of origin of endoparasites -- in the present phase, that is, in the orthoparasitic phase. To this origin [i.e. still standing amidst the process of creation of the endoparasitic way of life in the relevant group] belongs, for example, that particular case in which the terebrant Monodontomerus aereus Walk., which usually develops as an ectoparasite in ripe puparia of flies [a puparium is the last larval skin, hardened into some sort of barrel, in which the pupa of the fly rests], sometimes lays its eggs into not yet ripened puparia with the larval skin not yet separated from the pupa [in content and grammar this sentence is not clear in the original text. I have made the best of it.] ["the larval skin not yet separated from the pupa" implies that the parasite, finding itself on the developing pupa, is in this condition still an endoparasite, because it is under the (last) larval skin, which has still not hardened into a puparium, and therefore still being a true larval skin]. In such a case only a few chalcid larvae, having emerged in the cavity of host's body, and having consequently become endoparasites, can manage to attain maturity and emerge as an (winged) adult insect.
But it is problematic to suppose that somewhere in the evolutionary path of the terebrant-endoparasites there was still (as in the ectoparasites) a stage of mixed feeding. When this would be the case than one must hold that the, while inside the prey, not yet sufficiently developed terebrant larva, upon exiting that prey, would feed on the walls of the gall, that is, from an endoparasite would originate a phytophag. This is already very unlikely. And it follows that the immediately-parasitic line of evolution of endoparasitic terebrants was realized in a different way as was the case in the ectoparasites.
From what has been expounded follows that many terebrants of the group presently under discussion, especially of the ichneumonoids and braconoids, did not in their evolutionary history go through the egg-eating (oophagous) phase at all, and were able therefore to preserve their normal growth. In different cases, moreover, of solitary parasitism on large preys, the orthoparasitic terebrants were not only in the position to restore their sizes (as in Leucospis), but even to attain maximal sizes within all remaining terebrants, such as [such large terebrants as] Rhyssa and Thalessa.
Thus, development at the expense of the more or less fully-grown larval, prepupal, or pupal stage of the hosts, on which or in which these terebrants lay their eggs, is the characteristic feature of the immediately-parasitic, or orthoparasitic phase.
With all this we conclude our exposition of the Immediately-parasitic (orthoparasitic) Phase of hymenopterous evolution.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XLV.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII