

The Hymenoptera-Apocrita ( gall-wasps + parasitic hymenoptera [Terebrantia] + sting-bearing hymenoptera [Aculeata] ) form by far the largest group and subgroups as compared with the Symphyta (saw-flies, horn-tails, and allies). A large number of families has been established for them.
As to the classificatory System of recent Apocrita we may give the arrangement as it is adopted in RICHARDS and DAVIES in Imms' General Textbook of Entomology, 1977 :
|
Superfamily Trigonaloidea : Family Trigonalidae |
Superfamily Ichneumonoidea :
Family Ichneumonidae (ichneumon flies) |
Superfamily Evanioidea :
Family Evaniidae |
|
Superfamily Cynipoidea :
Family Cynipidae (gall-wasps) |
Superfamily Chalcidoidea :
Family Agaonidae (Agaontidae) (fig insects) |
Superfamily Proctotrupoidea :
Family Pelecinidae |
|
Superfamily Scelionoidea :
Family Scelionidae |
Superfamily Ceraphronoidea : Family Ceraphronidae |
Superfamily Bethyloidea :
Family Loboscelidiidae |
|
Superfamily Scolioidea :
Family Scoliidae |
Superfamily Pompiloidea :
Family Pompilidae (Psammocharidae) Family Rhopalosomatidae |
Superfamily Vespoidea :
Family Eumenidae |
|
Superfamily Sphecoidea : Family Sphecidae (digger-wasps) |
Superfamily Apoidea (bees) :
Family Colletidae (silk bees) |
In addition to these recent families many fossil families have been established.
Apocritan families only found in the fossil record, and recent families found in the fossil record (mainly Mesozoic) as well are, according to RASNITSYN, 1975 :
Ephialtitidae (Upper Jurassic). Superfamily Stephanoidea.
Megalyridae (Jurassic-Cretaceous-Recent). Superfamily Megalyroidea (according to RASNITSYN, 1975), Ichneumonoidea (according to RICHARDS and DAVIES, 1977).
Maimetshidae (Upper Cretaceous). Superfamily Ceraphronoidea.
Stigmaphronidae (Upper Cretaceous). Superfamily Ceraphronoidea.
Megaspilidae (Upper Cretaceous-Recent). Superfamily Ceraphronoidea.
Ceraphronidae (Lower Tertiary-Recent). Superfamily Ceraphronoidea.
Trupochalcididae (Upper Cretaceous). Superfamily Proctotrupoidea.
Proctotrupidae (Early Cretaceous-Recent). Superfamily Proctotrupoidea.
Cretevaniidae (Upper Cretaceous). Superfamily Evanioidea.
Kotujellidae (end of early or beginning of late Cretaceous). Superfamily Evanioidea.
Anomopterellidae (Upper Jurassic). ?Superfamily Evanioidea.
Baissidae (Early Cretaceous). ?Superfamily Evanioidea.
Ichneumonidae (Lower-lower Cretaceous-Recent). Superfamily Ichneumonoidea.
Ichneumonomimidae (Lower-lower Cretaceous). ?Superfamily Ichneumonoidea.
Bethylonymidae (Jurassic). Superfamily Bethylonymoidea.
Dryinidae (Lower-upper Cretaceous-Recent). Superfamily Bethyloidea.
?Pompilidae (Lower-lower Cretaceous-Recent). Superfamily Pompiloidea.
Mutillidae (Upper Cretaceous-Recent).Superfamily Scolioidea.
Angarosphecidae (Early Cretaceous). ?Superfamily Scolioidea.
Falsiformicidae (Upper-lower Cretaceous). ?Superfamily Scolioidea.
Eumenidae (Late Cretaceous-Recent). Superfamily Vespoidea.
Formicidae (Lower-upper Cretaceous-Recent). Superfamily Formicoidea.
Baissodidae (Early Cretaceous).
(End).
With the origin of the Terebrantia (parasitic Hymenoptera) and, consequently, with that of the suborder Apocrita, from certain Symphyta, we have already dealt, beginning with Part XXXIV of the present Series, and also at the end of the previous document (Part LXVI).
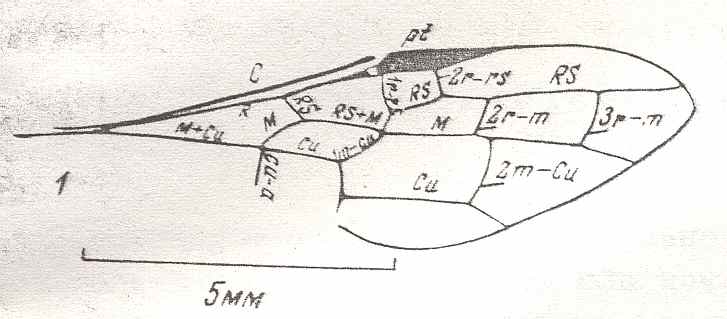
Figure 1 : Proapocritus praecursor Rasn. Lower or middle Jurassic of Sagul, central Asia (Kirgizen Republic, Oshk region, Batensk district, [settlement] Sagul ). Family Karatavitidae. Length of forewing 11 mm.
Anal veins not preserved. Veins and cross-veins indicated. From the pterostigma from left to right two cross-veins go down : 1r-rs and 2r-rs. The course of the Radial Sector, from its origin to its end, is indicated : RS - RS+M - RS - RS. M = Media, Cu = CuA (anterior Cubitus). 1r-rs, 2r-rs, 2r-m, 3r-m, cu-a, 1m-cu, and 2m-cu are cross-veins. The parallellogram-shaped cell in about the middle of the wing (bordered above by RS+M and below by Cu) is the cell 1mcu.
The very first section of the Radial Sector (branching off from the Radius) is here still in its primitive condition, namely obliquely directed toward the distal part of the wing. The Media, separating from the Cubitus, arches up toward RS and coalesces with it for a more or less short distance (RS+M). Then it separates from RS and heads off toward the wing-margin (below the wing-apex). The Radial Sector, after having itself separated again from the Media, runs directly to the wing-margin close to the wing-apex. While doing so, it gives off two cross-veins to the pterostigma (wing-spot), and two to the Media. The Cubitus (CuA), after having separated from the Media, continues, more or less zigzaggedly, to the posterior wing-margin. At the point of separation of Media and Cubitus, the latter gives off a cross-vein, cu-a, connecting it to the first anal vein (not preserved). Later the cubital vein gives off two more cross-veins to the Media, 1m-cu and 2m-cu. (After RASNITSYN, 1975) .
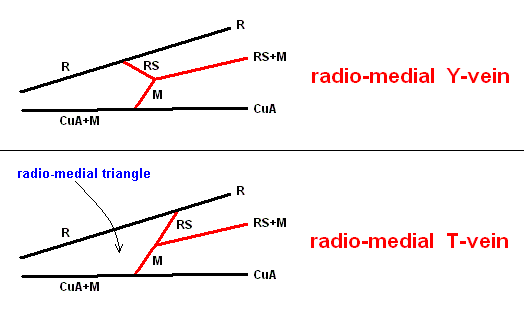
With this 'original' venation in mind we are well able to interpret the venation of the true Apocrita found in Jurassic and younger deposits (and amber), and also of recent Apocrita. One remarkable venational feature deserves attention right now. It is the direction of the very first section of the Radial Sector when it branches off from the Radius. In the beginning of the evolution of the Apocrita this direction is still the original ('primitive') one : As in Proapocritus this first section of RS is obliquely directed toward the distal part of the wing. Together with the Media and RS+M it forms what we will call the Radio-Medial Y-vein. Later in evolution, the first section of RS becomes directed, not obliquely distallly anymore, but, on the contrary, obliquely proximally, that is, it now obliquely points to the base of the wing, such that it is precisely in line with the arched-up part of M. And now this first section of RS forms, again together with M and RS+M, what we call the Radio-Medial T-vein. And the area enclosed by R, CuA+M, M, and RS, will be called the Radio-Medial Triangle. This structure became very wide-spread among Apocrita. See next Figure.
The next Figures of the wings of some recent Apocrita show us examples as to where (among others) the evolution of the apocritan venation has led.
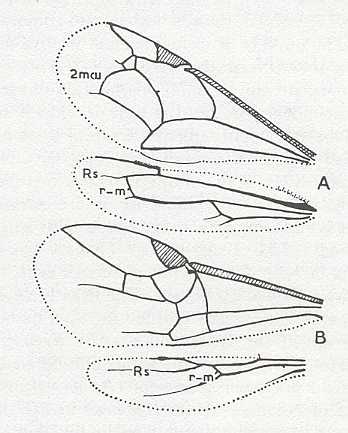
Figure 2 : Left wings of, A, an Ichneumonid and, B, a Braconid, to show the absence of 2m-cu in the forewing of the latter, and the different positions of r-m in the hindwing. ( Ichneumonidae and Braconidae are families of the Apocrita-Terebrantia).
In the upper wing we see RS ending at the end of the anterior wing-margin. Below it we see a minute appendage by far not reaching the wing margin. It is the [end of the] Media. Then we see the long cross-vein 2m-cu connecting the Media with the Cubitus (CuA). Also this cubitus does not reach the wing-margin. For an interpretation of the wing-venations depicted here, see FURTHER BELOW .
(After RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977.)
Figure 3 : Calicurgus fasciatellus, female, Britain. Family Pompilidae.
The wing venation of the forewings is relatively complete. Radio-Medial T-vein developed.
(After SHARP, in RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977.)
Figure 4 : Cerceris arenaria, female, Britain. Family Sphecidae.
Also here the wing venation of the forewings is relatively complete. Radio-Medial T-vein developed and and shifted distally, resulting in a large Radio-Medial Triangle.
(After RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977.)
Figure 5a : Eucoila eucera, female, Britain. Family Cynipidae.
In the forewings only one or two veins are left.
(After RICHARDS and DAVIES, in Imms' General Textbook of Entomology, 1977.)
Indeed, the reduction of the wing-venation in Hymenoptera-Apocrita can go as far as this (Figure 5a) and even further : There are apocritans, for example Platygaster (Platygasteridae), that have, even in the forewings, no veins at all.
Similar cases of strong reductions we saw in the wings of the family Cecidomyiidae (gall-gnats) in the Order Diptera. In most cases such reductions of the wing-venation are connected with a strong reduction in size of the insect.
Wings of Mesozoic Apocrita
We will now present a number of wings of fossil Apocrita, beginning with the family Ephialtitidae from the Jurassic. Let us first repeat what had been said (in the previous document) about what they are (for more details as to their relationship with the Karatavitidae see the end of the previous document) :
The family Ephialtitidae (upper Jurassic of Spain and southern Kazachstan) is supposed to be the most primitive family of the Hymenoptera Apocrita. The wing-venation [we only consider the forewing] usually is complete. In the forewing a rudiment of 1r-rs is often present, sometimes almost reaching the pterostigma. The cross-vein 2r-m is always developed. Not seldom the cell 2a is closed. The costal field is broad.
Second abdominal segment is well developed, it is comparatively little, or completely not, changed to become a hinge-segment in contrast to the next absominal segments.
The Ephialtitidae differ from the Stephanidae [a recent apocritan family] in their more complete venation (in Stephanidae 2--3r-m and 2m-cu are absent in the forewing) and in the absence of the, of the Stephanidae typical, specializations of the head, thorax, and legs.
The Ephialtitidae are morphologically in such a high degree primitive that we can, without special qualifications, see them as an ancestral group of all remaining Apocrita. As to their structure, they are still very close to the lower Hymenoptera ( Symphyta), especially to the Karatavitidae. Apparently, we may indicate only two reliable diagnostic characters by which the Ephialtitidae and Karatavitidae may be distinguished (but not all Apocrita from all Symphyta!) : (1) The position of the first segment of RS of the forewing -- In Karatavitidae it is directed obliquely to the wing-apex, whereas in Ephialtitidae it is perpendicular to R, or, more often, directed to the base of the wing, and (2) (as a second diagnostic character distinguishing the Ephialtitidae and Karatavitidae) if it were only the partial reduction of 1r-rs. Thus, the Ephialtitidae, judging from the available data, represent the "missing link" between Symphyta (as represented by the Karatavitidae) and the remaining Apocrita. In this, the morphological gap is, in both cases, albeit neat, not deep. Especially, the mentioned differences between the Karatavitidae and the Ephialtitidae are, apparently, not directly connected with the change of the way of life as it is supposed to have taken place in the Ephialtitidae, that is, with the transition from feeding [of the larvae] on wood to zoophagy (a similar position of the first segment of RS is known in a whole series of lower Hymenoptera, including also Siricidae ( Figure 124 [previous document] ) [of which the larvae still live in wood, i.e. are xylophagous] which are rather closely related to the Karatavitidae, and the reduction of the cross-vein 1r-rs as it is seen [not only in Ephialtitidae but also] in Cephoidea and Orussoidea.
Placing the Ephialtitidae with the Stephanidae, and contrasting both of them, as a single superfamily, with the remaining Apocrita, is based on the primitive structure of the abdominal hinge, being still very close, in a series of cases, to that what is characteristic of Symphyta. A similar structure of the abdominal hinge do possess also some mesozoic Megalyridae, but in them this feature is not stable (closely related forms may possess a broad or a narrow hinge) and usually is accompanied by a specialization (small size, reduced venation) and, probably, it appears in them secondarily and several times (secondary broadening of the hinge opening is characteristic also of some Chalcidoidea, also minute compact insects with reduced venation, but already in the context of the reduction of the second abdominal segment up to the hinge ring). The secondary broadening of the abdominal hinge was, apparently, realized as result of pedomorphosis (= the preservation in the imago of a [morphological] condition characteristic of the early pupa). This reversal of the character was eased in early Megalyridae, apparently, by the fact that a narrow hinge was acquired not long ago at the [evolutionary] establishment of the family, and did not yet "grow up" [was not yet (over)grown] by the many morphological correlations guaranteeing the stabilization of the character.
The Ephialtitidae and Megalyridae are, undoubtedly, closely connected phylogenetically, but to unite them into a single superfamily is hardly rational. More evident is the connection of the Megalyridae with the Ceraphronoidea. Therefore, after the Megalyridae, into the superfamily Stephanoidea we should include also the Ceraphronoidea, which themselves wholly earn the rank of superfamily. The formal difference of the Ephialtitidae from the Megalyridae is the presence of a free end of Cu in the hindwing.
Let us now, then, present figures of (mainly) the forewings of representatives of the families Ephialtitidae, Megalyridae, Maimetshidae, and other (fossil or recent) families of Apocrita (all Figures are taken from RASNITSYN (1975), and all species were for the first time described by him)
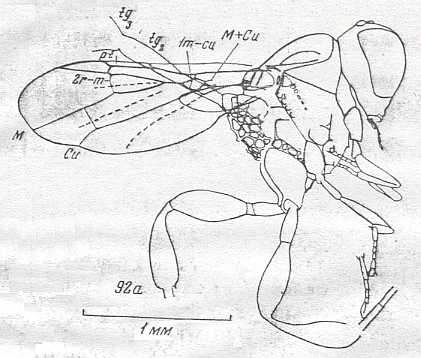
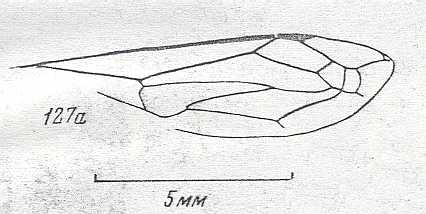
Figure 78 :
Wing of Curiosivespa curiosa.
Family Eumenidae. (Superfamily Vespoidea).
Turon (lower-upper Cretaceous) of southern Kazachstan (Kzyl-Zhar).
Length of wing about 10 mm.
Radio-Medial Y-vein has 'pulled' the Radius downward.
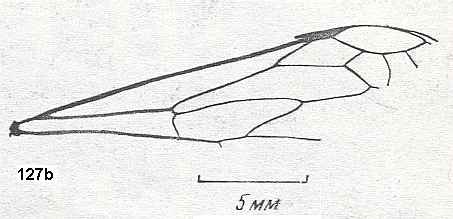
Figure 79 :
Fragment of wing of Curiosivespa magna.
Family Eumenidae. (Superfamily Vespoidea).
Turon (lower-upper Cretaceous) of southern Kazachstan (Kzyl-Zhar).
Length of wing 22 mm.
Radio-Medial Y-vein has 'pulled' the Radius downward.
Figure 80 :
Palaeomyrmex zherichini.
Family Formicidae. (Superfamily Formicoidea (ants)).
Coniac-Lower Santon (lower-upper Cretaceous) of Taimyr. Amber inclusion.
Length of wing 3.6 mm.
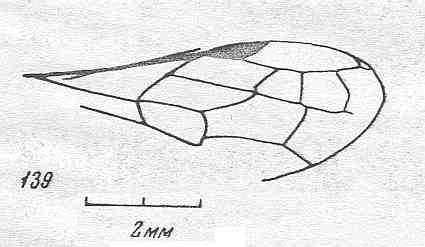
Figure 83 :
Wing of Vitimosphex incompletus.
?Family Baissodidae. Neocom (lower-lower Cretaceous) of Zabaikalj.
Length of wing 6.2 mm.
Radio-Medial T-vein very well developed. Radio-Medial Triangle broad.
Wings of Recent Apocrita
The next Figures (83A-89) depict wings of recent Apocrita
(Source : CHINERY, in Elseviers Insektengids voor West-Europa, 1983, unless stated otherwise.)
(See this LINK to the (above given) systematics of recent Apocrita ) :

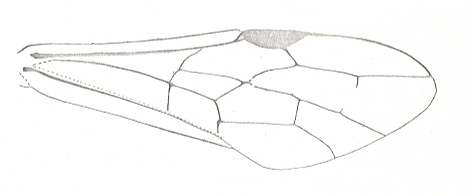
Figure 83A : Forewing of Odontaulacus editus. Superfamily Evanioidea. Recent. Subcosta absent. Radial Sector unbranched. Media, after having arched up, coalesces with RS for quite a long distance and then leaves it independently again. Basal section of RS markedly running backwards (and downwards), together with M forming the base of the Radio-Medial Triangle. Cross-vein 1r-rs absent. Cross-vein 2r-rs present. The cross-veins 2r-m and 3r-m atrophied.
(After COMSTOCK, 1918)

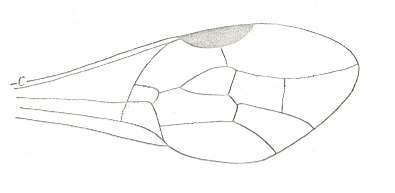
Figure 83B : Forewing of Aulacinus fusiger. Superfamily Evanioidea. Family Aulacidae (see systematics ). Recent. Subcosta absent. Radial Sector unbranched. Media, after having arched up, coalesces with RS for some distance and then leaves it independently again. Basal section of RS markedly long and running backwards (and downwards), together with M forming the base of the Radio-Medial Triangle. Cross-vein 1r-rs absent. Cross-vein 2r-rs present. The cross-veins 2r-m and 3r-m present. First anal vein without any cross-veins connecting it with with the posterior margin of the wing. Second and third anal veins absent. The cross-veins 1m-cu and 2m-cu present. Proximal part of wing (forewing) very narrow.
(After BRADLEY, in COMSTOCK, 1918)

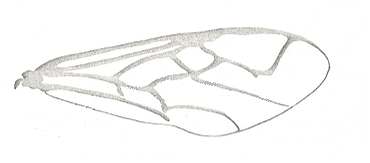
Figure 83C : Forewing of Evania appendigaster. Superfamily Evanioidea. Family Evaniidae. Recent. Subcosta absent (or coalesced with R). Radial Sector unbranched. Media, after having arched up a long way, coalesces with RS near the pterostigma (base of RS absent) for some distance while arching down again together with RS (merged with it) [M+RS] ), and then leaves it independently again, heading to the distal part of the posterior wing-margin. Upon leaving M, the Radial Sector arches up until it meets the thickened cross-vein 2r-rs, then heads toward the wing-apex, but long before reaching it it sharply bends upwards and ends up at the anterior wing-margin. Basal section of RS is absent, but the cross-vein 1r-rs is present as a thickened oblique vein. continuing the course of the up-arching Media. Cross-veins 2r-m and 3r-m absent. First anal vein without any cross-veins connecting it with with the posterior margin of the wing. Second and third anal veins absent. The cross-vein 1m-cu present as a thickened vein, and 2m-cu absent. Body-length about 9 mm.
(After BRADLEY, in COMSTOCK, 1918)

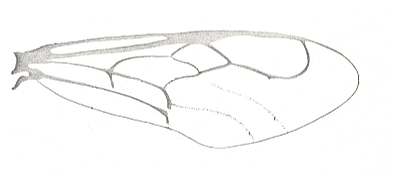
Figure 83D : Forewing of Acanthinevania princips. Superfamily Evanioidea. Family Evaniidae. Recent. Subcosta absent (or coalesced with R). Radial Sector unbranched. Media, after having arched up a long way, coalesces with RS (base of RS clearly present) for some distance, and then, when RS separates from it, the course of the Media toward the distal part of the posterior wing-margin is only present as a trace. Upon leaving M, the Radial Sector arches slightly up until it meets the cross-vein 2r-rs, then continues its course toward the wing-apex, but long before (reaching it) it sharply bends upwards and even backwards and ends up at the anterior wing-margin (meeting R1). Basal section of RS is present and runs backwards and downwards. Cross-vein 1r-rs is absent. Cross-veins 2r-m and 3r-m absent. Cross-vein 1m-cu present. After it, CuA bends down to the first anal vein and then continues its course toward the distal part of the posterior wing-margin only as a trace. First anal vein without any cross-veins connecting it with with the posterior margin of the wing. Second and third anal veins absent.
(After COMSTOCK, 1918)

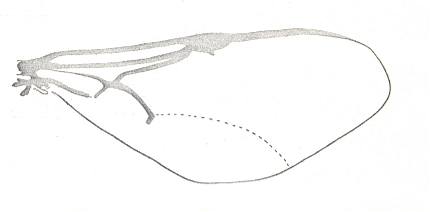
Figure 83E : Forewing of Semaeodogaster barticensis. Superfamily Evanioidea. Family Evaniidae. Recent. Venation highly reduced. Subcosta absent (or coalesced with R). The Radius extends far beyond the pterostigma, running along the anterior margin of the wing, but stops well before the wing-apex. Radial Sector absent. Media, after having arched up a long way toward the pterostigma, does not proceed further, neither does RS. CuA, after M has left CuA+M, continues its course, but already before mid-wing it becomes a mere trace, heading for, and reaching, the distal part of the posterior wing-margin. The first anal vein is very short, it stops about at the level where M arches upward from CuA+M. Second and third anal veins absent. The base of the (fore)wing is narrow, while the wing itself is more or less triangular.
(After COMSTOCK, 1918)

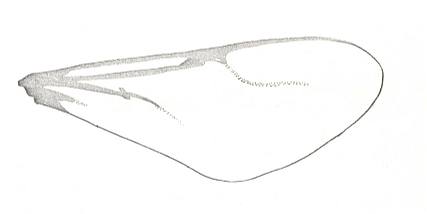
Figure 83F : Forewing of Hyptia. Superfamily Evanioidea. Family Evaniidae. Recent. Venation extremely reduced. Subcosta absent (or coalesced with R). The Radius extends far beyond the pterostigma(l area), running along the anterior margin of the wing, but stops well before the wing-apex. Radial Sector only present as a mere trace. The Medial vein is completely reduced, no trace of it exists. CuA is a strong vein only well before mid-wing, then it continues for a very short distance as a mere trace, hardly reaching mid-wing. First anal virtually absent. The second and third anal veins absent. The base of the (fore)wing is narrow, while the wing itself is more or less triangular.
(After COMSTOCK, 1918)

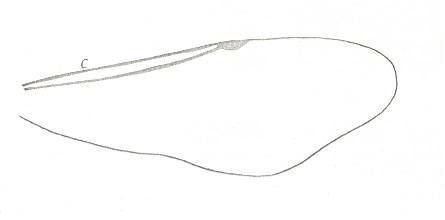
Figure 83G : Forewing of Evaniellus. Superfamily Evanioidea. Family Evaniidae. Recent. Venation almost completely reduced. Subcosta absent (or coalesced with R). The Radius is a strong vein. It runs from wing-base to pterostigma, but not beyond the latter. All other veins are absent. No traces of them exist anymore. The (fore)wing is narrow at its base, and its shape is more or less triangular.
It should be noted that indeed already in the Evanioidea we see this almost total reduction of the wing-venation, a phenomenon we are more familiar with in the Chalcidoidea.
(After COMSTOCK, 1918)
(continuing the listing of wing-venation in recent Apocrita)
To interpret the above two venations (forewings), we begin with the braconid (right-hand image). But, for this, see, first of all, another braconid wing, namely the bottom-bottom images in the table below . There we see that RS gives off two cross-veins, one to the pterostigma and one to the Media. The meeting-point of M and RS has shifted upwards all the way up to the Radius. And now, back again to the present Figure (85), we can correctly follow the course of RS after the point where it has separated from the Media : It sharply curves up until it meets the cross-vein connecting it to the pterostigma. Then it (RS) proceeds to the anterior wing-margin while having given off a cross-vein to the Media. The latter heads for the wing-margin just below the wing-tip.
As to the first wing of the above table (Figure 84) (Ichneumonidae) we might suppose that the meeting-point of M and RS has, like it was the case in the braconid just seen in the table below , shifted towards the Radius, while at the same time the section RS+M (still present in both braconids) has disappeared. [And, now in Figure 85 again :] The two cross-vein-like veins between RS and M (of which the proximal one is in fact a section of RS, while the distal one is the cross-vein 2r-m) in the braconid of Figure 85 can also be detected in the ichneumonid wing of Figure 84 [these veins] being placed here very close together.
The mentioned shifting of the meeting-point of M and RS can also already be seen in certain Symphyta such as in Eriocampa :

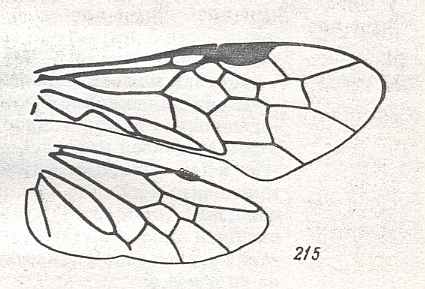
Figure 85b : Wings of Eriocampa Htg. Family Tenthredinidae, Symphyta. Recent. (After RASNITSYN, 1969)
Upper image : Schematic representation of the original (prototypic) venation of the forewing in Hymenoptera.
Interpretation of the venation of the forewing of Eriocampa : SC coalesced with R. Its free end still exists, ending up in C (Costa). The M+CuA trunk branches off from R near the base of the wing. Close to mid-wing it separates into CuA and M, and while CuA is heading more or less straight away to the posterior wing-margin, the Media arches up and reaches the branching-off point of RS [from R]. It then continues, together [that is, coalesced] with RS [and thus as the section RS+M ], into the direction of the wing-apex, but after this short common course RS separates from M and abruptly curves up [this section of RS looking like a cross-vein] until it meats the cross-vein 1r-rs connecting it [RS] with the base of the pterostigma. Then RS continues its course toward the end of the anterior wing-margin after it has given off the cross-vein 2r-rs also connecting it [RS] with the pterostigma. The Media, while [after having separated again from RS] proceeding to the posterior wing-margin [but still near the wing-apex], receives two cross-veins -- 2r-m and 3r-m -- from the Radial Sector. Further, below M, we see the veins CuA, A1, and A2+A3.

Figure 86 : Forewings of small Apocrita. Recent.
One is tempted to suppose that when the wings, and that is, especially the forewings, for some reason become very small indeed (1-2 mm), their venation will necessarily be reduced to a single vein or no veins at all, as in the wings above. But that this 'rule' not always holds is clear in fossil Apocrita such as the one depicted in Figure 57 . So we must assume that a strong reduction of the venation is not entirely connected with the size of such wings but also with a quite different flight-regime. So the formal difference of the wing in Figure 57, having a rather complete venation, from the wings in the above Figure (with a strongly reduced venation) must reflect a difference in flight-regime despite the fact that all these wings are equally minute.
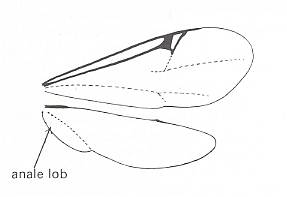
Figure 87 :
Wings of Phaenoserphus. Family Proctotrupidae.
Superfamily Proctotrupoidea, Apocrita-Terebrantia. Recent.
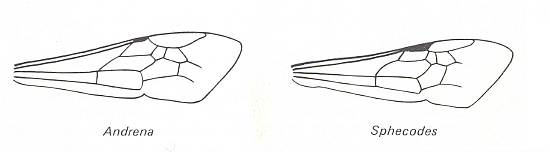
Figure 88 :
Left image : Forewing of Andrena. Family Andrenidae. Superfamily Apoidea (bees).
Right image : Forewing of Sphecodes. Family Halictidae. Superfamily Apoidea (bees).
Both : Apocrita-Aculeata. Recent.
In both wings (especially in the first one) the Radio-Medial T-vein is well developed, and the Radio-Medial Triangle is quite extensive. The Radial Sector branches off from R obliquely backwards, then, after joining the Media, runs into the direction of the wing-apex, then abruptly bends toward the anterior wing-margin (i.e. it abruptly bends up) meeting the cross-vein connecting it to the pterostigma, and then proceeds to the anterior wing-margin after having given off two cross-veins to the Media. The latter vein and the Cubitus do not reach the wing-margin. The anal area is strongly reduced and contains only one anal vein, namely A1, also not reaching the wing-margin. Largely the same we see in the next two wings (Figure 89).
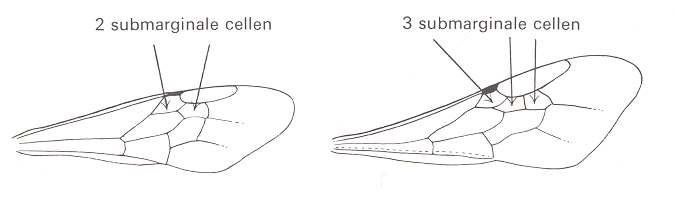
Figure 89 :
Left image : Forewing of Megachile. Family Megachilidae. Superfamily Apoidea (bees).
Right image : Forewing of Bombus. Family Apidae. Superfamily Apoidea (bees).
Both : Apocrita-Aculeata. Recent.

Figure 90 : Scolia maculata, Family Scoliidae, Dagger Wasps, Apocrita-Aculeata. 20-40 mm.
The apical and posterior marginal areas of the forewing are almost devoid of veins.
(After SEVERA, in ZAHRADNIK, Thieme's Insektengids)
Figure 90a : Detail of Figure 90. Right wings of Scolia maculata, Family Scoliidae, Dagger Wasps, Apocrita-Aculeata.
Forewing : Radio-Medial T-vein developed (that is, proximal part of RS directed backwardly). Up-arching (from CuA+M) Media markedly curved. RS+M short. Media heading for apical part of the wing, by far not reaching the wing-margin. After having separated again from M, the Radial Sector runs parallel to the anterior wing-margin for quite a distance until the cross-vein 2r-rs (running backwards). Then RS continues its course and then, as it seems, folds back toward the pterostigmal area. About the distal third of the wing devoid of true veins.
Figure 91 : The goldwasp Chrysis coerulans F., Family Chrysididae,
Superfamily Bethyloidea, Apocrita-Aculeata.
The apical part of the venation of the forewing is more or less reduced.
(After CLAUSEN, 1940, in MALYSHEV, 1966)
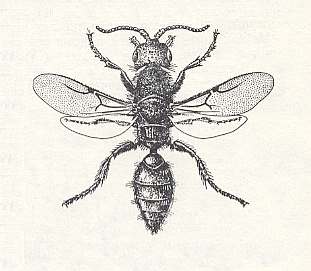
Figure 92 : Epyris extraneus Brid., Family Bethylidae, Superfamily Bethyloidea, Apocrita-Aculeata.
The venation of the forewing is strongly reduced.
(After WILLIAMS, 1919, in MALYSHEV, 1966)
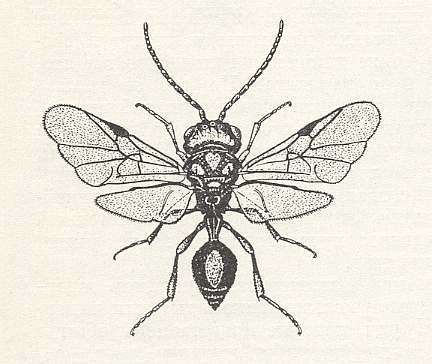
Figure 93 : Helorus paradoxus Prov., Family Heloridae, Superfamily Proctotrupoidea,
Apocrita-Terebrantia. The venation of the forewing is relatively complete.
As far as I know, this form (and also its relatives) is a minute wasp, and it is interesting to see that despite its small size its wing-venation is fairly complete. Compare with the Jurassic form depicted in Figure 57 .
(After CLAUSSEN, 1940, in MALYSHEV, 1966)

Figure 93A : Right wings of Camponotus ligniperda (female), Family Formicidae (ants). Apocrita-Aculeata. Body-length 7-14 mm.
Forewing : Radio-Medial T-vein well developed. Radio-Medial Triangle long. RS+M long. The Media not reaching the wing-margin. The end of RS curves uo to R. CuA not reaching the wing-margin. Cross-veins between Media and Cubitus absent. Cross-veins between Radial Sector and Media also absent.
[The wing-membrane is lightly colored but not structured.]
(Detail of figure after SEVERA, in ZAHRADNIK, Thieme's Insektengids)
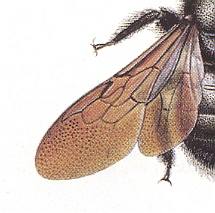
Figure 93B : Left wings of Xylocopa violacea, Woodbee. Superfamily Apoidea (bees). Apocrita-Aculeata. Body-length 21-24 mm.
Forewing : Radio-Medial T-vein developed. Radio-Medial Triangle long but narrow. Distal third of wing devoid of veins. (Detail of figure after SEVERA, in ZAHRADNIK, Thieme's Insektengids)

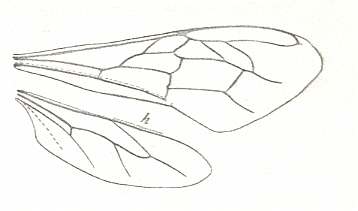
Figure 93C : Wings of Apis. Superfamily Apoidea (bees). Family Apidae. Recent. Forewing : Subcosta absent (or coalesced with R). Radial Sector unbranched (or its anterior branch curving backwards to the anterior wing-margin, while a minute vestige might indicate its second branch stopping just before the apical part of the wing-margin). The base of RS directed obliquely backwards, meeting the up-arching Media. After separating from the Media again RS arches upwards until it meets the cross-vein which is apparently 2r-rs (the cross-vein 1r-rs being absent). RS then follows its course into the direction of the wing-apexs (without reaching it). The cross-veins 2r-m and 3r-m present as oblique veins. The cross-veins 1m-cu and 2m-cu are present. The Media as well as the Cubitus do not reach the wing-margin. The first anal vein is present as a straight vein. The second and third anal veins absent. Proximally the (fore)wing is very narrow, but the (fore)wing as a whole does not have a marked triangular shape.
On the hindwing : h - hamuli.
(After COMSTOCK, 1918)
Sometimes the wing-venation in Apocrita has changed almost beyond recognition, such as we showed in Figure 2, above, which we here reproduce and analyze (with the help of a Jurassic form) :
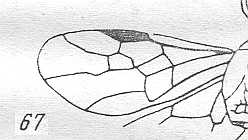 Figure 49 : Wing(s) of Mesaulacinus sculpturatus Rasn. Family Megalyridae (Apocrita-Terebrantia). Upper Jurassic of Karatau. (To see it in context with other Jurassic fossils [from the above long list] click HERE ). (After RASNITSYN, 1975) |
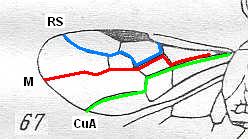 Figure 49a : Wing(s) of Mesaulacinus sculpturatus (Same as in Figure 49). Course of Radial Sector (blue), Media (red), and anterior Cubitus (green), indicated, in order to clarify the modified venations below. |

Figure 2 : Strongly changed venation in Apocrita. |
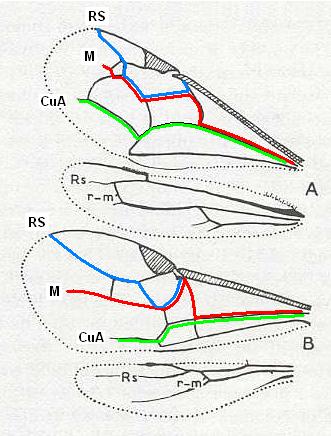
Figure 2a : Venation clarified. |
For the venation of some other representatives of recent Apocrita see the Figures at the beginning of the document, viz., Figure 3 , Figure 4 , and Figure 5a .
Biological status of the wing-venation in insects.
The wings of insects are in the great majority of cases [if not, all cases] organs of flight, and as such belong to the strategy s.str. of the given insect species or higher taxon. That is to say, the possession of wings belong to that large set of morphological and physiological features that together make up the strategy, that is the very way of the noëtic pattern (which is that very strategy as a noëtic prescription, and as such existing in the Implicate Order) of how for it to be able to exist and persist in the domain of time and space, the Explicate Order. So the biological status of the wings of insects is functional. And because the wings are light-weight membranes (airfoils) they must be strengthened by ribs in order to maintain their plate-like nature when they strike through the air. Apart from mere folds existing in some places in the wing-membrane, these ribs are 'sclerotized folds', wing-veins. So the presence of wing-veins is also a functional feature of the insect. And, of course the structure of the wing-base (basiala) and its articulate [hinge] attachment to the thorax are highly functional. And, as has been said earlier, also certain details of the wing-venation in the wing itself may be functional, such as the pterostigma (sclerotized wing-spot) in many insect wings, the 'hinge structures' in the wing-plate itself, etc. But the rest of the wing-venation, that is the rest of its details, seems to be devoid of any function whatsoever. And it is these details that have been dealt with in the present and all the foregoing documents on the evolution of insects, and with which we will continue to deal. The status of these venational details is formal, not functional. And because insects are, in the fossil record, documented chiefly by their wings (in most cases forewings), and that is [documented] by their wing-venation, we must take the formal [versus functional] status of its details into account when concluding things about evolution from fossil insects.
Let us now work out further the mentioned formal status of the details of the wing-venation in insects.
Inspecting the wing-venation of recent insects all over the world, we see that the venation of all insects is a morphological, or geometrical for that matter, derivation of a single original scheme, discovered by the American entomologists Comstock and Needham at the end of the nineteenth century. It is the now well known scheme of seven main veins or main-vein systems [in its most original condition supplemented by an eighth not too clear a system, the Jugal vein system at the very posterior basal part of the wing surface, best preserved in the hindwings of certain insects. We will exclude this system from consideration in our further expositions unless when needed.]. It is, as we know now, the longitudial-vein scheme of (1) Costa (C, marginal vein, in most cases strong and thick [convex], and often only extending along the front edge of the wing), (2) the Subcosta (SC) (a more or less weak concave vein [i.e. a vein lying in a groove of the wing-membrane] lying between the Costa and the next vein (R), (3) the radial system consisting of the Radius (R) (the chief supporting vein of the wing, being convex, i.e. lying on a ridge of the wing-membrane) and the Radial Sector (a strong vein branching off from the Radius at about its middle or before it, and itself often branched), (4) the Media (M) (a weaker, concave often branched vein), (5) the Anterior Cubitus (CuA) (a stronger convex vein), (6) the Posterior Cubitus (CuP) (a weak concave vein branching off from the common cubital trunk near the wing-base), and, finally, (7) the anal vein system (A) consisting of at least three anal veins (A1, A2, A3). In the original condition, these longitudinal veins are connected with each other by a system of cross-veins which generally are much weaker than those longitudinal or main veins.
This comstock-needham scheme of insect wing-venation, this venational prototype, can be detected in the wings of all insects (the Pterygota), either as just described, or as a morphological derivation of it, where veins may have been (evolutionarily) atrophied (disappeared) or partly so, or where their course has been (evolutionarily) changed, sometimes dramatically, or, finally, where all veins have disappeared.
Although having the wing-membrane provided with veins is, as has been said, a functional feature, the status of the 7-system vein-scheme, the comstock-needham scheme of insect wing-venation, is not, as far as I could detect, functional, but purely formal (like it is also the case with, for instance, the five-fold (instead of four-, six-, seven-, etc.-fold) symmetry of starfishes and of many flowers). But all further venational (evolutionary) developments in insects (that is in the Class of Insecta) must, apparently, remain within the limits of this basic venational scheme. It seems that this scheme (and consequently its 'legal' derivations) is not only somehow qualitatively compatible with the qualitative content of the general strategy which we call "insect" (pterygote), but is the only venational scheme that is so compatible. So although the wing-venation is as such non-functional, it is nevertheless not entirely free to mutate into any venational pattern whatsoever. It must always be such that it is formally derivable from the basic comstock-needham scheme of venation.
Upon inspection of recent insects we see yet another restriction of mutation of the wing-venation. It seems that, although all insects follow in their venation the comstock-needham scheme, every insect Order -- be it that of dragon- and damselflies (Order Odonata), of grasshoppers, locusts and crickets (Order Orthoptera), of butterflies and moths (Order Lepidoptera), of gnats, mosquitoes and flies (Order Diptera), or that of wasps, ants and bees (Order Hymenoptera) -- nevertheless has its special type of wing-venation distributed among all of its representatives. So it is easy to recognize 'odonate wings', 'dipterous wings', 'lepidopteran wings', 'hymenopterous wings', etc. In each such an Order certain gross venational features are typical and constant. And so it seems that whatever evolutionary changes might take place in the wings of some given insect Order they always take place within the morphological confines of the venational type of the Order, that is, they never bring the venation outside this type [ This has only taken place, apparently, when one Order originates from another in evolutionary history]. Indeed it is possible to discover the prototypic wing-venation of any given Order of the Pterygota. And, while remaining to comply with the comstock-needham venational scheme, every Order has its own prototype of venation from which the venation of all the representatives of this Order can formally be derived. We here do not mean that necessarily all venational patterns of those representatives can be derived from each other (they cannot), and through them from the prototype. We just say that every venational pattern occurring in a given insect Order can be formally derived directly from the venational prototype of that insect Order. In the previous document we have argued that any evolutionarily established wing-venation is, in the Explicate Order, not completely stable. In many cases factors in this Order slowly change certain details as geologic time goes by. And by the process of injection and projection (i.e. the interaction between the Explicate and Implicate Orders) a given trend of venational change may step over onto another species about to be projected. These changes are, however, just minor changes in the venation.
As has been explained in earlier documents, the strategies of complex noëtic patterns to exist in the Explicate Order (as organisms) are 'forged' in the Implicate Order, and they then turn out to together form a set of noëtic strategies (noëtic descriptions) of which the members noëtically relate to each other derivationally. That is to say, they are not actually derived from each other but are only formally derivable from each other. And the derivational direction is, as we assume, from 'higher', that is, more complex and more sophisticated strategies, to 'lower', more simple and less sophisticated strategies, while their subsequent projection into the Explicate Order takes place in reverse order, resulting in the evolution of higher strategies from lower ones. Such a strategy, a strategy sensu stricto, consists of an integrated set of all relevant functional characters (including behavior). And, indeed, all non-functional characters do not belong to this set (they constitute no part of the strategy s.str.). They are merely implications of, or additions to, this set. One such non-functional character is the wing-venation in insects (minus, of course, certain functional details of it). It originates in the Implicate Order as a possible pattern of veins (as all 'thing' in the Implicate Order in the form of noëtic patterns or descriptions that are as such immaterial). These possible patterns of veins are now, in the Implicate Order, distributed among the noëtic strategies of winged insects. But, as we have found out, they must comply, first of all, with the general comstock-needham venational scheme, and also with the particular prototypic venational pattern of the insect Order to which a given species (insect strategy) belongs. So this prototypic venation, or a derivation of it, will be added to the strategy of a given insect species of this Order. And precisely which derivation (that is, the protype itself or one of its derivations) will be assigned to a given insect species (insect strategy) depends upon whether such a derivation is qualitatively compatible with the qualitative content of that species. And although the qualitative content is different in different species, one and the same derivation of the Order's prototypic venation may be perfectly compatible with the (different) qualitative contents of many different species. So it is to be expected that the representatives of a whole series of different but related species (such as those of some higher taxon) possess virtually the same wing-venation, where the small differences have (as mentioned) been caused by factors active in the Explicate Order during the existence of these strategies in this Order. So any given wing-venation has three basic roots as to its whatness and origination : (1) the wing-venation prototypic of the Class Insecta (= the qualitative content of the general insect-strategy), the comstock-needham scheme, (2) the wing-venation as it is in the prototype of the given insect Order (= the qualitative content of a given less general insect-strategy), whereby this Order-prototype necessarily implies the Class-prototype, the comstock-needham scheme, and (3) the gradual small venational mutations as a result of factors active in the Explicate Order.
Let us recapitulate things.
In the Implicate Order there exists a very large number of different noëtic (immaterial) patterns that are each one of them a description of a strategy of how, for this pattern, to exist in the Explicate Order (and so acquire ontological completion). Upon projection into the Explicate Order such a pattern will exist there in the form of material individuals of a corresponding organismic species. Now if there exists, in the Implicate Order, a given noëtic pattern (among many other such patterns) that is a strategy, a noëtic prescription, such that it specifies a "winged insect" (i.e. the noëtic pattern is such that it can and will exist in the Explicate Order as a species of winged insect), then this description not only must prescribe -- together with all other structures making up the given strategy -- the possession of wings (because in the present case this naturally belongs to the set of attributes making up the strategy), but also must prescribe the presence of a set of supporting ridges, venation, in the wing-blade in order for it (upon projection of the species) to be able to function as an organ of flight. So also the presence of (whatever) wing-venation forms part of the given strategy (the strategy sensu stricto). All this is necessarily implied by the whatness or qualitative content of that strategy. What is not, however, necessarily implied by this qualitative content, and therefore not strictly belonging to this content, is the precise pattern of the veins. This pattern is, as has been said, non-functional. And although the precise position and course of the veins -- the particular wing-venation -- may influence, during flight, the air-flow pattern over the wing-blade, and thus in some degree may determine certain aspects of the flight-regime of the insect possessing this particular venation, it does as such not belong or contribute to the strategy sensu stricto, but only to the idiosyncrasy of the given species (or higher taxon). So the precise nature of the wing-venation in the wings of a given insect species is only determined, in the Implicate Order, by at least the two mentioned restrictions : (1) compliance with the comstock-needham venational scheme, and (2) compliance with the Order-prototype of venation, whereby this second restricion automatically implies the first. Of course a third restriction is that some given venational pattern, some given wing-venation, can only (noëtically) suppelement a given strategy when it does not interfere with any pre-existing function co-constituting or supplementing this strategy. It must be fully compatible with the qualitative content of this strategy.
So when we have a strategy standing for a given species of winged insect, the wing-venation of it must either be identical to the prototype of the insect Order to which the species belongs, or stand in a direct derivational relation to it, meaning that it is formally and directly derivable (not derived) from this prototype. And given that, it must be fully compatible with all elements of the strategy, that is, it must not interfere with or damage this strategy. The strategy must remain strategy.
In the Implicate Order it is the strategies (s.str.) that become ordered in a formal derivational sequence. The derivability goes there from higher strategies to lower strategies (generally by subtraction of elements), while upon projection it is reversed. The wing-venation, however, belongs to those features that are not elements of the strategy (s.str.) and so it is not fixed as to the direction of derivability in one or the other Order : In the Explicate Order as well as in the Implicate Order derivability of a given wing-venation always means the derivability from the venational prototype ('archetype') of the insect Order of which the species possessing this venation is a member.
These findings about the derivability of a given wing-venation from the Order-prototype (and ultimately from the Class-prototype) as a necessary condition for its existence in a given insect species, do not follow from our noëtic theory of evolution. Indeed, if they were a consequence of that theory, then this fact would add to the evidence in favor of it. What we in fact have done is merely to describe these 'findings' about derivability which form a part of common usage in taxonomy and paleontology already for a long time, in terms of our noëtic theory of evolution. Indeed, our theory must be such as to accommodate these facts of derivability, and our considerations took care of that. We must, however, dwell upon the derivability of any given wing-venation a little longer. First of all, when in the practise of evolutionary taxonomy one speaks of the derivability of some given venation V2 (or any other morphological structure for that matter) from another venation V1, one has -- if no other characters do contradict it -- in mind the actual evolution of the recent representatives of V2 from earlier representatives of V1, and thus an actual evolutionary transformation of the venation V1 into V2 is assumed. We, on the other hand, in our noëtic theory, do not let "derivable from" necessarily imply (or mean) "having actually been formed from", nor do we let it imply (or mean) "actually derived from", but nothing more than just "formally derivable from". In all this we must understand that of a given wing the wing-venation as a whole (= the whole venational pattern) is probably always functional in the sense that it, together with the size, form, and shape of the wing, and with hairs and thorns and bristles on it, constiutes a functional organ of flight. In fact we can take the following hypothesis : The correlation between the developmental height of the insect Order, the wing statics (= how the wing is actually structured), wing movement, wing length, wing shape, wing-beat frequency, and way of flight, are regularly so connected that they, in the representatives of the different insect Orders result in every individual case in a well operational flight system.
Thus, the flight ability in more primitive or ancient insects (with more primitive wings and wing-venation) is not necessarily higher than in more 'advanced' insects. It is in most cases simply different. Only the details of the wing-venation are considered by us as non-functional (and this is, of course not entirely certain). Based on the venation of the wings of the most ancient insects (found as fossils in carboniferous deposits) we may hold that the most primitive venation is a set of veins (the full comstock-needham scheme of longitudinal veins) that are more or less evenly distributed on the wing-plate. All longitudinal veins are heavily branched and are, along their entire lengths, connected with each other by numerous cross-veins, resulting in small cells all over the wing surface. This dense system of cells is called the archediction ( In fact it is here still a primitive condition of a true archedictyon which is much more dense as it is [so much more dense] in Palaeodictyoptera). The next Figures depict sich an ancient primitive wing.

The drawing and interpretation of this fossil is given in the next Figure.
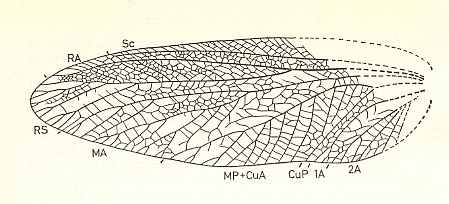
Figure 93D : Forewing of Zdenekia grandis Kuk. Lower Carboniferous (Namurian) of Tsjechoslowakia. Family Paoliidae. RA = Radius (radius anterior), MP = posterior Media, MA = anterior Media. The marks placed outside along the wing-margin indicate the domains of the longitudinal-vein systems.
[Judging from Figure 5 (p. 30) in ROHDENDORF and RASNITSYN, 1980 (where also the hindwing is given) of this same specimen, the high density of the mesh of vein-vessels as drawn in the Radial and Subcostal area of the wing is not (in drawing) continued in the rest of the wing, probably in order not to conceal the course of the main veins there. In the mentioned Figure 5 (reproduced here [next Figure] ) the mesh of vein-vessels is drawn with a homogeneous (high) density throughout the wing.] (After SHAROV, 1966)
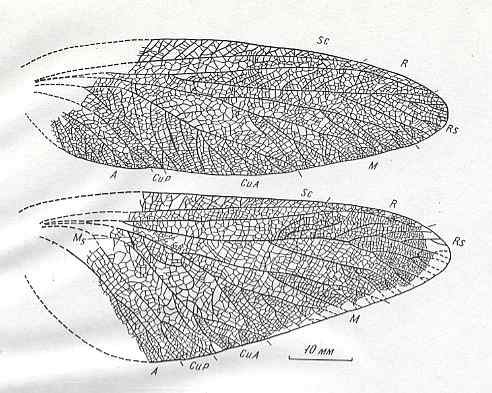
Figure 93DD : Wings of Zdenekia grandis Kuk. Lower Carboniferous (Namurian) of Tsjechoslowakia. Family Paoliidae. Sc = Subcosta, R = Radius, Rs = Radial Sector, M = Media, CuA = anterior Cubitus, CuP = posterior Cubitus, A = anal system (Analis). These marks placed outside along the wing-margin indicate the domains of the longitudinal-vein systems.
(After Kukalova, 1958, reproduced in ROHDENDORF and RASNITSYN, 1980 (Figure 5))
About the Paoliidae (extinct Order Paoliida), to which Zdenekia belongs, ROHDENDORF and RASNITSYN, 1980, Istoritsjeskoje razwitije klassa nasjekomich [ Historical development of the Class of insects], p. 29-30 report the following :
The Order Paoliida is the most primitive group, ancestral of all other winged insects. They practically are known only by their wings rendering the size and delimitation of the Order very imprecise. At rest the wings were, apparently, folded roof-like over the abdomen, and possessed a mechanically imperfect shape and venation which gave them a look similar to the leaves of [higher] plants.
In the forewings a clearly convex position on the membrane is adopted only by C, R, and M5+CuA, whereas clearly concave are SC and CuP. In the hindwings all veins after R are uniformly weakly concave. Sometimes here was developed a free strongly convex M5, whereas CuA took a clearly concave position.
The branching of the main trunks of the venation was often irregular. Strictly pectinate, and more so dichotomic, systems are not characteristic. An irregular archedictyon is common [among them], but also wings were found with more or less regular cross-veins [this being a first differentiation within the primitive venation]. The anal region of the hindwing is lightly broadened, not folded back in the resting position of the insect.
The structure of the body is, corresponding to the assumed ancestral nature of the Order, reconstructed simply as maximally primitive in winged insects (complete biting [gnawing] mouth-apparatus, thorax with external sternites, free coxites and styli of the ninth segment in both sexes independent of the genitals, etc.). Individual development is assumed to proceed according to the archemetabolic type : with larval and nymphal stages and with imaginal molts [in recent insects only preserved in mayflies]. The way of life is the same as [assumed] in the ancestral forms generally of winged insects, namely dendrophilous (tree dwellers) and phyto-embryophagous [feeding on spores and the like]. Eggs were, apparently, deposited inside the tissues of plants with the help of a cutting ovipositor, as in certain dragon- or damselflies, in many Homoptera [cicadas and the like] and in lower Hymenoptera [sawflies and the like].
Justification of assigning this or that fossil remains to the Paoliida consists in the possession of a primitive venation and the absence of the ability to fold back the [specially formed] anal lobe of the hindwing. This is of course too broad a diagnosis because similar features may perfectly be preserved in a whole series of forms ranging from the true Paoliida to the direct ancestors of the Cimiciformes [psocids, cicadas, bugs, and related forms]. However, in the absense of data about the structure of the body we must live with it.
The mentioned diagnosis is satisfied by the families Paoliidae (Namurian and middle Carboniferous of Europe and North America), Cacurgidae (middle and upper Carboniferous of Europe and North America), Herdinidae (middle Carboniferous of North America), and Evenkiidae (upper Carboniferous of the Tungushka Basin). A large number of Carboniferous insects, chiefly Namurian and middle Carboniferous, may be seen as being close to the paoliiforms, but their precise position in the taxonomic system is, at present [1980] not sufficiently clear. Of these insects we must specifically mention Metropator Handl. from the lower Namurian of the USA, which for a long time was assigned to the Order Mecoptera (Scorpionflies).
As we indeed see, in the above Form, Zdenekia (Paoliidae), all the seven main-vein systems (Costa, Subcostal, Radial, Medial, Anterior Cubital, Posterior Cubital, Anal) are present [and it thus complies with the comstock-needham venational scheme] and are more or less evenly distributed over the wing-surface. So this primitive venation shows little, if any, internal differentiation. The general tendency in evolution is the development of internal differentiation, resulting in an uneven distribution of these longitudinal veins and also of the cross-veins over the wing-surface. This inhomogeneous distribution is accomplished by the shift of the course of several existing longitudinal veins, and by the disappearance of some of them, or by the lessening of their branching, and, finally, by the shift or disappearance of many cross-veins.
This evolutionary tendency and corresponding processes result in the at least formal derivability of later wings from earlier ones. But, as is clear from empirical evidence, these processes differ in different insect Orders. So while probably all insect wings are formally derivable from wings such as depicted above, every insect Order has its own characteristic starting-point (as such still derivable from the mentioned wings) which we have called its prototypic venation. And from this latter venation the wing-venation of all members of the Order can formally be derived directly. And now also the precise nature of this derivation is clear : It consists of reduction (total or partial) of longitudinal veins, of their branching, and of the cross-veins, often combined with displacements (up to coalescences) of existing longitudinal veins and cross-veins. In the context of formal derivation "reduction" here means "subtraction of venational elements" in comparing different venations with each other, while in the context of real evolution it means atrophy of venational elements as a result of them becoming incompatible with the rest of the (changed) qualitative content of the species involved. On the other hand, certain regions of the wing may evolutionarily develop further such that new elements are added. The requirement of formal derivability of the venation of a given member of some insect Order from the latter's venational prototype is in the Implicate Order, together with the requirement of this derived venation with the qualitative content of the strategy noëtically representing the corresponding insect species in the Explicate Order. So when we say that the venation of some given insect species can be directly derived from the prototypic venation of the Order to which that species belongs, we do not mean that that species actually has directly descended from early representatives carrying that prototypic venation, but only that the derived venation complies with the noëtic requirements implied by the qualitative content of the strategy that, as a noëtic patterm, represents that species. In actual evolution the derived venation may have evolved from the prototypic venation not directly, but through many entangled detours. So formally the derivation from the prototype is direct, but materially not necessarily so.
Evolutionary extremes in wings of insects.
An instructive example of the possible large extent of evolutionary transformation to which an insect wing may be subjected, while still remaining an operational organ of flight (in contrast to the forwings of beetles for example, having changed into stiff elytra, or the hindwings of Diptera having changed into halteres), is given by the hindwings of the earwig Forficula auricularia L. (insect Order Dermaptera). While here the forewings -- like those of beetles -- have evolutionarily been transformed into stiff shields, the hindwings, which are still functional organs of flight, have been radically changed in shape and venation, mainly to make them suitable to be folded as a compact package under the very small elytra when the insect is not flying. See next Figure.

Figure 93E : Left hindwing of the earwig Forficula auricularia L. Recent.
The wing is spread out evenly, and the sclerotized structures are kept dark. Fold-lines are indicated by dashed lines. The wing-fan is partitioned by convex (kx) and concave (kv) radial folds ["radial" here has no relation with "radial" in "radial veins"], which draw together like a fan when the wing is placed under the elytron. Further folds, such as the ring-fold ( Rf ), ensure the wing becoming a small package. 1. An = first anal vein (A1). V. sp. = Vena spuria.
[We will not discuss here most details of the wing indicated in this drawing, but only consider some gross features : It seems that the anal area of the wing (perhaps combined with the jugal area posterior to it) is strongly extended and has acquired numerous new veins. This has taken place in the hindwings of many representatives of other insect Orders as well (such as grasshoppers), but here it is especially conspicuous that the extension of the anal area took place at the expense of the rest of the wing. Almost nothing is left of it. Nevertheless the wings remain functional flight organs.]
(After NACHTIGALL, 1968)
Another example of extreme evolutionary transformation of insect wings is represented by the wings of thrips (Order Thysanoptera) :
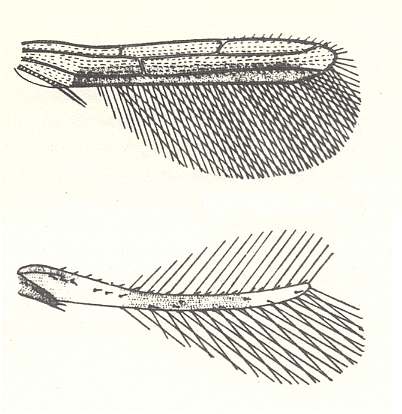
Figure 93EE : Bristle wings.
Top image : Right forewing of Aeolothrips kuwanaii. Order Thysanoptera. Recent.
Bottom image : Right forewing of Euthrips orchidi. Order Thysanoptera. Recent.
The wing lengths are only a few tens of a millimeter.
(After NACHTIGALL. 1968)
Wing-venation a functional structure after all?

Figure 93EEE : Contortion of wings during flight.
A blowfly of the genus Calliphora is in the process of landing on a lump of meat. It plunges itself in an unimaginably fast tempo onto it and bends its wings at downbeat sharply downwards. For such movements the wings possess a preformed kink spot at their base. This 'crash-landing' is not typical, but shows very beautifully how enormous the ability to manoeuvre is of such a fast-flying insect.
(After NACHTIGALL. 1968)
Further down we will pursue the question of the functional status of the wing-venation in insects still further, after we have got some idea about the evolution of all the different wing-venations, and their ultimate origin from some supposed prototype, i.e. from some first primitive venation present in the wings of the very first insects.
Evolution of the wing-venation in Pterygota.
In order to properly determine the condition of the wing-venation as it is found in the Order Hymenoptera [and these same considerations do also apply when any other insect Order is about to be studied as to the wing-venation of its representatives] it is instructive to reproduce here the discussion of SHAROV (a Russian author on paleoentomology) in his english written book "Basic Arthropodan Stock with Special Reference to Insects", 1966, (from which we already reproduced two Figures of Zdenekia).
This discussion is, first of all, about how to determine the precise structure of the wing-venation of the very first insects having functional wings, that is, it is about the venational prototype of the subclass Pterygota (winged insects), and, secondly, about the venational prototype of the main groups of Pterygota, viz., the Palaeoptera (insects that do not fold their wings over the abdomen when not flying, such as dragonflies, damselflies, mayflies, and the extinct [paleozoic] Order Palaeodictyoptera), and the Neoptera (insects which are able to fold their wings over the abdomen when not flying), and, finally the discussion is about the venational prototype of the subgroups of Neoptera, viz., the Paraneoptera (such as psocids, cicadas, aphids), the Polyneoptera (such as grasshoppers, locusts, and cockroaches), and the Oligoneoptera (holometabolic insects, such as butterflies and moths, mosquitoes and flies, scorpionflies, beetles, wasps, bees, and ants). In fact all this is about establishing vein homologies.
We will be informed about the specific difficulties that are encountered when trying to determine prototypic venations. And one of the conclusions of this discussion is the supposed fact that the wing-venation of the above mentioned Paoliida, especially that of the above figured Zdenekia grandis Kuk. , is closest to the true prototypic venation of the Pterygota. So this true prototype is reconstructed with the help of Zdenekia by taking it just one more step backward, for example in depicting a still free vein M5 (most posterior branch of the Media), of which in Zdenekia only its very base is (evolutionarily) preserved (the rest having been merged with CuA).
Also important in this discussion on vein homologies is the (original) corrugation of the wings, such that longitudinal veins, lying on a ridge (convex veins, indicated by "(+)"), alternate with veins lying in a groove (concave veins, indicated by "(-)"). It may be that the main longitudinal veins and their main branches carry with them the constant character of being convex or concave [that is, one main vein is always, that is in all insects, convex (except when secondary transformations have taken place), whereas the other main vein is always (in all insects) concave (also except when secondary transformations have taken place). As long as this is true, it helps to identify those main veins and their (main) branches. It is clear that paleontological evidence is of great help here.
Investigations, as reported in the discussion to be reproduced here, may help us to recognize the general tendency in the evolution of wing-venation in insects, and will give us a better idea of "venational derivation".
Let us now, then, give SHAROV's discussion [with little changes and additions].
The original scheme of venation of the wings of insects which serves as a basis for determining and establishing the homologies of the main trunks and their branches is associated with the names Comstock and Needham (1898-99). The name Redtenbacher (1886) was undeservedly forgotten although his ideas, as has now become clear, are much closer to the truth than the views of Comstock and Needham. To Redtenbacher we owe the names of the main veins now used. He also indicated the constancy of alternation of convex and concave veins -- a phenomenon the importance of which was first noted by Adolf (1879). In Redtenbacher's view, based on comparative study of the venation of representatives of different Orders, the more ancient insects are characterized by richer venation than representatives of geologically younger groups in which venation is considerably reduced. Comstock and Needham rejected both these views. Moreover, Comstock (1918) in his well-known monograph "The wings of Insects" described these views as follows : " The acceptance of these two erroneous views, the theory of alternating concave and convex veins and the belief that the first winged insects had many wing-veins did much to retard the progress of the effords to establish a uniform terminology of the wing-veins" (p. 6). Accepting from Redtenbacher the terminology of the veins, Comstock and Needham on the basis of study of the tracheation of the wings in nymphs and pupae -- [that is, how and where certain tracheae (tubes for bringing oxygen to the places in the body where constantly needed) enter the developing wing-bud, and which tracheae, in the wings, develop into veins] -- proposed their well-known scheme of venation of the hypothetical wing in which the wing[-venation] corresponds to the trachea[e] and each trunk has a definite but very small number of branches. See next Figure.
Figure 93F : Hypothetical primitive type of wing-venation of Pterygota.
A, after Comstock and Needham (1898-99). B, after Lameere (1922).
In the right-hand image we have : C = Costa, Sc = Subcosta, R = Radius, Sr = Sector radii (Radial Sector), M = Media, Sm = Sector mediae (Medial Sector), Cu = Cubitus, Scu = Sector cubiti (Cubital Sector), further the vein "P" and the sector of P, "Sp", the vein "U" and the sector of U, "Su". " + " = convex, " - " = concave.
In the left-hand image we have Sc1, Sc2, being branches of the Subcosta, and R1, R2, R3, R4, R5, being branches of the Radius, etc. (From SHAROV, 1966)It was considered that all the various types of venation of Pterygota are derivatives of this original type. Where the number of branches did not correspond to the scheme, it was considered that the branches had either fused or divided, and the venation of such forms was artificially fitted into the scheme mentioned.
During its existence the system of venation proposed by Comstock and Needham underwent some changes. Tillyard (1919) established that in Mecoptera (Scorpionflies) there is a further branch [of] M almost completely merging with Cu except for the basal part represented by the oblique vein between M and the anterior branch [of] Cu. In addition, he showed that the vein designated by Comstock and Needham as 1A, in fact represents the posterior branch [of] Cu. Lameere (1922) rehabilitated the view of Adolf and Redtenbacher on the importance for identification of the concave and convex position of the veins and considered the concave veins to be branches (sectors) of the convex ones, for which purpose he even proposed that the designation of these veins be changed (see image B in Figure 93F above). Unlike the scheme of Comstock and Needham, the number of M and Cu branches was increased to eight. However, he made the same mistake as Adolf considering corrugation of the wing to be its original state and overlooking the fact that in the individual parts of the wing it might have originated from different groups in parallel and, in such a case, the same veins in one group [of insects] might be concave and in the other convex. Martynov (1938) noted that in Orthoptera [locusts, etc.] in particular, differentiation by convexity and concavity "only starts and does not run along the same path as in Palaeoptera often not corresponding to it at all" (p. 134) [ In Palaeoptera -- dragonflies, damselflies, mayflies -- the corrugation of the wings is especially evident].
Martynov (1924, 1925) already noted that it was very risky to base oneself on tracheation of the wing rudiments in seeking to elucidate the homologies and evolution of venation, and that much caution must be exercised in judging venation from tracheation and that paleontological material must first and foremost serve as the basis for establishing vein homologies. A similar conclusion was reached by Smart (1956) who demonstrated experimentally that the arrangement of the tracheae in the nymphal wings is variable and therefore cannot be the basis for determining the homologies of the wing veins. Tracheation of wing rudiments offers even less support for concluding that [the] venation [ontogenetically developed from it] was the starting state in the first Pterygota, since owing to its high plasticity [the pattern of] tracheation changes almost as rapidly and in the same direction as does venation. [So the pattern of tracheation in a wing-bud may also be subjected to (further) evolution (as does the venation), and does not, therefore, necessarily reflect some more primitive state of earlier venation, that is, it does not reflect the venation of some remote ancestor, and so does not necessarily reflect any prototypic venation.]. Therefore, the only reliable way of forming a correct idea of the original type of venation is to make a comparative study of fossil venation and [of the venation in] existing representatives of different groups of Pterygota.[To this discussion we (author of present website) may add the following concerning the tracheation in the wing-pads of nymphs (the corresponding tracheation pattern in pupae is not always constant) :
The tracheal pattern in the developing wings (wing-buds, wing-pads) is not fundamental, that is, it does not, in individual development (ontogeny) precede the determination of the course of the veins, and so does not reflect some ancestral condition of the pattern of veins. Nevertheless the tracheal pattern might well be a very good guide to establish vein homologies (Smart, 1956), because the main tracheae normally pass along the lacunae which, in the wing-pad, precede the veins. Lacunae are not induced by tracheae (Holdsworth, 1940). A vein-lacuna (in contrast to for instance a prothoracic blood-lacuna) is not in a 'watertight' way characterized by the presence in it of a trachea. Tracheae do not precede cross-veins.
There are indications that phylogenetically the tracheae preceded the veins (lacunae), rendering the tracheae in this respect the most fundamental after all. However, the tracheae have had their own evolution as well (up to a certain degree), and it is now (i.e. in recent insects) such that (in all probability) the lacunae are formed first, in which subsequently grow the tracheae.
So the course and number of tracheae in a wing-bud of a representative of some given insect species do not as it were recapitulate phylogeny, but may nevertheless be helpful to identify the course of veins in the wing of the adult insect in cases where, in such wings, complicated coalescences of veins have taken place.]As already noted by Redtenbacher, the geologically more ancient insects have a richer and more ramified venation. The representatives of the oldest Order with already formed venation -- Protoptera [Sharov here has in mind the Paoliidae and relatives] -- also have richly ramified venation. It is interesting to note that ramified venation is also characteristic of the earliest representatives of Palaeodictyoptera and also of ancient representatives of Neoptera. See C and E in the next Figure.
Figure 93G : Comparison of the hypothetical primitive venation of Protoptera [Paoliidae and relatives] and the venation of the most ancient representatives of Palaeoptera, Paraneoptera, Polyneoptera, and Oligoneoptera (Holometabola).
A - original primitive type of venation [that of the Paoliid Zdenekia taken one step back in evolution].
B - Breyeria barborae Kuk. ( Palaeodictyoptera).
C - Protoprosbole straeleni Laur. ( Paraneoptera).
D - Metropator pusillus Handl. ( Mecoptera).
E - Eoblattina complexa Bolt. ( Protoblattodea).
(From SHAROV, 1966)Thus, it must be acknowledged that Redtenbacher was right in relation to this feature of venation of ancient Pterygota. It should be noted that the forms with rich venation show high variability in the number of branches of each trunk, apparently also characteristic of the venation of the original type. It might have been expected that this type of venation must be closest to that of Protoptera [Paoliidae and allies].Description of the original type of wing-venation of the Pterygota.
Comparative study of the most ancient representatives of the different phylogenetic branches of Pterygota made it possible to give the following scheme of such an original type of venation. [ To scrutinize this (hypothetical) scheme we have magnified image A of the previous Figure] :
Figure 93H : Hypothetical primitive venation (forewing) of Pterygota (based on Paoliidae ).
The venation consists of the full set of longitudinal veins. The Costa is very short and has a small pre-costal field anteriorly to it. It has even some branches [ In the vast majority of insects the Costa coincides with the edge of the wing (leaving no room for a precostal field), where sometimes this Costa runs all around the wing, while in other cases it is present only at the anterior wing-margin or a little beyond]. The posterior main branch of the Media is free, not yet partly or completely merged with CuA. There are no true cross-veins. Between the longitudinal veins and branches there exists a primitive archedictyon (not drawn) which is a more or less dense mesh-work of veinlets. (+) = convex (position of) vein, (-) = concave (position of) vein, (0) = neutral (position of) vein. The signs set around the wing-margin denote the domains of the main-vein systems. Ju = jugal domain of venation.
During the description and discussion (below) of this venation we will refer to this Figure by the LINK "original venation".
(From SHAROV, 1966)A short convex Costa (C) abutted against the anterior margin of the wing with several branches arranged in the pre-costal field. Parallel to the anterior margin almost up to the tip of the wing ran the concave Subcosts (Sc) with numerous short branches from it towards the anterior margin. At the base of the wing C and Sc were joined together, C apparently arose as a result of separation of one of the Sc branches. The branching of the convex Radius (R), the neutrally situated Media (M), and the concave Cubitus (Cu) was dichotomic, and the dichtomy was most fully marked only in the Media. The first branching of R, M, and Cu, occurred approximately at the same level [all rather close to the wing base]. The anterior branch of R was, as it were, the continuation of trunk R and was convex. In the distal part it formed several branches running towards the wing tip. Comstock and Needham regarded it as the first of five branches of the Radius and correspondingly designated it R1 [see image A of Figure 93F, above]. Lameere proposed that it should be considered as the continuation of R, which was adopted by Martinov and other Russian entomologists. This, of course, was more reasonable since this branch corresponds to all the posterior branch [of] R and not to its secondary branches [i.e. it corresponds to the whole main structure of that posterior branch]. But it would have been more logical to designate the anterior branch [of] R by analogy with the corresponding branches of the Media and the Cubitus as Radius Anterior (RA). [see original venation]. The posterior branch of the Radius was situated in the same neutral or base plane as the branches of the Media. The anterior and posterior branches of the Media and their ramifications were arranged almost symmetrically. The anterior branch of the Cubitus -- CuA -- had a convex position, whereas the posterior branch, being as it were the continuation of the Cu trunk, ran at the bottom of the cubital groove. In its distal part CuP [posterior branch of Cubitus] formed several branches. The anal trunk (A) very early divided into three main branches the anterior one of which, the first anal (1A), occupied a convex position, while the other two a neutral one. The first of them -- the second anal (2A) -- formed branches running mostly backwards [i.e. branching off from the right-hand side of it], whereas the jugal (Ju) ran in an anterior direction [i.e. obliquely in the direction toward the distal part of the wing, or, equivalently branching off from the left-hand side of the main vein] from the main branch. [see original venation]. [ End of description of the venation of the forewing of the first Pterygota]The branching of the anal veins in the hindwing was apparently more copious than in the fore one.
The wings were fairly compact and leathery. Between the veins was the meshing of the archedictyon.Further evolution from the hypothetical type.
The change from such a hypothetical type to the venation of Protoptera (Paoliidae and allies), which is regarded by the author as the original one for the different types of venation of Palaeoptera and Neoptera, consisted in the main in fusion of MP with CuA while the base of MP was preserved in the form of a short oblique vein (see the forewing of Zdenekia in Figure 93D , where it is indeed drawn as a very short oblique 'cross-vein' [in the original photograph of the fossil -- not in our reproduction of it -- it can be discerned, albeit with some difficulty] ). The fact that this vein belongs to the longitudinal veins [not to the cross-veins] of the wing was convincingly demonstrated by Tillyard (1919) for Mecoptera. He regarded it, starting from the notions of Comstock and Needham on the four-branched Media, as an additional fifth branch ( M5 ) of this vein. Lameere (1922) regarded it as the base of the sector of the Media, and he also noted the presence of this vein in Plecoptera. Martynov (1937) and Zeuner (1939) designate this vein in Orthoptera as MP. However, F.M. Carpenter (1944), noting that in Cacurgidae this vein is convex, did not agree with this interpretation and took it as CuA, which at the base was supposed to merge with M. Such an interpretation is inconsistent with the following two aspects : firstly, no case is known in which the base of CuA is present in the form of a vein diverging from CuP towards M. Secondly, it is not possible to see how in such a case we can interpret the part of the vein leaving CuP and situated more proximally to the oblique vein under discussion. This can only be the base of CuA. In some Protoblattodea, see image E of Figure 93G , the fusion of MP- and CuA-branches was still not so complete as in the majority of other Pterygota, and in them it is still possible to distinguish MP- from CuA-branches [ In the wing of image E of Figure 93G we see the branches of the posterior Media in the left half of the wing, whereas the branches of CuA we see next to them at the right side]. As for the convex position of the MP base, this vein, when it is preserved, has the same position also in Palaeodictyoptera, see image B in Figure 93G . When MP was [still] a free branch it was apparently neutral in its position but after fusion with CuA its free base was elevated by the convex CuA. Unfortunately, neither among existing Pterygota, nor among fossils known to us, are there any representatives with MP free.Further evolution from Protoptera.
Evolution of venation from Protoptera [Paoliidae and allies] to Palaeoptera [ Palaeodictyoptera (extinct), dragonflies, damselflies, and mayflies] consisted in (1) narrowing and loss, in the majority of representatives, of the pre-costal field and of the MP-base, although in individuals they are still preserved ( image B in Figure 93G , pre-costal field in the upper right corner of the wing ), that is, although the wing depicted here belongs to the Palaeodictyoptera, and thus to the Palaeoptera, it still has a small pre-costal field, and the base of MP is still present), and (2) acquisition by the anterior branch, MA, of a convex position and by the posterior one of a concave position.
In the phyletic branch from Protoptera to Paraneoptera [psocids, cicadas, aphids, bugs, and allies] in addition to the loss of the pre-costal field there was sharp reduction in the number of anal veins and a reduction in the size of the hindwings. In the majority of Heteroptera (bugs) [The text says "Homoptera" (cicadas, aphids, etc.) but this must be an error.] the cubital groove is sharply pronounced, separating the anal region (clavus) [from the rest of the forewing], but in the more primitive representatives (image C in Figure 93G ) the CuP branched at the end, and the cubital groove was not deep.
In the evolution from Protoptera to Oligoneoptera (holometabola), on the other hand, the cubital groove was almost completely flattened (image D in Figure 93G ). The base of MP as already noted, is maintained in many Oligoneoptera.
In the evolution from Protoptera to Polyneoptera [grasshoppers, cockroaches, etc.] a crucial factor was formation of an anal fan from the veins of the anal group. We must describe as mistaken the view of Martynov (1937, 1938) that in Blattodea [cockroaches] the anal fan [in the hind wings] was formed from the jugal veins whereas in Orthoptera [grasshoppers, locusts, and crickets] the anal and jugal veins were responsible, sharply distinguishing cockroaches from Orthoptera. As was made clear by the detailed study by Smart (1951, 1953) in the anal fan both of Blattodea and Orthoptera, 1A and 2A and their branches occupy approximately equal parts of the fan. The error arose from the fact that Martynov took CuP and 1A of Blattodea as 1A and 2A, respectively.
As for the venation of the forewings of Polyneoptera, its evolution in different branches had some interesting special features. The venation of some Protoblattodea (image E in Figure 93G ) is still very close to that of Protoptera [Paoliidae and allies]. The majority of Blattodea lost the pre-costal field and the base of MP, although in some of the most primitive representatives the MP-base can still sometimes be observed. Among the Paraplecoptera [a fossil Order related to stoneflies (Plecoptera) ], representatives of Cacurgidae and related families are still very close in venation to Protoptera which, incidentally, was the reason for including Paoliidae into the Superorder Cacurgoidea (Kukalová, 1958). In representatives of the superfamilies Ideliidea and Liomopteridea the base of MP was reduced, and together with this there was displacement in the first fork of MA closer to the base of the wing up to the level of the origin of RS or even more proximal, and the posterior branch assumed a concave position which lead the author (Sharov, 1961, 1962) to regard it erroneously as MP. The base of the genuine MP is usually not preserved, and only in representatives of the families Archiprobnisidae and Megakhosaridae (Sharov, 1961) is it represented in the form of an oblique cross-vein together with the concave false MP. Changes of the same type in the position of the branches of MA also occurred in Orthoptera but the MP in them is preserved, and the pre-costal field of the forewings underwent further development.The primitive archedictyon of Protoptera was transformed in Palaeoptera and Neoptera either into a more refined alveolar archedictyon or was reduced and formed cross-veins. Apparently, this explains the fact that even in the oldest Palaeoptera and Neoptera, together with representatives which have maintaind the archedictyon we encounter representatives with simple cross-veins (Figure 93G ). However, instances are known of the appearance of a secondary archedictyon when the wings aquired a covering function as has been established in some Orthoptera (Sharov, 1962).
Smart (1953) was the first to draw attention to the phenomenon of taking over of a vein belonging to one system by the veins of another system. For example, cases are known in which RS loses its connection with R and attaches itself to M or MA. Such individual cases were regarded by Zalesskii (1943) as a reflection of ancient features of venation. As a result, he proposed that the names and designations of the veins be changed. However, This new terminology was adopted by no one else and was used only by this author himself.
Smart (1951, 1953), following Snodgrass (1935), designates 1A as the post-cubitus (Pcu) while he takes the anterior supplementary branch 2A characteristic only of Blattodea and Mantodea as 1A. The author sees no grounds for such substitution of terminology, agreeing in this respect with Ragge (1955).
[ End of reproduction of SHAROV's ideas about the primitive, original, venation of insects, and its subsequent evolution.]
Prototypic Venation and Derivation.
The purpose of the above long quotation, and also of what comes next, is to establish what exactly "venational derivative" (one venational pattern being derivable from another) should mean. For this we need to know the prevailing tendency (or tendencies) of venational change in evolution (such as displacements of veins or parts of them, reduction or addition of veins, addition or reduction of cross-veins). For this we must inspect the oldest fossils of winged insects, and/or must find clues in the earlier stages of individual development of recent representatives of generalized (i.e. 'primitive', 'original') insect Orders, that is, we must inspect the tracheation(al pattern) of wings of nymphs ["nymphs" are larval stages of insects with incomplete metamorphosis, i.e. insects lacking a pupal stage]. In fact, it always remains uncertain whether "derivation" should correspond to reduction or to addition of veins or parts of them. And also the direction of vein-displacements (from A to B, or from B to A?) is not always known with certainty.
But a general and always-holding condition or pattern in the morphology or behavior of organisms that may safely be termed a "derived" condition or pattern, is when this new condition represents an increase in d i f f e r e n t i a t i o n within the original morphological or behavioral condition or pattern. More simply expressed : "derived" means (among less clear cases of "derived") "less symmetric". And this is especially true of the wing-venation : shifting, reduction, or addition of veins may result in the left-hand part of the wing being different from its right-hand part, or its anterior half being different from its posterior half, or, generally, parts of the wing being different from other parts of that same wing. Functionally, differentiation within a given whole means division of labor among its parts. Gross features of the differentiation of parts within the wing-blade are easy to detect, but small contributions to differentiation must be as such recognized, such as, for instance, the transition from dichotomic branching of certain veins to a pectinate branching of them. See next diagram.
It is clear that the pectinate mode of branching is less symmetrical than the dichotomic way of branching, and thus contributes to the overall differentiation of the venation where it occurs. Of course many venational transformations as have been taken place during the evolution of the wings of insects are much more dramatically in this respect, and thus more evident. But of course, if venation B is identical to venation A, the former can be formally derived from the latter. And if it is certain that some particular vein present in venation C has disappeared in venation D, the latter condition can formally be derived from the former.
Functional significance of the wing-venation revisited.
As has been said earlier, the air-current flowing over the (upper and/or under) surface of the wing-blade during flight may be deflected by (following) partial deformations of this surface, deformations that are allowed to take place by the particular course and pattern of the veins or folds, or just by the pattern of relief as a result of the presence of more or less thick, convex, or concave veins. Generally we may expect that the insect-wing, while beating through the air, will be deformed in a specific way, grossly depending on local (on the wing-surface) small (transient) differences of air-pressures, and specificly depending on the mechanical and elastical properties of the wing-blade, where these properties vary from place to place on that blade as a result of existing venational subpatterns in it and relief on it. And we assume that the deformation of the wing during flight is specific, because it is largely determined by the specific pattern of veins (and folds), the venation. And this specific deformation, or even contortion, of the wing of the insect in flight certainly constitutes an element of the specific flight-regime of that given insect. So the wing-venation of a given insect co-determines the particular flight-regime, it reflects part of this regime. If we now look to insects such as Tachinidae (larvae of which live endoparasitic), Calliphoridae (blowflies), Glossinidae (tsetse flies), and Sarcophagidae (flesh flies) (all four families belonging to the Order Diptera), we see that their wings have virtually the same structure and venation, whereas their ways of life differ very much. So venational differences, and thus differences of subtle features of flight-regime, do not go parallel with differences in the way of life. And thus such a particular subtle version of a [more broadly taken] flight-regime, and thus the particualar wing-venation that [co-]determined it, does not belong to the particular strategy of a given insect species. However : only does it not belong to it insofar as the strategy of that species distinguishes itself from the strategies of other species. But as a common and necessary ingredient, common to some larger or smaller group of strategies, that particular subtle version of the flight-regime, and thus the particular wing-venation co-determining it, certainly does belong, as an essential ingredient, to the strategy, i.e. to that particular strategy as well as to all other strategies of the group (to which this strategy belongs). So, to continue with the example just given, although the particular flight-regime, or, particular aspect of the flight-regime, precisely and only insofar as it is determined by the wing-venation, is not a part of the strategy of, say, some given blowfly species insofar as the strategy of this species differs from the strategies of all other flies of the mentioned group of families, it is nevertheless a necessary common ingredient of all the different strategies in this group (and is absent in the strategies in other groups). So in this sense we may say that a given wing-venation belongs to the strategy of the insect species of which the representatives possess this wing-venation (while it also belongs to the different strategies of other species also possessing this same wing-venation). And consequently we may hold that the wing-venation, as a particular pattern or structure, comes from the Implicate Order, because it is there where strategies exist (and from where they are projected into the Explicate Order).
On the other hand, the very small differences in the details of the wing-venation of different individuals or of closely related species do not come from the Implicate Order but are caused by factors active in the Explicate Order (as explained earlier, that is, in the previous document). And, as it is to be expected, these small differences are very variable.
Having all this in mind (differentiation in the wing-blade, functional significance of venation), we want to establish the prototypic venation of the class Insecta. When we have found it we can then derive the venation of all insects from this prototypic venation, and can also identify each vein in the wing of any insect whatsoever. Such a hypothetical prototype might well be the one proposed by Sharov and depicted here in Figure 93H, above . And indeed we see that this prototypic venation contains little, if any, differentiation of parts or areas of the wing. Also the way of branching is largely dichotomic, that is, symmetrical. What is notable, however, is the large number of branches of the main veins. This is, because this hypothetical prototype is largely based on the venation as seen in very old insect fossils. As such this approach is correct. But we must take into account that at the time (in the Earth's history, that is, around the middle of the Carboniferous) in which the insects, now represented by the mentioned fossils, lived, the class Insecta was already in full swing, meaning that already many specializations had taken place, in their wings as well as in other body parts. For example, the transition from the existing mesh-work between the main veins to genuine cross-veins (effecting some degree of differentiation within the wing-blade) has already taken place, as the next carboniferous fossil insect wing shows :
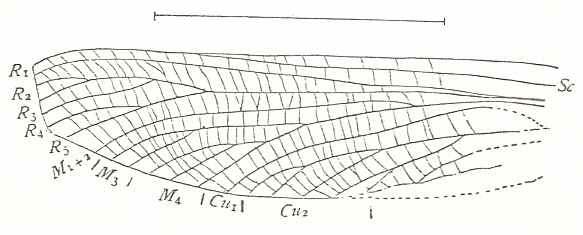
Figure 93I : Wing of Eurythmopteryx antiqua. Lower Upper Carboniferous from the Pratt Mines, near Birmingham, Alabama, USA.
The line measures 49 mm.
(After HANDLIRSCH, 1906-8, in COMSTOCK, 1918)
We have no fossils of truly f i r s t winged insects, and therefore we cannot a priori hold that the oldest fossils that are known to us necessarily represent a venation that is prototypic of the class Insecta. So, with the above proposed prototypic venation in mind (as a serious possibility) we must consider other means of finding the true prototypic venation. Such other means consists in investigating the formation of wings and their venation during earlier phases of individual development of recent representatives of one or another generalized (i.e. primitive, original) insect Order. A good example of such a generalized, but still present today, insect Order are the Plecoptera (stoneflies).
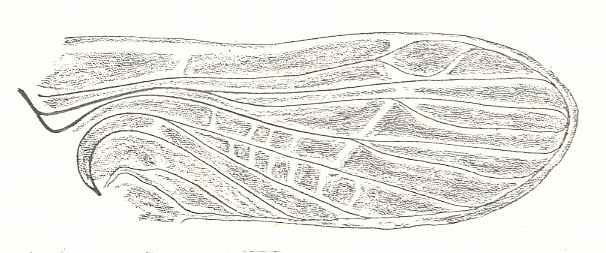
Figure 93J : The forewing of a nymph of Nemoura (Plecoptera). Recent. The dark lines are the tracheae having entered the wing-pad. The white cavities foreshadow the veins of the adult. (After COMSTOCK and NEEDHAM, 1898-9, in COMSTOCK, 1918)
This is what Comstock and Needham (1898-1899) have done for the first time in a series of articles (in American Naturalist), later extended by Comstock in his well-known "The Wings of Insects" (1918). The work of these authors was extensive and covered wings and their tracheation of nearly all insect Orders. As a result of these investigations they came up with a hypothetical primitive type of tracheation and venation, such that from their scheme of primitive venation the venation of all insects could be derived. See next Figure.
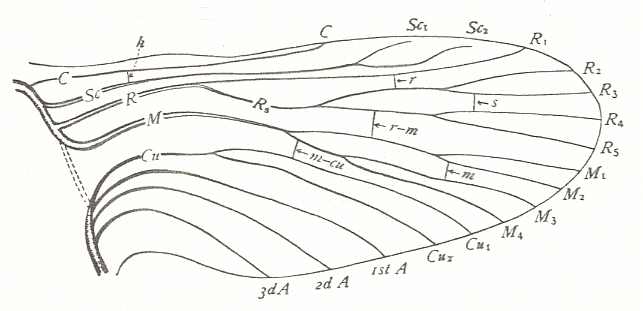
Figure 93K : The hypothetical primitive type of wing-tracheation and venation with the named cross-veins added. (After COMSTOCK, 1918)
And this hypothetical primitive venational scheme has its main veins numerically much less branched than they are in the hypothetical primitive venation proposed (much later) by Sharov ( Figure 93H, above ). So did then the few-veined primitive wings, apparently present in the lower Carboniferous or Upper Devonian, subsequently evolve into the numerous many-veined wings of the insects found as fossils mainly in Upper Carboniferous strata, such as Zdenekia ? This would then be an instance of phylogenetic derivation, not by reduction, but by addition of venational branches. But, as we can see, when comparing the prototype proposed by Comstock and Needham with the forewing of Zdenekia we see in the latter only a slight, if any, increase in differentiation, and the appearance of a loose archediction. It is not easy to be sure whether such an archediction is primitive or not with respect to wings having no such archedictyon but cross-veins instead, while it is relatively easy to hold that the very fine-meshed archedictyon of the true Palaoedictyoptera is derived from a more coarse one such as in Zdenekia. Perhaps in the hypothetical primitive type of venation devised by Comstock and Needham the cross-veins should be removed and a loose archedictyon added. For a typical member of the paleozoic insect Order Palaeodictyoptera see next Figure.
Figure 93M : Representative of Palaeodictyoptera -- Stenodictya sp. Upper Carboniferous. Reconstruction based on the photograph in the work of Laurentiaux (1952a). The wings possessed a fine-meshed archedictyon, not visible in the present Figure. It is visible in the next Figure. Note the lateral lobes on the sides of the abdomen and especially on the prothorax. Note further the piercing mouthparts of this insect.
(After SHAROV, 1966)
Figure 93MM : Blow-up of a part of the previous Figure (93M), depicting the right forewing of Stenodictya sp. (Palaeodictyoptera).
Subcosta is long, with many short branches. Radius-proper unbranched. Radial Sector 5-branched. Media 2-branched. Cubitus 2-branched. Five anal veins. Between the veins there is a fine-meshed archedictyon everywhere. There are no true cross-veins.
The wing is thus relatively few-veined, and the veins are distributed more or less evenly over the wing-blade, that is, they do not bring about a marked differentiation of parts of the wing-blade. So, apart from the achedictyon, and from the Media being only 2-branched, the venation of this wing comes very close to the hypothetical primitive type of Comstock and Needham ( Figure 93K ). It differs markedly from SHAROV's hypothetical primitive type ( Figure 93H ). The main veins of the latter generally have much more branches.
But despite its ancientry (Upper Carboniferous) and despite its allegedly primitive venation, Stenodictya was a specialized insect, as is witnessed by its piercing mouthparts (see previous Figure).
(After SHAROV, 1966)
Let us look a little closer to the hypothetical primitive venational type of Comstock and Needham insofar as it is based on the pattern of tracheation in developing wings of nymphs of species belonging to generalized (primitive) insect Orders.
" It is evident [...] that in the perfecting of a wing as an organ of flight the position of a vein in the adult may become quite different from that of the corresponding trachea of the immature form. In other words, although there is no doubt that the courses of the principal wing-veins of primitive insects were determined by the position of the principal tracheae of the wings, the wing-veins have been more or less modified to meet the needs of adult life, while at the same time the tracheae of the immature wings serving the purpose of respiration, and lying more or less free within the wing-sac, have not been forced to follow closely the changes in the cuticular thickenings of that sac. The operation of this principle is shown only to a slight extent in the wing figured here [a nymph of Psocus (Order Psocoptera [booklice and their allies] ) ]. But when we study more highly specialized forms, it is seen that the divergence of these two sets of structures is sometimes very wide, and must be taken into account in an interpretation of the characters presented by a wing."
An extreme example of this non-correspondence of tracheation and venational pattern is presented by the wings of a caddis-fly (Order Trichoptera, which is an Order that is close to the Orders Lepidoptera, Mecoptera, and Diptera, and of which the larvae live in self-made cases in fresh water) :
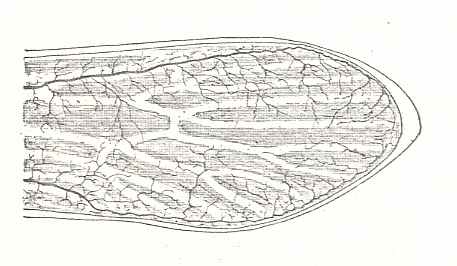
Figure 93N : A wing of a pupa of a caddice fly (Trichoptera). The dark branches are the tracheae of the wing-bud. The pale areas foreshadow the course of the wing-veins in the adult. (After COMSTOCK and NEEDHAM, 1898-9, in COMSTOCK, 1918)
" While this increases the difficulty of determining the homologies of the wing-veins, it is often of great aid in taxonomic work, for it may afford an indication of the degree of divergence from a primitive type in the structure of a wing. And when a series of forms is studied the course of this divergence is often clearly indicated." [in forms in which the divergence is not so great as in the caddis fly just depicted.].
As regards such non-correspondences, Comstock (1918, p. 24) says : " Fortunately in the case of those Orders where the tracheation is reduced, the venation of the wings of adults so closely resembles in its more general features that of the Orders in which the tracheae are well preserved that there is no difficulty in recognizing the identity of the principal veins.
There are cases, however, in which the evidence presented by the tracheation of the wings is misleading and can not be accepted. This does not imply that we are to accept the testimony of the tracheae if it suits our purpose and to reject it if it does not. But rather that we are to consider other evidence as well as that presented by the tracheation."
As is evident from all the above considerations, it is hard to establish with certainty the original primitive type of wing-venation of the very first winged insects, that is, the prototype from which the venation of all winged insects (Pterygota), fossil and recent, can be derived. It is also evident that there has taken place both specialization by reduction and specialization by addition of (longitudianal) veins. A clear case of specialization by addition is given by the Order Neuroptera. Other Orders provide evidence of specialization by reduction. And as already noted, the chief feature to observe that indicates specialization, and thus a derived condition, is that effects local and global asymmetry in the wing, that is, differentiation of parts of the wing. And when we assume that the wing-venation, including all of the constant details of it, is truly functional, we may see the formation of asymmetries in the wing as elements of the formation of a wing adapted to a very specific flight-regime that is typical of a smaller or larger group of related insects.
To me the original primitive type of wing-venation of all insects proposed by Comstock and Needham in 1898-1899 seems to be the most probable because it is based on ontological studies. However, that does not make improbable the prototype proposed by Sharov . The latter is mainly based on paleontological evidence, but, as we have said, we do not know fossils of the truly first winged insects, and so the oldest fossils known to us could be misleading. And although it seems probable that all winged insects (pterygota) have descended from a single ancestor, the possibility of a polyphyletic origin of them cannot be excluded. And then, perhaps, both prototypes are equally valid.
And further, we must realize that, in following the prototype proposed by Comstock and Needham, although we can indeed identify in all insects all the principal veins insofar still present (C, SC, R, RS, M, Cu, and A), experience shows that we cannot expect that we can, always, in all insects, precisely identify all the main branches of these principal veins (for instance the four branches of the Radial Sector, R2, R3, R4, R5, or the four branches of the Media, M1, M2, M3, M4) named by Comstock and Needham in their prototype. We may identify, for example a two-branched Radial Sector, but we generally cannot retrieve whether these branches are R2 and R3, or R4 and R5, or R2 and R5, etc. So in our effords to identify the veins in fossil and recent insects on the basis of the prototype of Comstock an Needham we must be content with identifying the mentioned venational chief systems (C, SC, R, RS, M, Cu, and A), and sometimes with being able to distinguish between, for instance, "MA" (anterior part of medial system) and "MP" (posterial part of medial system), as Sharov has done in his prototype . So in trying to determine the degree of reduction or proliferation of veins in some given wing-venation with respect to the prototype of Comstock and Needham, all we can do is to count the branches of the principal veins that are actually present, without naming them individually.
And, of course, from the venational prototype of all insects are derived all order-prototypes, that is, the venational prototype of each insect Order.
In the next document we will apply these findings to the insect Order Hymenoptera. There we will see how the wing-venation of representatives of Symphyta as well as of Apocrita (fossil and recent) can be derived from the venational prototype of the Order Hymenoptera.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for further study of Organic Evolution, Part LXVIII.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII
Back to Evolutionary Part LVII
Back to Evolutionary Part LVIII
Back to Evolutionary Part LXII
Back to Evolutionary Part LXIII
Back to Evolutionary Part LXIV
Back to Evolutionary Part LXVI