

Parasitic egg-eating (endo-oophagous) phase
The decrease of the size of (certain) terebrants as a result of malnutrition of their larvae in small egg-clutches of their hosts led in further evolutionary development the terebrant-egg-eaters to their (individual) development at the expense of the content of only one single egg of the host. This fact was, apparently, inseparably connected with the ability, appearing in these terebrants, to lay their eggs inside the eggs of the host.
The transition from predatory external feeding to endoparasitism opened up a series of new possibilities, first of all the possibility of the development of several and even many very small egg-eating larvae inside a single host-egg. In this way among the terebrants the smallest forms evolved, and indeed, not only (the smallest) among terebrants, but among the whole Class of insects.
Such are the terebrant-egg-eaters of the family Mymaridae ( Fairy Flies), of which some have a length of only 0.1 mm, that is, in spite of the fact that they possess the complete organizational complexity of Hymenoptera, they are three times smaller than the unicellular infusorium Paramecium.
While in previous phases the terebrant-larvae were only in a position to attack eggs having a sufficiently weak shell [because they were already enclosed in galls and the like], now, for terebrants developing inside the eggs of the host [the egg of the terebrant now being laid inside the egg of the host], the quality and condition of the shell of the host-egg became less important for these terebrants, because the adults obtained the ability to successfully bore with their ovipositor through the shell of the egg that had to be infected. This gave the terebrants the possibility to widen their range of potential hosts, including those that carry eggs possessing a hard shell -- Lepidoptera [butterflies and moths], herb-bugs [Hemiptera], many grasshoppers, and others. And so in this way new relationships may be created. The terebrant-egg-eater of the phase presently under discussion, while (still) attacking larger egg-clutches (for example the egg-capsules of grasshoppers), infected in them only some individual eggs to which also the feeding of its larva was limited.
This parasitic egg-eating, or endo-oophagous, phase, is represented by very small forms of terebrants, chiefly belonging to two large groups : to the Chalcidoidea, to which belong the above mentioned Mymaridae, and to the Proctotrupoidea (= Serphoidea). In some cases, as we shall see, also among the gall-wasps, Cynipidae, one encounters development at the expense of the egg-stage of a host.
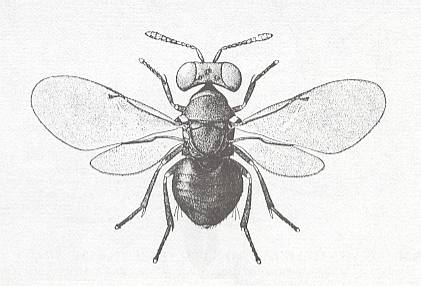
Although not perhaps precisely belonging to the present phase, we here depict a typical chalcid, Blastothrix sericea, in order to provide the reader with some image of this large group (Chalcidoidea), that, as a superfamily or family can be found in many evolutionary phases. They are very small, generally dark shiny stocky wasps, often having wings with a very reduced venation with only one vein.
The tendency of the egg-eating terebrants to find egg-clutches of various hosts expressed itself with such a force that some of these even went down into the water and move in it with the help of legs or wings in order to find and infect eggs that had been laid in submerged parts of plants, that is, in eggs of water-beetles, dragonflies, and aquatic bugs. Thus the Chalcidoid Prestwichia aquatica Lubb. ( Trichogrammatidae ) develops at the expense of eggs of large species of water-beetles (Dytiscidae), laid in leaves and stalks of various aquatic plants. See next Figure.
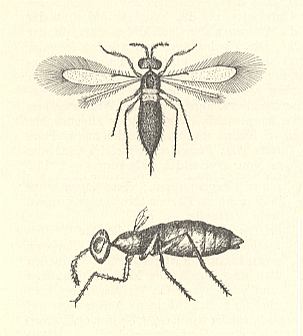
Swimming in the water with the help of its legs, the female of Prestwichia finds the eggs of the water-beetle which are present under characteristic incisions made by the beetle's ovipositor in the integument of the plant, and lays in them several tens of eggs (maximally 50), [each] provided with a fairly long stalk. All of the development of the terebrants takes place in one egg of the water-beetle such that inside the emptied transparent shell of the latter's egg the prestwichias reach their maturity (their adult state), copulate there [but see, later, the remark of Wesenberg-Lund], and then gnaw an exit hole and swim to the water's surface (Rimsky-Korsakow, 1916).
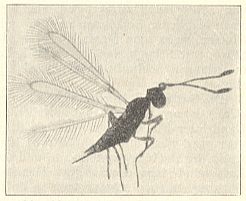

The females of the mentioned mymarid lay their eggs in the eggs of large water-beetles as well as in those of small ones (especially Hybius), where from the latter emerge in total from 4 to 6 individuals, whereas in the first up to 20 individuals develop. Usually C. cinctus stays all of its live, lasting up to 15 days, in the water without access to atmospheric air (Rimsky-Korsakow, 1925, Ivanova-Kazas, 1954).
Extract from
Above we reproduced Malyshev's short exposition of aquatic Hymenoptera. And because the ecological transition from a terrestrial life -- typical of Hymenoptera -- to a (more or less) aquatic life is, in a certain respect, a sharp transition (because, among other things, the respiratory conditions become quite different), we will dwell upon it longer, and reproduce an exposition of aquatic Hymenoptera given by Wesenberg-Lund.
Wesenberg-Lund is an authority on aquatic insects, and therefore his exposition will represent a valuable contribution to what has already been said above. It was written in German, and starts on page 576 of his book on aquatic insects. We will translate (and comment where necessary) into English :
One might assume that insects that have adapted themselves to living in fresh water, escape the attacks of ichneumon-flies [parasitic hymenoptera-terebrantia], to which terrestrial insects are so heavily subjected. This is, however, not the case at all. The better we come to know the aquatic insects, the more we get the impression that almost every species of them is ecologically connected with a particular ichneumon-fly, that parasitizes on it and [thus] lives of it. In saline water there are only a few wasps. However, the larvae of Ephydra (Diptera-Cyclorrapha, Ephydridae) are often heavily attacked.
Chalcididae
Until now (1943) little was known about aquatic ichneumon-flies. The first ones were described in 1863 by Lubbock. Then many years went by, until recently in further investigations one had found numerous aquatic ichneumon-flies. But the way of life of many of these is unfortunately still little known. Our knowledge of this group chiefly comes from Hendriksen (1918 and 1922), whose works formed the source of most of our expositions here.
Attacks of ichneumon-flies in water or on land apparently differ by the fact that aquatic insects almost always are infected in their egg-stage, and only exceptionally in their larval stage. Most ichneumon-flies of the water are only a few millimeters long. They bore their ovipositor into the eggs of aquatic insects, from which then, instead of an individual of the involved species of the latter, emerge one or many very small ichneumon-flies. Many insects of which the larvae and pupae live in the water, as for instance certain Diptera (Stratiomys, Eristalis a.o.), lay their eggs above the water, often on Phragmites. Therefore, many parasitic wasps (ichneumon-flies) live in the reed zone. Wasps that infect eggs not submerged in water cannot be considered aquatic insects, because they never volontarily go into the water, while they sometimes parasitize animals that are in certain of their stages aquatic insects. Many aquatic insects deposit their eggs directly onto the water's surface. Also these eggs can become infected without the wasps needing to go under water. Other aquatic insects, for instance many dytiscids (predator aquatic beetles), bore their eggs into living plant tissue under water. When in the spring the plants (Iris, a.o.) grow out above the water, the eggs now end up above the water-surface (while they were laid below it) and here go through the largest part of their development. Such eggs are almost always heavily infected by ichneumon-flies. In many places in north Seeland (Denmark) I have collected hundreds of such eggs. Often of about 50 eggs not a single one was not infected, while eggs that develop under water are less often infected. So it seems that the parasites of such eggs chiefly of them infect those eggs that find themselves (now) above the water-surface.
So it is not surprising that the ichneumon-flies of the water only rarely possess features pointing to a relationship of the animals with water. Their body, it is true, often is non-wettable, but this is not a result of special hairs or the like, but rather of the structure of the chitin. However, in many of them the underside of the tarsi (feet) is covered with felt. The wings are somtimes absent or are reduced. The wasps generally are bad swimmers. They rather crawl than swim. Their tracheal system is normal. Certain forms (Smicra) infect, it is true, eggs (of Stratiomys), but the egg of the ichneumon-fly only begins to develop when the host-larva has emerged. But most ichneumon-flies [of this 'watery group'] develop in the host-eggs [that is, consume them when they are still eggs]. Braconidae and Ichneumonidae do not parasitize eggs but lay their eggs into the larvae of their hosts.
Many parasitic wasps, and chiefly those that parasitize eggs of Odonata (damselflies and dragonflies), look for such eggs under water. They are in several respects clearly adapted to living in the water and are skilled swimmers. The ichneumon-flies of the water are distributed into five [terebrant] families : Chalcididae, Proctotrupidae, Ichneumonidae, Braconidae, and Agriotypidae.
Chalcididae and Proctotrupidae always are very small insects, almost all smaller than 1 cm and often only a few millimeters long. They are distinguished from each other especially by the fact that the ovipositor of the females in chalcids is placed far anteriorly on the underside, in Proctotrupids at the tip of the abdomen. Further, the antennae ar in chalcids always, in Proctotrupids not always, bent [the parts forming an obtuse angle].
The forewings have no spots (macula). The whole venation consists of just a single vein at the anterior wing-margin. Winglessnes is widely distributed. Further, the chalcids are characteristic by their metallic colors. The majority of them parasitize terrestrial insects [and many of them are phytophagous], and only a small minority of them can be considered aquatic wasps, and also these chiefly infect eggs or cocoons, placed above the water level, of aquatic insects. Some of these have been reared from cocoons of Gyrinidae [beetles living as adults on the surface of water], a single species
Of the large division of Trichogrammae only the species Trichogramma evanescens Westw. has certain relationships with fresh water (Kryger, 1918). It is often encountered on the brown eggs of Sialis (Alder Flies). Their females are winged, the males usually wingless. The small males crawl over the Sialis eggs and wait for the females to mate with them. The animals do not limit themselves to Sialis eggs though, but lay their eggs also into those of Stratiomyidae (certain Diptera, with aquatic larvae), Megaloptera other than Sialis, butterflies, as also into the eggs of the beetle Rhynchites betulae (Cuculionidae). So these wasps are in fact terrestrial animals that parasitize eggs at the water (rather than in the water).
Only a single species, the famous Prestwichia aquatica Lubb. (see Figure 1 above ), really goes under water and infects there the eggs of numerous aquatic insects, as for instance water-beetles, dragonflies, and aquatic bugs (Hemiptera). Numerous treatises on the life history of this animal have appeared. The male is wingless. The females appear in no less than three forms, of which one has well-developed, the second shortened, and the third rudimentary wings. The very small size of these animals is best expressed by the fact that from one single Dytiscus-egg or an egg of an aquatic bug up to 34, but at least 11-16 wasps emerge (Ruschka and Thienemanm, 1913). The wasps have a length of less than 1 mm. When they lay their eggs in those of Odonata, then from each one of them only one wasp emerges. Males and females swim with the help of their legs (Rousseau, 1907), which are, however, not provided with special swimming hairs. The winged females do not use their wings for swimming. Most often they crawl on plants or walk on the water-surface. But it is also observed that they go under water, crawling under the water-surface and then slowly swimming downward. The animals can be held for five days in aquaria without leaving the water. Their tracheal system is normal, with open spiracles at the metathorax. Probably also respiration through the skin plays an important role. Their body is certainly unwettable. Under water the animals are always surrounded by air, and instantly dry when they have come at the surface. The females are much more abundant than the males. Rimsky-Korsakow (1917) asserts that often, when many wasps develop in one host-egg, mating takes place in that egg, before the wasps emerge. The correctness of this observation is disputed by other authors (Heymons, 1908, Hendriksen, 1918). Development lasts only a short time. Several generations in a year develop.
Proctotrupidae
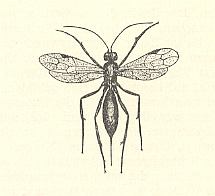
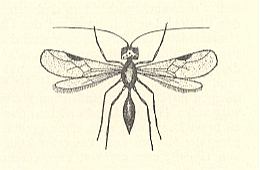
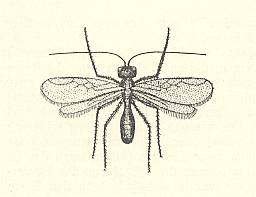
Also this family chiefly consists of very small forms, some of wich have relationsships with freshwater. But only a few of them can be considered truly aquatic. They have been reared from eggs of Odonata and aquatic bugs. Their chief genera are Limnodytes, Litus, Anteris, Anagrus (see next Figure), and Anaphes (= Polynema) (see Figure 2 and 3 ).
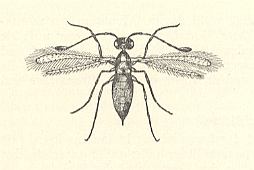
The genus Limnodytes lays its eggs in those of pond-skaters (Gerridae, Order Hemiptera, bugs). Both sexes are winged. They are aerial as well as aquatic animals. In the air they fly, in the water they swim with the help of their wings.
Apparently best adapted to an aquatic life of all wasps is
Polynema natans Lubb. (= Anaphes cinctus Halid. = Caraphractes cinctus). See Figure 2 and 3 above . Also this species is hardly one millimeter long. Both sexes are winged. For swimming they use their wings provided with long hairs. Earlier it was indicated that these animals do not possess a tracheal system, and that their wings, supposedly filled with blood, serve as gills. This turned out not to be so. The tracheal system of these animals is well developed. These tiny creatures belong to the prettiest microscopic objects. When a larger number of them swimms in a fish-tank they are most lovely to watch. It is certain that mating also takes place under water. The animals, it is true, come, from time to time, at the surface, but are able to live certainly at least several days under water (see Figure 3, above ). This species parasitizes eggs of damselflies (Calopteryx) and aquatic Hemiptera (Notonecta, backswimmers). According to the investigators, having enquired into the parasites of eggs of Odonata, from each one of the infected eggs only one ichneumon-fly emerges. Also when several ichneumonfly-eggs have been laid in one odonate-egg, only one of them will develop. In the much larger Hemiptera-eggs one finds in one such egg 4-5 wasps, often 2 males and 3 females. When the whole odonate-egg has been consumed, which lasts only a few days, the wasp-larva pupates. The pupal stage lasts 10-12 days. In north Seeland (Denmark) P. natans is very common in odonate-eggs. One can recognize infected eggs already by their blue-black color. Leaf-stalks of Nuphar, densely populated by eggs of Erythromma najas (Odonata-Zygoptera, damselflies), often take a bluish shine as a result of the wasp-attacks. Where, and in what stage, the animals hibernate is not known. When in autumn the shores of the Furesee [a lake] are, after a storm, covered with foam, I often have found in it thousands of 1 mm long ichneumon-flies. They, however, have not yet been determined (as to what species they belong).
Two species of the closely related genus Anagrus parasitize in odonate-eggs laid under water. For a representative of this genus see Figure 4, above .
The Proctotrupidae are characterized by the fact that inside the host-egg they go through several very peculiar larval stages, belonging to the most adventurous forms of the insect world. Because they develop inside the insect-egg, these larval stages naturally, are very small indeed, being only some fractions of a millimeter long.
Ichneumonidae
Among the family Ichneumonidae, including so many large forms, and that preferably parasitize in larvae and not in eggs, there still are a few that parasitize aquatic insects. But they most often develop in those stages of them that live above the water : Hemiteles in cocoons of Gyrinidae (beetles, as imagines living on the surface of the water) (Thienemann, 1916, Butcher, 1933), see next Figure.
Atractodes develops in pupae of flies (Fliegenpuppen) (Diptera) (Ruschka and Thienemann, 1913), or in Stratiomys-larvae (Diptera-Stratiomyidae) that always float on the surface. Also in larvae of Trichoptera (caddis flies) now and then are found parasites, chiefly, it is true, in those that ended up outside the water. Thus Hemiteles biannulatus Gray was reared from Limnophilus-larvae (Siltala and Nielsen, 1906), Trichocryptus aquaticus Thomson from Hydrocampa nymphaeata (Hendriksen, 1918). At the same place where Hendriksen had found this species, I have, during several years, observed numerous Hydrocampa-cases, containing pupae of about 1 cm long of parasitic wasps. Probably they belong to the mentioned species.
Braconidae
Further we have (among aquatic braconids) Liposcia, Gyrocampa (see next Figure), Dacnusa, and others.
And finally we have Hygroplitis rugulosus Nees reared from Hydrocampa-cases (Hendriksen, 1918, Ruschka and Thienemann, 1913, Schulz, 1907, Rousseau, 1907, Brocher, 1910). One has observed that Dacnusa and other species swim in the water using their wings. The wings of these and other species living nearby the water often are hairy, or their margin is provided with long hairs. Also the tarsal segments are widened, in order that their legs can be used as swimming devices.
Agriotypidae

On the first warm days of spring toward the end of April in north Seeland (Denmark) above rapidly flowing forest-brooks one sees almost-black wasps of about 1 cm long flying around. They also sit on rocks and from there they crawl -- surrounded by an air-envelope -- into the water, where their trail quickly vanishes in the turbulence of the water. At the same places numerous Silo-cases (i.e. cases of certain caddis flies) are present. Surely, the wasps look for the larvae living in these cases in order to lay their eggs onto them. The emerging wasp-larva feeds on the caddis fly larva. The latter, however, dies not directly, but still manages to pupate. Only then the Agriotypus-larva totally consumes its prey. It has no anus and no tracheae. The fully-grown larva carries two short hooks at its last abdominal segment. When the host-larva is paralized [The original text reads "verzehrt", which certainly must mean here "paralysiert", as this expression is indeed used (by Wesenberg-Lund) in the subscript of his accompanying Figure 490 (see next Figure here), despite the fact that the ability to paralyze the prey is more typical, not of terebrants (parasitic wasps), but of aculeates (sting-possessing hymenoptera)], the wasp-larve provides the Silo-case with a wide flat band, of which the length varies from 1 to 5-6 cm. See next Figure.

The cocoon [i.e. the case] anteriorly has, between the side-wall and the anterior wall, a cleft. Through this and the sieve-disc [Siebplatte] the band [which Wesenberg-Lund now calls the tube (Röhre)] emerges. How the larva manages to push the tube out is at least to me [Wesenberg-Lund] difficult to understand. If one cuts off this band the parasite dies. The band is filled with air. The fully-grown parasite is surrounded by a layer of air. Hendriksen (1918) notes to this that the Agriotypus-pupa, having an open tracheal system, must provide itself with air, because the caddisfly-pupa has tracheal gills and therefore not stores air in its case. The band would then be the means by which air is led in [for the benefit of the wasp-larva]. It mysterious to me [W-L] how this very flat tube would be able to lead the air contained in the water into the pupa-case. Moreover, one is then forced to hold that countless other aquatic parasites also would need such an air-pipe. I have seen countless cases ['houses'] of Silo and Goëra that were occupied by Agriotypus, where these chiefly were, as it seems to me, cases that were outside the water. The tube is certainly produced by the silk glands of the wasp-larva. It is natural to compare this band with the mast on the Hydrophilus- (a vegetarian water-beetle)-cocoon. The presence of this bands indicates that a [caddis fly] case is occupied by Agriotypus. In autumn the larva spins its cocoon and before the beginning of winter changes into the adult insect. As such the animal rests 4-5 monthes in its cocoon inside the caddisfly-case, and only at the end of April frees itself from its prison.
With all this we have come to an end of our exposition of the Parasitic egg-eating (endo-oophagous) phase of the evolution of the Hymenoptera.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXXVII.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV