

[Also in these enquiries into the wing-venation and its transformations in Diptera-Nematocera we will consider the subcostal vein (Sc) at most only occasionally. This vein, especially its terminal branches (Sc1, Sc2), is -- in Diptera -- often difficult to discern.]
The superfamily Chironomidea includes three families : the Chironomidae [ = Tendipedidae, Midges], the Ceratopogonidae [ = Heleidae, Biting Midges], and the Simuliidae [ = Melusinidae, Blackflies].
1. The venational groundplan of the wings in the family Chironomidae can be represented by the wing-venation in Trichotanypus nigriventris KIEFF. :
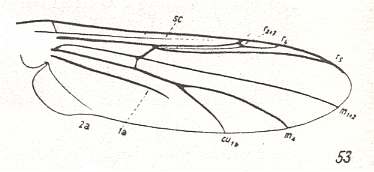
Figure 1 : Wing-venation of Trichotanypus nigriventris KIEFF. Order Diptera, Subfamily Pelopiinae, Family Chironomidae, Superfamily Chironomidea.
(After HENNIG, 1954)
Subcosta (Sc) reducing. R5 and R4 present. R2+3 branches off from R4. R2+3 very short, not forked, ends up in R1. So the Radial Sector (Rs) is 3-branched. The Media is 2-branched : M1-M2 fork absent (leaving only M1+2). M3 absent. M4 present. The cross-vein tb -- or better, its anterior part, compare with Figure 1 in Part XVI -- shifted toward the wing-base. In any case, R2+3 and tb are preserved almost only in the subfamily Pelopiinae (Figure 1). First anal vein (1a) shortened, does not reach the wing-margin. The second anal vein (2a) is weak, it also does not reach the wing-margin.
The same venation we find in Pelopia :
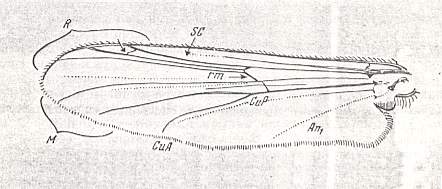
Figure 2 : Wing-venation of Pelopia sp. Order Diptera, Subfamily Pelopiinae, Family Chironomidae, Superfamily Chironomidea.
(After HENDEL, from ROHDENDORF, 1951)
A next state of reduction of the venation we see in Polypedilum :
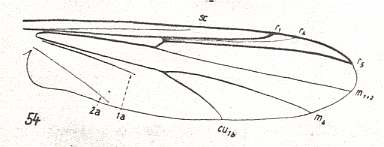
Figure 3 : Wing-venation of Polypedilum nubeculosum MEIG. Order Diptera, Family Chironomidae, Superfamily Chironomidea.
(After HENNIG, 1954)
Subcosta (Sc) reducing. R2+3 absent. R4 very weak. R5 present. So Radial Sector (Sc) only 2-branched. Media 2-branched : M1+2 and M4. The cross-vein tb (in fact its anterior part) shifted still farther toward the wing-base : it is now in fact situated in the very wing-base. First anal vein (1a) short, not reaching the wing-margin. Second anal vein (2a) thin, apparently just reaching the wing-margin.
The maximal reduction of the venation in this family can be seen in the very small wings of the Corynoneurinae ( Corynoneura and Thienemanniella) :
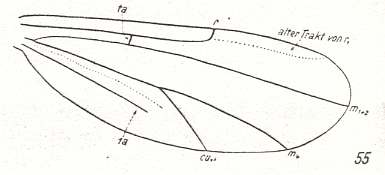
Figure 4 : Wing-venation of Thienemanniella sp. Order Diptera, Subfamily Corynoneurinae, Family Chironomidae, Superfamily Chironomidea.
(After ENDERLEIN, 1937, from HENNIG, 1954)
Subcosta totally reduced. R1 shortened, its old tract still visible. Radial Sector absent. The Media is 2-branched : M1+2 and M4. First anal vein (1a) shortened, not reaching the wing-margin. Second anal vein absent. The cross-vein tb still situated in the wing-base, almost in line with M1+2, hardly recognizable as a cross-vein. Wing not particular elongate.
To understand the state of the venational groundplan of the (recent) Chironomidae, we can best compare its venation (as it is in Trichotanypus, Figure 1, or in Pelopia, Figure 2) with that in Culicidae (Culex, or Anopheles), which, like the Chironomidae, have elongate wings :
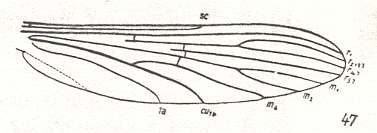
Figure 5 : Wing-venation of Anopheles maculipennis MEIG. Order Diptera, Family Culicidae, Superfamily Culicidea.
(After HENNIG, 1954)
If we let the subcosta lose its terminal part, if we shorten the fork R2+3-R4 and let R2+3 run into R1, if we let the radial veins (R and Rs) be pressed more or less against the anterior wing-margin and their end-points be shifted into the direction of the wing-base, if we totally reduce the fork M1-M2 leaving only the vein M1+2, if we shift the cross-vein tb (in fact its anterior part) into the direction of the wing-base, and, finally, if we reduce the terminal part of 1a, we obtain a venation similar to that of Trichotanypus (venational groundplan of Chironomidae) depicted in Figure 1, and here produced again) :

Figure 6 ( = 1) : Wing-venation of Trichotanypus nigriventris KIEFF. Order Diptera, Subfamily Pelopiinae, Family Chironomidae, Superfamily Chironomidea.
(After HENNIG, 1954)
With this we do not mean that the Chironomidae have actually descended from the Culicidae, but only the fact that their groundplan-venation can formally more or less neatly be derived from the venation as it is in the family Culicidae.
The fact that some typical venational characters of Chironomidae and also their typical wing-shape were already present in the lower Jurassic is indicated by Architendipes tshernovskiji ROHD. :
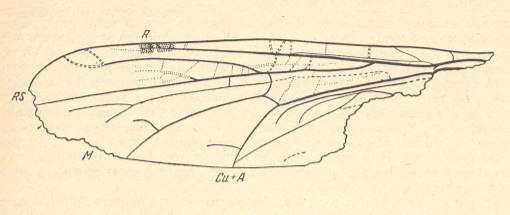
Figure 7 ( = 1) : Wing of Architendipes tshernovskyi ROHD. Order Diptera, Family Architendipedidae, Superfamily Chironomidea. Length of wing 4.5 mm. Coll. PIN No. 358/197. Lower Jurassic of Issic Kul, central Asia.
(After ROHDENDORF, 1962, from ROHDENDORF, 1964)
Of course the above fossil does not contain sufficient data to show that it undoubtedly is a true chironomid, but the shape of the wing and the type of reduction of the venation point in that direction.
2. The venational groundplan of the family Ceratopogonidae can be represented by the wing-venation as it is in Atrichopogon rostratus WINN. :
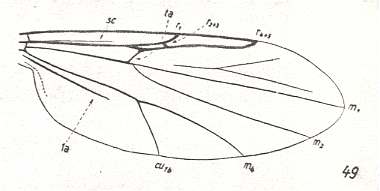
Figure 9 : Wing-venation of Atrichopogon rostratus WINN. Order Diptera, Family Ceratopogonidae ( = Heleidae), Superfamily Chironomidea.
(After HENNIG, 1954)
R5 and R4 coalesced, resulting in one single vein, R4+5. Endpoints of R1 and Radial Sector shifted toward the wing-base. R2+3 branches off from R4+5, it is very short and ends up in R1. The fork M1-M2 is present and deep, that is, its bifurcation point has shifted toward the wing-base. M4 present. The cross-vein tb, that is, its anterior part, has been shifted all the way into the wing-base. First anal vein (1a) present, but shortened, not reaching the wing-margin. Wing moderately elongate, in fact relatively broad.
HENNIG, 1954, p. 283/4, discusses the status of the fork lying between R4+5 and M1 as we see it in Atrichopogon rostratus (figure 9). The main lines of this discussion we now give :
The proper identification of a fork lying between the Radial Sector and M1 in many Heleidae has caused headaches in many authors. Here we have not to do with simply the formation of creases or grooves in the wing-membrane, but, as coloration and growth of hair indicate, with a true vein-like stiffening of it. Interpreting this fork and its arms as R4 and R5 or MA2 (VIGNON & SÉGUY) would ask for big problems, because it would then be incomprehensible why these veins precisely in Heleidae have been preserved, while they evidently have been lost in all their close relatives (also in those that are, apart from it, much more primitive) [What is meant here, evidently, is the f o r k R4-R5 (two veins), not R4+5 itself (one vein). The interpretation as R4 and R5 lets the venational groundplan of the Heleidae have a 4-branched Radial Sector.]. Against such an interpretation we can also bring up the following : The mentioned fork especially occurs in forms that are otherwise relatively apomorph (derived, advanced). According to me all these problems are solved easily by the following considerations : Often we have already found out that the structure of the wing-surface (the formation of creases and grooves) has, it is true, original connections with the course of (true) veins, but that these veins can free themselves relatively easily from their creases, while [after such things have happened] the original structure of the wing-surface is being retained relatively tenaciously. Apparently, when needed, things can lead to a re-activation of the old wing-surface structures, without having to assume the reappearance or preservation of veins that have been lost or shifted long ago, while they have been lost in all close relatives. In Heleidae the development of the wing-venation clearly proceeds in the direction of the Radial Sector shifting to the Radius. The Media does not join in to this. As a result a relatively broad vein-free field is formed between Rs and M which is not favorable to the stabilization of the wing-blade. Nevertheless the shifting of the Radial Sector to the Radius continues. Compensation of the mentioned unfavorable implications of this process takes place by the re-activation of an old crease-structure (or, in any case, some sort of structure) in the wing-surface that originally was occupied by the (now) shifted Radial Sector. So one can, in interpreting this structure, as it is now in Heleidae, refer back to the Radial Sector (that was once there) without, however, because of it, identifying this structure with certain definite branches of the Radial Sector. The forked structure only indicates the former position of the Radial Sector. It is, as it were, the old bed of Rs, now newly occupied.
Something completely comparable happens in the wings of the family Melusinidae [=Simuliidae] in the region between M2 and M4. (HENNIG, p. 284)
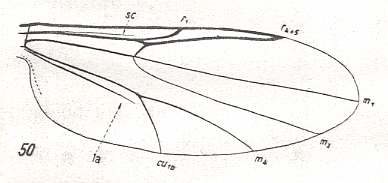
Figure 10 : Wing-venation of Bezzia ornata MEIG. Order Diptera, Family Ceratopogonidae ( = Heleidae), Superfamily Chironomidea.
(After HENNIG, 1954)
This venation (Bezzia) is identical to that of Atrichopogon in Figure 9 , except that now R2+3 is totally reduced, leaving the Radial Sector to be just a single vein. Wing moderately elongate.
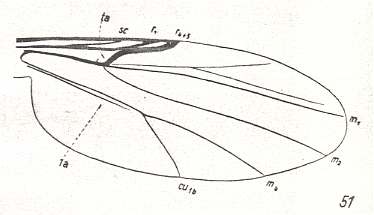
Figure 11 : Wing-venation of Forcipomyia ciliata WINN. Order Diptera, Family Ceratopogonidae ( = Heleidae), Superfamily Chironomidea.
(After HENNIG, 1954)
The Radial Sector have now come so close to the Radius that they at last merge :
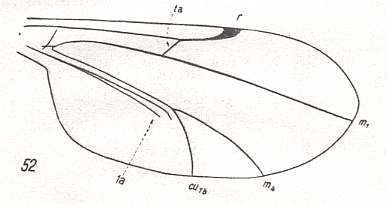
Figure 12 : Wing-venation of Brachypogon vitiosus WINN. Order Diptera, Family Ceratopogonidae ( = Heleidae), Superfamily Chironomidea.
(After GOETGHEBUER, 1934, from HENNIG, 1954)
Now anterior to M1 there is only one simple longitudinal vein present, which can be rightly labelled as being R1-5 + MA. So here the devolopment, already begun in the ancestors of the Holometabola, is carried to its extreme!. In this extreme case the Subcosta (Sc) is apparently completely lost, that is, the wing-venation in this region can be so interpreted because its shortening is already apparent in other cases ( figure 9 and figure 10 ).
3. The venational groundplan of the family Simuliidae can be represented by the wing-venation as it is in Prosimulium rufipes MEIG. :
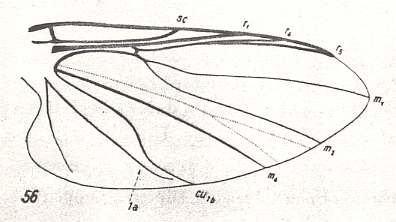
Figure 13 : Wing-venation of Prosimulium rufipes MEIG. Order Diptera, Family Simuliidae ( = Melusinidae), Superfamily Chironomidea. Basal cell not drawn.
(After HENNIG, 1954)
Subcosta (Sc) strong, reaching the wing-margin. Therefore the venation in this form cannot be derived from that in Ceratopogonidae, because there, even in their venational groundplan (see Figure 9 ) the Subcosta is alreading in a state of reduction.
Radial Sector 2-branched. It is markedly pressed toward the Radius and anterior wing-margin. Apparently the two branches of Rs can be identified as R4 and R5. The Radio-medial cross-vein ta ( = r-m) is clearly present, albeit short. The M1-M2 fork (if this identification is correct) is extremely long, that is, its bifurcation-point has been shifted markedly toward the wing-base. The cross-vein tb ( = m-cu) is absent. The fork M4-CuA ( = M4-Cu1b) is, like the M1-M2 fork, deepened, that is, its bifurcation-point has shifted markedly toward the wing-base. These shifts undoubtedly are connected with the secondary broadening of the wings in Simuliidae. Also the presence of a fork in the area between M2 and M4 is connected with this. The presence of this fork should be explained in the same way as we did (following Hennig) with respect to the occurrence of a fork in the region between Rs and M in Ceratopogonidae.
The undulation of CuA ( = Cu1b) and the strong development of the first and second anal veins are certainly connected with the broadening of the anal area of the wing.
The Radial Sector being 2-branched only occurs in Prosimulium and Parasimulium, while in all other Simuliidae it is just a single vein :
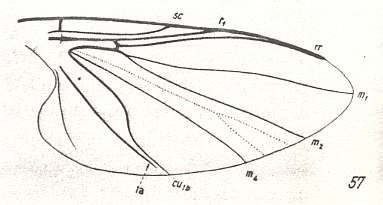
Figure 14 : Wing-venation of Melusina reptans L. Order Diptera, Family Simuliidae ( = Melusinidae), Superfamily Chironomidea.
(After HENNIG, 1954)
Subcosta (Sc) still well developed, reaching the wing-margin. Radial Sector just a single vein. The rest of the venation is identical to that in Prosimulium ( Figure 13 ).
As could be expected, the same venation can be found in species closely related to the one just depicted :
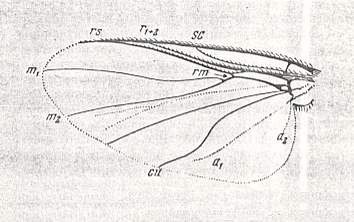
Figure 15 : Wing of Simulium sericatum MEIG. Order Diptera, Family Simuliidae ( = Melusinidae), Superfamily Chironomidea.
(After HENDEL, from ROHDENDORF, 1951)
We can see that the wings in the families Chironomidae, Ceratopogonidae, and Simuliidae undergo a fairly strong reduction of the venation, but differently in each one. It is unlikely to assume that their venation (that is, the venation as we see it in the superfamily Chironomidea) represents a single sequence of reduction. Each family has characteristic wings, reflecting a venational history of its own.
In the next document we will consider the wing-venation in the family Bleohariceridae.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XX.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII