

The development of the terebrant-egg-eaters at the expense of single eggs or of small clutches of the host led, as we already saw, to a diminution, sometimes extraordinarily, of the size of the representatives of whole families or even superfamilies such as the Chalcidoidea, (part of the) Cynipoidea, and Serphoidea. But, as is known, in the suborder Terebrantia there also exists a series of other large systematic units of which the representatives fall into the categories of medium or even large sizes. Already this one fact shows that the development of the terebrants of the latter group took place under other conditions and followed other paths than those of the egg-eaters. Meanwhile, in the archaic inquilinoid phase where the development of the terebrant-inquiline basically took place at the expense of the tissue of the original gall [taken over by the inquiline], and also in the case of feeding by the solitary larvae of the egg-eaters on large egg-clutches of the host, sufficient favorable conditions were created for the preservation of normal [i.e. complete] growth of the terebrants, which is visible for example in the Evanoidea.
Closer inspection of the life habits of the terebrants of this second group -- which are not egg-eaters -- indicates that the preservation by them of sufficiently large sizes, and sometimes even an increase of their growth, has been realized along different paths. One of the basic and, apparently, most widely distributed way to reach a complete satiation of the larva of the terebrant was the laying of the egg by the terebrant into the egg of the host or into its very young larva such that the latter did not die but continued developing normally. Now, the parasitic larva, finding itself inside the prey, successfully emerged from its egg, and was in the position to develop later at the expense of the more or less grown host-larva and sometimes even of the pupa of the host. Thanks to that the terebrant could attain proper growth. Not themselves being thus egg-eaters, the terebrants of the present group originated, as we must assume, basically from precisely those primitive egg-eaters that layed their egg not on the surface, but inside the egg of the host and after that began to develop at the expense of its more or less grown larva.
This strategy of delayed parasitism is very widely distributed. It is encountered also among the most primitive terebrants from widely separated systematic groups, as such pointing to their common origin from a single basic kernel of terebrants. Here are some examples.
The relict cynipoid Ibalia leucospoides Hoch, from the subfamily Ibaliinae, being a giant among the other cynipoids, lays its eggs in the egg-clutches of the blue horn-tail Sirex cyaneus F., which arranges them [i.e. its egg-clutches] in special deepenings in the bark of a tree. Having lowered its ovipositor into the egg-tunnel of the horn-tail, the Ibalia carries its egg over into the egg of the horn-tail or into its newly-born larva. In a single lowering of the ovipositor several eggs or larvae of the prey may be infected. The length of the period of the egg-stage of the Ibalia is very variable, namely from 6 weeks until almost a year. The third-instar larva of the Ibalia leaves the body of the horn-tail larva and finishes feeding outside it, and thus in its fourth instar the larva of the Ibalia does not eat anymore and may remain in this state for about a year. The overall length of the period of development of the Ibalia from egg to adult is not ascertained precisely, but one can safely assume that it takes no less than three years. All developmental stages of the Ibalia can be found in nature the whole year around. Thus, the connection of this primitive cynipoid with egg-clutches of the host is very indicative.
As regards another remarkable relict, Orussus of the superfamily Orussoidea, and of the family Orussidae, see next Figures, sometimes, on the basis of morphological features placed into the suborder Symphyta [Saw-flies and allies], but on the basis of behavior into the suborder Terebrantia, there are up to now [1966] only very fragmentary data.
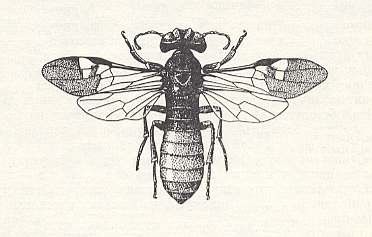

From a series of observations, reported by Burke, 1917, it follows that the species Orussus hopkinsi Rohw. and O. occidentalis Cress., living in America, are solitary parasites of larvae of beetles of the family Buprestidae (Buprestes), that live in the trunks of poplar, pine-tree, and other trees. As it was shown, the larvae of the mentioned Orussidae are encountered at different times of the year, but in each case the orussid larva was already fully-grown and was found in some cases next to a large larva of the beetle, and in other cases already next to its remains. So in spite of the different seasons only fully-grown orussid larvae were found and thereby next to their preys. This leads us to think of parasitism of young orussid larvae inside the larvae of the host, to which also Burke himself was partly inclined to think. This state of affairs is possible, however, only when the egg-laying by the parasite takes place in a corresponding young stage of development of the host, similar to what was noted above with respect to Ibalia. This fact, consequently, points to a significant biological affinity between both relicts, which do not seem to display any genealogic affinity.
From the obtained data Cooper, 1953, drew an unexpected conclusion : Because Burke had actually not seen young larvae of Orussidae in the act of feeding, their being carnivorous could not be taken as proved. According to Cooper it is more probable that the larvae of Orussus feed on wormhole left behind by the larvae of Buprestidae in their channels. But Cooper himself had not seen young, nor fully-grown larvae of Orussus, and had only seen two females of O. sayii Westw., having lowered their ovipositor into a dry log, lying in a storage depot, while eggs were not observed at the place where the Orussus did have their boring activity. Moreover, feeding on wormhole (which is in fact coprophagia [= feeding on excrements] ) is not at all typical to larvae of Hymenoptera.
In a similar way, evidently, we can see things also in a relict from the group of evanoids, namely from the third family of them, the Aulacidae. See next Figure.
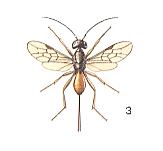
As in the previous cases, we might think, here the small female Aulacus, not possessing a corresponding organization, cannot lay its eggs into larvae of horn-tails and beetles living deeply hidden in wood, but can infect only their egg-clutches placed in the bark of trees. This assumption becomes even more probable by the fact that the relatives of the Aulacidae -- the other evanoids, Gasteruptiidae and Evaniidae, are in their development, as was already mentioned, connected with the egg-stage of their hosts. It is therefore interesting to note that the European species Aulacus striatus Jur., having a length of 7-9 mm, is encountered on logs and trunks of trees, containing eggs of the horn-tail Xiphydria prolongata Goef., at the expense of which also this aulacid develops. But until now it is not known whether it does so as an internal parasite of the larva or as an external one.
Anatomic enquiry has shown that the ovaries of Aulacus striatus contained about 200 very small (having a length of 0.14 mm) eggs. These latter have a spindle-like shape and are provided with a short stalk.
In connection with this we must note that the representatives of the small, structurally primitive, family Stephanidae, parasitizing on larvae living in wood, are, with respect to their structure, especially of their head, extraordinarily similar to the Orussidae, as was pointed to earlier when the parasitism of the latter became known. Together with this the connection of the Stephanidae with certain representatives of the archaic group Evanoidea became clear, especially with the just mentioned Aulacidae.
This fact, consequently, also confirms the above mentioned connection of the Orussidae with the Terebrants, and absolutely not with the phytophags-horntails (Symphyta).
So it is very probable that also the Stephanidae, as to their method of egg-laying and as to their development at the expense of larvae living in wood of trees, belong to that same group of "delayed-parasitic" forms, as do the Ibalia, the Aulacidae, and the Orussidae. According to Berland, 1951, the Stephanidae are very abundant in the tropics where they parasitize on larvae of xylophags (wood-eaters), and in the same way, apparently (in Oceania) also on solitary bees. This latter habit brings them close to the evanoid terebrants of the type Gasteruptiidae.
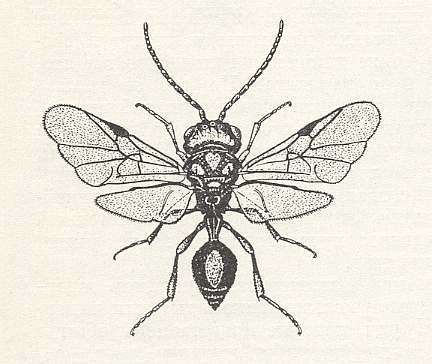
Similar cases of development, not at the expense of the host-egg, into which the egg of the terebrant is laid, but at the expense of a later stage of the development of the host, are observed also in other families of terebrants represented by comparably large forms and also solitarily parasitizing. Thus, the braconids Chelonus (see next Figure) and Ascogaster -- solitary parasites of Lepidoptera [butterflies and moths] -- lay their eggs in the eggs of butterflies, but the development of their larvae only begins when the caterpillars, emerged from the infected eggs, have become almost fully grown.

Of the ichneumonoid terebrants, Oocenteter tomostethi Cush., of the family Tryphonidae, lays its eggs into eggs of the saw-fly Tomostethus, and develops only in the fully-grown pseudo-caterpillar of the latter. Also the ichneumonid tribes Bassini and Exochini develop at the expense of more or less fully-grown larvae of the host, although the first (Bassini) lay their eggs into eggs of flies, while the second (Exochini) lay them into the eggs of Tortricidae (butterflies).

Thus, Apanteles militaris Walsh. can drive 70 eggs a second into its prey. And -- [presumably as another example] -- from a dead head of a caterpillar can exit more than a thousand (1200) adult individuals of A. acherontiae Cam. Finishing its development, the larva of the Apanteles tears up the skin of the host and exits into the open where it builds its cocoon from spinnings, and sometimes, in addition, together [i.e. several individuals] encapsulate themselves with spinnings, forming cotton wadding-like clods, reminding of egg-cocoons of spiders.
In this egg-laying the ovipositor is lowered from below or from the side of the abdominal segments of the prey, after which the female Helorus folds her legs together, and then the agitated prey may carry her for some time. The female Helorus only lays one egg in the larva of the lace-wing, but during the course of her life, lasting 4-6 weeks, she may lay about 50 eggs. The laid egg of the terebrant freely floats in the cavity fluid of the prey, but then, after minimally two days, from it emerges the peculiar "many-legged" larva, reminding us of the larva of Ibalia. The length of the period of the first instar of the larva of Helorus is very variable. Thus, when it happens to be in a larva of Chrysopa (lace-wing) that is hibernating, then also the Helorus-larva hibernates in it without undergoing further changes. In summer conditions, on the other hand, and where an almost fully-grown Chrysopa-larva is infected, the first stage of the Helorus-larva may last all in all 3-6 days. However, in this latter case, as well as in the first case, further development only takes place when the Chrysopa-larva finishes feeding and makes a cocoon. Soon after the first moult of the parasite, in the body of the Chrysopa-larva appear opaque white round particles, and with the increase of their number the fat-body and necessary tissues of the prey disappear. One must assume that the parasitic larva gives off some secretion that causes the disintegration of the host's tissues. About in the middle of the second instar of the Helorus-larva, lasting all in all 2.5-3 days, all movements of the prey stop, and it soon dies. The parasitic larva of the third instar eats the whole content of the prey in two days, and after that it exits from it into the open, leaving of the prey only its 4-5 posterior segments. In this way several generations of Helorus paradoxus develop in one year.
With all this we have concluded the exposition of the Delayed-parasitic (metaparasitic) Phase of hymenopterous evolution.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XV.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII