

Extensive intrusion of metatypic characters into the order- and family-groundplans in epizoic Diptera (Braulidae, Hippoboscidae, Nycteribiidae, and Streblidae).
Introduction
In Diptera there are a number of families of which the representatives are epizoic, which means that they live on the bodies of other animals which can be insects, birds, or mammals. So we have the Braulidae of which the imagines live on the body of honey-bees, the Hippoboscidae that live on birds or mammals, the Nycteribiidae and Streblidae, which live exclusively on bats. All these are families of the Order Diptera, and while the Braulidae can considered to be acalyptrate cyclorraphic flies, the other families belong, as far as can be determined, to the calyptrate cyclorraphic flies. Many of them are wingless. And, except the Braulidae, they do not lay eggs but fully-grown larvae which directly pupate.
As a result of their peculiar way of life these diptera have changed to such an extent that most of them do not look like flies at all. And this not only because many of them have lost their wings, but because their entire body structure (their organization) has changed dramatically in accordance with their epizoic way of life. It is because of these heavily transformed organizations, having become totally different from almost any other dipterous organization (and, moreover, totally different from each other as well) that new families are erected for them, or even new supra-orders. It is, however, clear that they represent peculiar off-shoots of existing or extinct families originally consisting of normal diptera, that is to say, certain species of these original families have found new and peculiar environments (for example bee communities), and in them new ecological niches to live in. As a result of adaptation to these highly strange and new habitats and way of life the species underwent intense transformations, so intense, that is, that they can be recognized as being in fact certain diptera only upon very close scrutinization. So in the present context we consider the 'families' Braulidae, Hippoboscidae, Nycteribiidae, and Streblidae not as separate families in their own right, but as deviant off-shoots of families originally consisting of normal diptera. And it is the groundplan of one or the other of those original families that we are speaking of in what comes next. So, said in terms of groundplans (prototypes) we can describe things as follows : In each of the mentioned epizoic group of diptera the family-groundplan, and even the order-groundplan (the groundplan "Diptera"), has evolutionarily been, to a very great extent, 'over-written' (over-formed) by metatypic characters, that is, in addition to the emergence of new metatypic characters, many prototypic characters of the family- as well as of the order-groundplan have been replaced by metatypic characters mainly of an adaptive nature. So there is little left of the prototypic characters of the order- and family-groundplans, meaning that these groundplans have become in a stronger degree implicit than they already were, as a result of the far-reaching demands of adaptation. In the Braulidae, for example, not only the wings are lost, but also the halteres, and, in addition to that, the 'fly-shape' of the body.
After having considered all this, we can say that the characters of one or the other of the mentioned epizoic groups of diptera that are adaptive to this or that type of epizoic life are metatypic characters of the groundplan of the original family (this groundplan including the dipterous groundplan) from which the epizoic group (the 'new' family) has descended. But these same characters must be interpreted as being prototypic with respect to the new family, that is, as having their place among the prototypic characters of the new family, into which the original family had been transformed. This means that we have to do with a new groundplan having evolved from another as a result of a transgression from one ecological niche into another, while in most cases, as we have seen, the appearance of a new groundplan has to do with the noëtic derivation of it, in the Implicate order, from another groundplan on the basis of their respective stability fields, and as such not a matter of the Explicate Order because generally groundplans do not have special ecological significance.
The morphology and ecology of the epizoic diptera are so peculiar that they constitute a great challenge to evolutionary theory : Any such theory that respects itself must be able to at least describe them evolutionarily in its own terms. And of course this also applies to our own theory involving Implicate and Explicate Orders. And, to begin with, it is clear that the morphologies, adaptations, and feeding habits of these epizoic diptera cannot be described (exclusively) in terms of random mutations and natural selection, because too many necessary and integrated adaptations are involved in each group of these diptera.
Let us now, one after the other, describe and comment on the four mentioned groups -- 'families' -- of dipterous epizoons.
Braulidae (Bee-lice)
Sometimes on the body of our honey-bees one can find pinhead-sized shiny-brown diptera which look a little like mites. It is the bee-louse, Braula caeca NITZSCH. The essential characteristics of these strongly transformed diptera are : winglessness, absence of halteres, strong legs, very small inconspicuous complex eyes, a relative narrow thorax, and a wide weakly curved abdomen. See next Figure.

Figure 1 : This micromount of the bee-louse (Braula caeca) clearly shows the absence of wings and the comb-shaped claws of the strong legs.
(After SCHUMANN, in Neue grosse Tier-enzyklopädie, 1971.)
That this animal is an acalyptrate fly (Diptera, suborder Cyclorrapha), and not some other insect as the name might suggest, is evident from the body-structure, the presence of the frontal sac, that is, the ptilinum (which, on inflation serves to thrust off the anterior end of the puparium at the time when the contained imago is ready to emerge and to force the fly through soil, etc.) and the ptilinal suture, the structure of the copulation-device, and the developmental stages (larva, pupa). However, its precise place in the taxonomic system of the Diptera is not known for certain. Some features point to the Borboridae or the predatory Chamaemyiidae (both acalyptrate flies). Certain is that they do not belong to the Pupipara, because the females lay eggs (not larvae or pupae) and the larvae -- which are normal maggot-like -- are free-living.
Pupipara
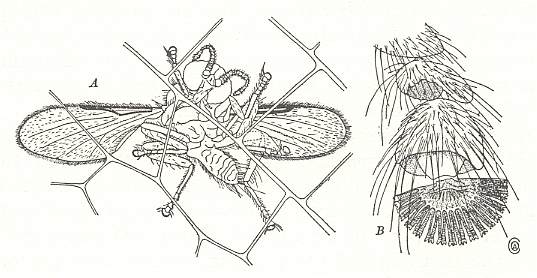
Figure 2 : A blood-sucking midge, Pterobosca adhesipes, which attaches itself to the wings of dragonflies.
A -- Adult in position on the wing of Agrionoptera.
B -- Tip of tarsus, showing the sucker-like process.
(After BRUES, 1946)
The next account of this group and the three families that are meant by it is taken from OLDROYD, The natural history of flies, 1964, pp. 229.
Hippoboscidae (louse-flies, keds)
There are about a hundred species of Hippoboscidae scattered throughout the world, and almost everything of interest about them concerns the adult flies. They are always flattened dorso-ventrally, with a tough, leathery appearance, and strong legs equipped with long, curved claws. They are called 'louse-flies' when they occur on birds, and 'keds' on mammals, the latter especially the wingless species.

Figure 3 : The epizoic swift louse-fly, Crataerina pallida, possesses strongly developed claws, and vestigial wings. The object below is a puparium of this species.
(After SCHUMANN, in Neue grosse Tier-enzyklopädie, 1971.)
The adaptation of these flies to a parasitic life has evidently been going on for some time, and is very complete, affecting every detail of their external appearance. The flattened shape and strong, spider-like legs enable them to move quickly about on the skin of the host, pulling themselves along by grasping the hairs or feathers, with a crab-like motion.
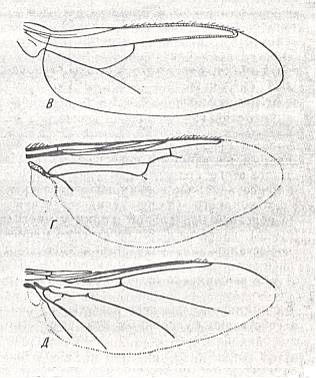
Figure 4 : Wings of the epizoic dipterous family Hippoboscidae (louse-flies).
Upper image : Lipoptena cervi L. Length 5 mm.
Central image : Hippobosca camelina Lch. Length 10 mm.
Bottom image : Ornitheza sp. Length 4 mm.
(After ROHDENDORF, 1951)
Broadly speaking, those that fly actively are found on a variety of hosts, and few, if any, are physiologically confined to one host in the sense of being unable to tolerate any other blood. As with fleas, and perhaps other parasites, it is really the host's habitat to which the parasite is adapted, and it will accept other hosts if they occur in similar surroundings. So the number of hosts available to any particular Hippoboscid is determined by the type of habitat.
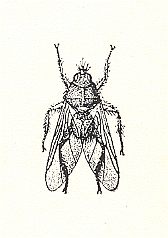
Figure 5 : Ornithomyia avicularia L. (Hippoboscidae). 5 mm.
(After OUDEMANS, from SENDEN, Muggen en Vliegen, Part 26 of Wat leeft en groeit)
[Here it is again evident that organic evolution is not a process exclusively focussed on biological improvement. The evolutionary process is mainly focussed on creating diversity. Life already exists for a very long time, so one of its characteristics must be diversity, because only then Life as such can survive extensive destructions of certain habitats as a result of major climatological and geological changes and catastrophes. The reason why some Hippoboscids have evolutionarily lost functional wings while others, living on the same type of host, have retained them, is just to retain diversity.
Crataerina pallida (Figure 3) is surprisingly common in the nests of the European swift, and may weaken or even kill nestlings by its voracious appetite for blood. Pupae overwinter in the deserted nest, and the new generation of adult flies emerges after the birds have returned in the following summer. Crataerina is thus obviously adapted to birds that return to the same nest, or at least to the same colony. If the nest is not reoccupied the hungry flies crawl -- being unable to fly -- in search of food.
All the Hippoboscidae of birds are likely to be taken on birds of prey, to which they have transferred when their original host was killed, so that hawks, owls, and falcons appear to be infested with a great variety of parasites.
The Hippoboscidae that live on mammals are the lesser section of the family, and apparently a later evolutionary experiment. Like the bird flies, they have their active, fully winged members, and their flightless ones. The genus Hippobosca itself consists of relatively large, handsome flies, with a striking pattern of brown and yellow, and with fully developed wings. They can be found on mules, horses, cattle, camels, and dogs. Other species of Hippobosca live on various antelopes and gazelles, and one species, H. struthionis, has transferred itself to the ostrich. This last example gives convincing support to the idea that it is the habitat that is significant to the fly, and not the specific nature of the host itself. Apparently to Hippobosca the ostrich is not a bird, a member of another zoological class of animals, but is simply a gazelle with feathers!
The active mammals of the steppes have active, fully winged Hippoboscids. The less active deer of woodland and open forest have their own group of flies, which have developed the curious habit of breaking off the wings, a habit also present in the bird-feeding acalyptrate Carnus hemapterus. Adults of both sexes are fully winged for mating and finding a suitable host, but then they break off the wings near the base, and commit themselves to the fortunes of their host. They are able flyers, and it is not easy to imagine why they should have evolved the mechanism that is needed in order to shed the wings in this way. It is usually said that they benefit by being able to move freely about their host, unimpeded by the wings, but it is not obvious why Lipoptena, and the African Echestypus, should need this advantage, when Hippobosca is able to keep its wings.
There is something to be said for the complete loss of wings, as in the sheep ked, Melophagus ovinus. Here the parasite has to live in a dense, tangled fleece, creeping about as if on the floor of a primeval forest. Melophagus has gone to the extreme of adaptation to this life, not only becoming wingless, but loosing the halteres too, and reducing the eyes further than any other Hippoboscid, see Figure 11 . These wingless, flattened flies look like ticks, and are somtimes confused with the true sheep tick, Ixodes ricinus. Their proper name is the sheep ked, and in a similar way Lipoptena cervi is known as the deer ked, especially after it has shed its wings.
Hippoboscidae infest the kangaroos and wallabies of Australia, and the lemurs of Madagascar, mammals that are at opposite ends of their evolutionary line. The stage of evolution reached by the respective Hippoboscidae cannot be matched with that of their hosts, and once again it looks as if the flies spread into a habitat rather than on to a host, and made do with the mammals that happened to be there, regardless of their zoological classification.
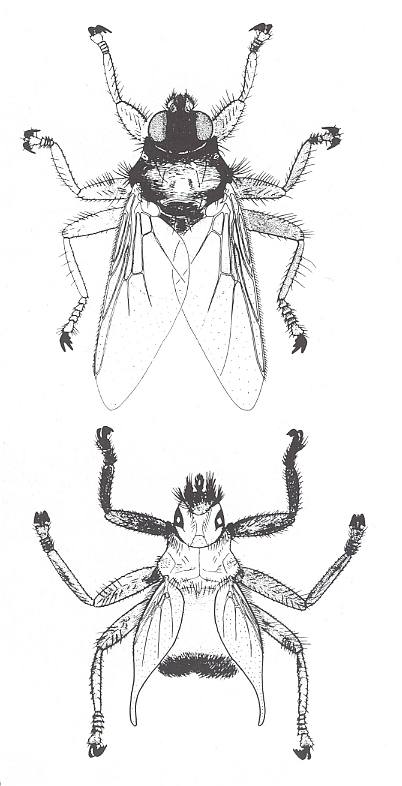
Figure 5a : Two common African louse-flies, parasitizing on birds.
Ornithoctona laticornis (upper image) possesses well-developed wings, while these are significantly reduced (in size) in Crataerina acutipennis (bottom image), a parasite of the swift. 6 mm.
(After SKAIFE, LEDGER, and BANNISTER, in Afrikanische insekten, 1979)
[The evolutionary origin of Hippoboscidae can be described in about the same way as that of the Braulidae: The family of ancestral flies, not yet epizoic, successively occupies its intrinsically predetermined potential ecological niches. Many such niches do not significantly differ from the niche occupied by the evolutionarily first member of the family. And occupation of, and subsequent adaptation to, these niches, result in the addition of further metatypic characters to the family-groundplan core. That is to say, most of such characters are merely added to -- but without going to belong to -- the existing prototypical characters that constitute the family-groundplan, while only a few of them actually replace some prototypic characters. This means that the acquired adaptations and other adventive structures do not destroy the original family-groundplan. However, one or two species of the ancestral family can discover yet another potential ecological niche, namely to be epizoic on warm-blooded animals (Also here the discovery of this niche is being introduced by more or less regular interactions with these animals). And via injection, noëtic reaction, and projection we see new species appear which are fully adapted to this new way of life. But this time the adaptation is so far-reaching that almost all original family-prototypic characters have been replaced by these adaptive structures, resulting in the appearance of a new family-groundplan as we see it in the Hippoboscidae. But in contrast to the Braulidae, in the Hippoboscidae the order-groundplan (the groundplan "Diptera") generally remains explicit.]
Streblidae
In contrast to the Nycteribiidae (considered later), most of the Streblidae can be recognized as flies, though the females of Ascodipteron make up for this by looking quite unlike insects at all (see Figure 8 below).
Instead of being flattened like the Hippoboscidae, most Streblidae of the Old World have a cylindrical body, the thorax being nearly globular. Many of the Streblidae of the New World are more flattened, and Nycterophila coxata could be mistaken for a flea. The tips of the tarsi (feet) are flattened, with powerful claws, and the whole body is covered with a neat array of bristles.
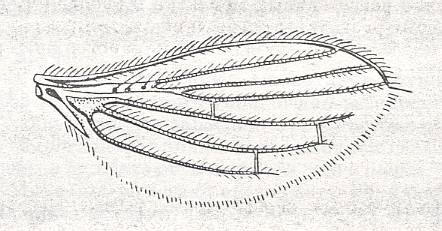
Most Old World Streblids have wings, and fly well on occasion, and their wing-venation is not widely different from that of other flies. They solve the problem of what to do with their wings by folding them in pleats along the back, in a way that is very convenient for them as they move about the fur of their host bats.
Streblidae are found only on bats, and then only in warm countries. JOBLING showed that their distribution falls neatly within the winter isotherm of +100C in the Northern and Southern Hemispheres, this being the critical temperature at which the bats begin to hibernate. During hibernation the body-temperature of the bats falls to within a fraction of a degree of that of their surroundings, and this is too much for the Streblidae : In the few instances where they have occurred outside the mentioned limits there have been special circumstances, an exceptional year, or the protection of a cave against low temperatures.
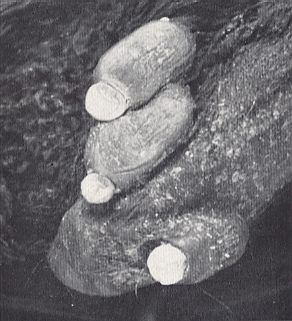
The female fly not only remains on its host, but burrows into it with enlarged mouthparts until the parasite almost disappears from sight. Wings and legs are shed, and the sclerotized body is reduced to an almost unrecognizable remnant. On the other hand, the first abdominal segment swells and becomes a membranous bag, which envelops the rest of the fly, and converts it into a flask-shaped object from which the scientific name is derived. The male flies remain normal in appearance, and fly actively. Ascodipteron drops its larva to the ground where it pupates, in the same way as the larvae of Hippoboscidae and of tsetse-flies. All other female Streblidae attach their mature larvae to a wall or other surface, and this becomes encrusted with puparia from which the adult flies emerge in due course. Since they are already in the roosting place of the bats they have no difficulty in finding a fresh host.
[As regards the origin of the Streblidae in terms of one or more species of the ancestral not yet epizoic family discovering another potential ecological niche, of injection, of noëtic reaction, and of projection, we can say the same as we did with respect to the Hippoboscidae. Here the evolutionary sequence leading to Streblidae might be along the lines just suggested by Oldroyd : non-blood-sucking flies ==> flies sucking blood on bats and laying eggs in the dung of bats ==> flies sucking blood on cave-dwelling bats and laying fully-grown larvae in the cave.]
Nycteribiidae
A similar origin perhaps may be postulated for the Nycteribiidae, also restricted to bats. The fact that these two families share the same hosts only serves to emphasize the striking differences between Streblidae and Nycteribiidae.
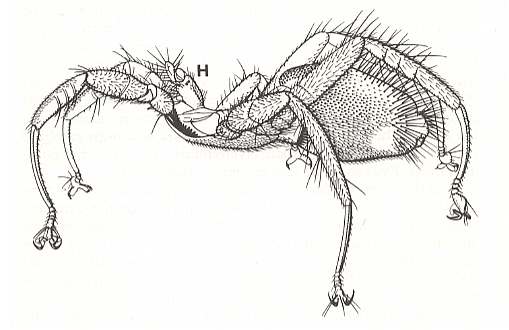
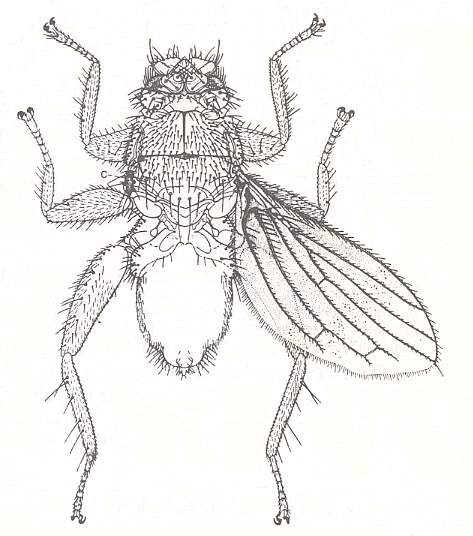
Nycteribiidae never have wings, and have evidently been without them for a very long time, because the thorax has lost its usual box-like structure as the flight-muscles dwindled. Streblidae still look like flies (except for Ascodipteron), but Nycteribiidae look like six-legged spiders. The upper surface of the thorax is little more than a framework of hard chitin, joined together with large areas of soft membrane, and the head is a grotesque structure apparently sitting on top of the thorax (See Figure above). Indeed, any one seeing a Nycteribiid for the first time is likely to mistake the under surface for the upper, and fail to find the head at all ! The eyes are greatly reduced, and may be absent altogether. When they are present they are quite unlike those of other adult flies, being either a single, round facet, or two little lenses on a black mount.
In the Nycteribiidae so many family-prototypic characters have been overwritten (replaced) by new adaptive structures that the original family-groundplan has become highly implicit or even totally deleted, resulting in the appearance of a new family-groundplan, that of the Nycteribiidae. Even many order-prototypic characters have been overwritten, resulting in the destruction of the basic 'fly-look'. See also next Figure.
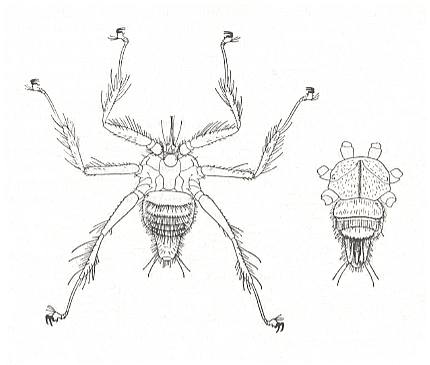
Figure 9a : Penicillidia jenynsi (Nycteribiidae), from Formosa (Taiwan). Dorsal view of male (left), and ventral view of thorax and abdomen (right).
(After RICHARDS & DAVIES, in Imms' General Textbook of Entomology, 1977)
We said "... have been overwritten (replaced) by new a d a p t i v e structures". But when we compare things with the other representatives of the Pupipara (Hippoboscidae and Streblidae) we run into the problem of deciding whether a given structure is adaptive or not. Let's go into this matter :
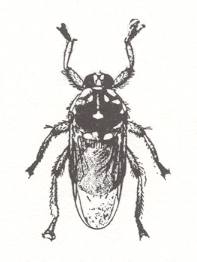
And even where they do not possess wings and are not at all fly-like, as is the sheep ked Melophagus ovinus, the result is not a spider-like appearance as in Nycteribiids, but a compact short-legged creature :
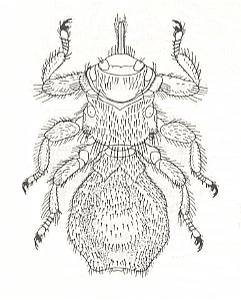
Nevertheless we cannot assert that all these forms are in a lesser degree adapted than are the Nycteribiids. And the differences of body-habit between the winged Hippoboscids on the one hand and the Nycteribiids on the other cannot be attributed to differences in the groundplans of the respective ancestral families (giving rise to respectively Hippoboscids and Nycteribiids) because already within the Hippoboscidae we see both the fly-like and the non-fly-like body-habits. Also in Streblids we encounter both body-habits. And the three epizoic dipterous families (Hippoboscidae, Streblidae, Nycteribiidae) apparently come from comparable not too different cyclorraphic ancestral families, whose (small) differences cannot be responsible for the great morphological differences which separate these three epizoic families. We wonder therefore whether all the morphological structures in Nycteribiidae are really adaptive structures.
In the present document we have discussed the way of life and the overall morphology of true epizoic diptera. We did this because they are significant for a theory of organic groundplans.
In the next document we will continue the development of the theory of groundplans and discuss in what way two totally different (larval) groundplans of predatory insects are nevertheless perfectly suited to fit into the same (larval) ecological niche. The mentioned groundplans are of predatory larvae of insects which are not taxonomically related : They even belong to different insect orders, viz., Planipennia (Neuroptera) and Diptera.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXVII.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C