

Scheme of evolution resulting from the ongoing noëtic transformation of groundplans, and the scheme of evolution as expressed by the genealogical tree obtained from phylogenetic systematics.
Comparing our interpretation of organic evolution as expressed in the Physical-Noëtic Scheme of the origin of, and relations between, organic groups WITH the interpretation of evolution by Phylogenetic Systematics (sensu HENNIG) as expressed by its hierarchic system of monophyletic groups (SEQUEL).
Phylogenetic Systematics and the evolutionary development of Organic Groundplans
After having expounded the theory of Phylogenetic Systematics sensu HENNIG, and also having expounded the relationship of phylogenetic systematics, as we have it (metaphysically) re-interpreted, with the Implicate Order and with the evolution of organic groundplans, we will now follow HENNIG, Phylogenetic Systematics, 1966, p.219-228, where he himself discusses the ability of phylogenetic systematics in his sense and the ability of the resulting phylogenetic system to shed some light on the problem of the essence of 'organic types' or groundplans and their phylogenetic formation. Of course, HENNIG's discussion acknowledges only one single order of Reality, the material world. Nevertheless it is very instructive and stimulating as to a better understanding of types and their relationship with the phylogenetic system. We will follow his text, sometimes loosely, and present comments and explanations :
Having discussed the ideas of a number of authors concerning certain laws or rules in long-term evolution and the origin of the higher taxa, HENNIG continues :
But we can also proceed from our idea of phylogenesis, which says that all evolution of taxonomic groups can be understood only as progressive differentiation. However long phylogenesis may continue into the future, the descendants of existing species of Diptera will always be holomorphologically only extensions of the structural plan [groundplan] of the Diptera, the descendants of the mammals only extensions of the structural plan of the Mammalia. In this sense the evolutionary possibilities (further differentiation, "variation") are undoubtedly more limited than they were for the common ancestors of the Diptera and Mammalia [Here it is assumed that the Insects and the Vertebrates did not emerge totally independently of each other, but at last go back to a single common ancestor how far remote it might be], whose descendants had both the dipteran and mammalian, as well as many other [because the Diptera and Mammalia are not simply sister-groups], paths open to them.
The main stem of life ascends in another sense, which we best understand by comparison with the morphology of the individual organism. In the latter, development is by progressive internal complication, the parts always differentiating in different directions through division of labor, and thereby becoming the more intimately tied together into a higher unit. Similarly in phylogeny there is first a breakdown of diversity -- progressively more sharply separated special types develop which, taken together, build up (precisely by their division of labor) an ever more narrowly closed total fauna of a living space, a time period. These are considerations that no longer work with the individual developmental series and the arrangment of forms within them. Rather the object is the stems themselves, and from their development to discover laws of living morphosis. This would be morphology of stems.
Von HUENE (1940) expressed himself similarly :
Thus it seems to me that the total evolution of life through the ages is pervaded by a superordinate principle that orders the totality in a uniform and direction-giving sense (cf. Spemann's organizers, on a small scale), and which acts cooperatively in all possible orders of magnitude. In this way there arises a four-dimensional total form (time being the fourth dimension) of the totality of organisms, much as the individual gradually develops all organs by growth from a single cell. The organs do not develop in a directionless way ; they develop within the framework of the whole, and some disappear before the body as a whole reaches maturity. As the particular is dominated by the uniformity of the whole in the development of the individual (which in turn is only a subordinated part of another whole), so does the total evolution of organisms also show a higher organization, an organic course of life.
Attempts to grasp the total form of higher taxa -- not to be confused with the "type" of idealistic morphology -- have scarcely gone beyond the programmistic character expressed in the statements quoted above. One of the first positive observations is that of ZIEGENSPECK : "Characters that in phylogenetically . . . lower groups [ I take this as not meaning lower taxa, but primitive groups] still occur within close circles of kinship, in the derived forms become so specialized that they are inviolable family characters" (quoted from MEZ, 1926).
In trying to determine our own position on this important question, we must note first of all that we do not believe [that] showing how species arose, or at least could have arisen, is a sufficient explanation of the evolutionary process [that is, the appearance of a new species (as one of the two new daughter species originated by the cleavage of some given species) is not the beginning of the development of some a l r e a d y d e t e r m i n e d type. A particular type, i.e. this type and not that one, if there is any such type at all, only develops conditional upon what happens to come next]. It no more explains the origin of higher taxa than does the origin of mutations in certain individuals explain the process of speciation. The aim of this whole book is to develop this fact as clearly as possible. But this is not the decisive factor in the position of those upholding the type doctrine [HENNIG means here that the adherents of the "type doctrine" will not acknowledge the mentioned fact]. They contend that in speciation -- or even in the initial process of altering the form of an individual -- there are already decisive differences depending on whether the species is destined to be the stem species of a new "type" or merely one of the differentiation products of an old stem.
" The breadth of evolution of successive groups shows a distinct narrowing, since the basic divergences of organization become progressively smaller. The type of the mammals is more uniform and closed than that of the reptiles, which in turn is unquestionably uniform compared with the type of the Amphibia-Stegocephalia.... The same phenomenon is repeated in every systematic unit of higher or lower order " (BEURLEN, 1937).
This, no doubt, is how the law should be understood, and in this form there can be little objection. But we may ask whether any knowledge has been gained that merits calling it a law. Also the impossibility of framing exactly the concept of similarity and difference in form is evident here. When the last [seen from 'below' in time] common stem species of the Mammalia and Diptera split up into successor species, the latter would scarcely have differed from one another more than do living species that are related to one another in the first degree. But who can say how much the descendants of the living mammals a hundred million years from now will differ from one another within their still common structural plan, and what yardstick would we use to measure their differences in comparison with the differences among [now] living species?
One thing emerges from all this : The Fechner-Rosa law [law of progressive reduction of variability] has inner relations to two other laws of evolution, Dollo's law of irreversibility and Cope's law of the unspecialized. Our assumption that the descendants of the living species of Diptera could evolve further only within the framework of the dipteran structural plan (and that of its families, etc.) is based on the assumption that the law of irreversibility is valid [In the course of the evolution of a given group more and more possibilities are successively realized, and the assumed narrowing of the field of remaining possibilites can only be understood when possibilities, once realized and later perhaps disappeared again, cannot for a second time be realized anymore]. The area of applicability of this law was discussed in the first part of this book. It undoubtedly has its limitations. With the restrictions that now must be made, it is merely a probability statement concerning the course of evolution of characters : the more complicated an organ the less probable that it can arise again in the same way after it is once lost. This is how MÜLLER (1939) reduced Dollo's law to a probability statement from the genetic viewpoint :
" Thus a very complicated path leads from the original stage to the existing type, so it is scarcely possible that the original condition can be restored by a back mutation of the main gene. For smaller and very young phylogenetic changes, however, reverse developmental stages may occur and even be observable" (quoted from WETTSTEIN, 1941).
Like Dollo's law, Cope's "law of the unspecialized" (or "law of primitive stem forms" as PLATE [1928] prefers to call it) is related to the Fechner-Rosa law of progressive reduction of variability. If the possibility of producing new structural plans decreases progressively -- with progressive removal [= progressively increasing distance in time] from the origin of the pattern of the stem form -- then it is understandable that new types that persisted in the further course of evolution often arose from stem forms morphologically similar to the original form. Cope's law leads to the problem of the relation between species cleavage and change in form, which is discussed below.
ABEL (1929) gathered Rosa's, Dollo's, and Cope's laws, together with the "law of orthogenesis", under the general term "law of biological momentum". It cannot be denied that there are internal relations among them, but it is doubtful whether there is the slightest gain in ABEL's proposal, especially since there is nothing to justify the analogy to physics implied in the name.
All the laws so far mentioned belong to the circle of problems that we have called "character phylogenetics", i.e. they are statements regarding conformities to law in the evolution of individual configurations either as wholes or in their parts. We may ask whether there are not also laws that determine the course of evolution as a whole, whether for example phylogenesis moves toward a particular goal or even several goals. Whether we can speak of a law if this question can be answered in the affirmative must remain uncertain, since the action of a law is not restricted to a particular case whereas phylogeny as a whole is a nonrepetitive process.
The most general evolutionary trend, the tendency toward gradual perfection, or "higher development", has been called a law. As always in such cases, these two concepts [tendency, law] are in no sense unequivocal, and have not always been used in the same sense. It seems to me that the only possibility of speaking of a "higher development" in phylogenetics is to give this concept, with von BERTALANFFY, the sense of an "elevation of the stage of organization", an increase in internal differentiation, the "achievement of a higher level of integration" (FRIEDRICHS, 1937). The concept of "perfecting" [see first sentence of this paragraph] would have to be separated from this. It can be related only to the possibility of the organism dealing successfully with its environment, as formulated by PLATE (1928) and not always consistently by FRANZ (1935). [Here we clearly see distinguished (1) the holistic organization of the organism as this organization is some non-adaptive formal new groundplan or type and as it results from internal differentiation and subsequent (formal) integration, from (2) the new adaptive functional complex of features.]
We then face the problem of investigating the relation between higher development [which is a purely formal process and, translated in our theory, wholly a matter of the noëtic Order] and perfecting [which is a biological process]. [. . .]. Only if it can be shown that higher development is not always connected with perfecting -- much more difficult to prove than many theoreticians of phylogenetics seem to think -- could we see a general law of phylogenetic evolution in the tendency toward elevation of the stage of organization [We ourselves would not so much emphasize the "elevation of the stage" of (bodily) organization, but rather first of all its diversification].
So far we have dealt exclusively with the form of organisms [ = that particular expression of organisms ...], which in general language usage we call individuals [carrying a hierarchy of formal groundplans and a complex of adaptive structures]. We must now recall that species and higher taxa are in a certain sense also individuals in the phylogenetic system. If higher development by elevation of the stage of organization, increase in internal differentiation, can at least be considered as the goal of phylogeny, we must ask whether this development relates only to individuals in the usual sense, or whether the individuals of higher order do not have a form (species, genus, family, etc. form) capable of higher development. WOLTERECK (1940), for example, regarded the origin of species and higher taxa as an expression of a general tendency toward diversification (anamorphosis) in the course of phylogenesis, but this is not a positive answer to our question. Mere diversification in species and higher taxa is not the same as the origin of new specific, generic, and family structural patterns -- which must not be confused with the "type" (the average so to speak [that is, the empirical, contingent, holomorphological average, not the formal groundplan] ) of these groups. So far as I know, FECHNER (1873) was the first to consider the possibility of regarding the higher taxa as the possessors of individual group forms [I take this to mean : possessing a holomorphological pattern all by themselves].
Paleontologists in particular have urged that the superindividual "form" of higher phylogenetic units be worked out. MARINELLI (1939), for example, proposed making "the total form of an animal stem the object of morphological consideration". He wrote :
If this proves to be generally correct we would have in the evolution of higher groups the same principle seen in the development of the individual : pecularities originally belonging to a single cell are distributed among the descendant cells which thus become distinguishable as a group from other cell neighborhoods that have received a different set of characters. The essential thing in this development is not at the morphological level ; it is that the functions dependent on these pecularities supplement each other in such a way that the organism becomes a truly functional unit within its environment. Only if the same could be proved for the higher taxa, with a differentiation and mutual augmentation of functions connected with the "related differentiation of structures" to make the total group an action unit, would a comparison between the higher taxa and individual organisms be more than a loose analogy
[In our theory of the involvement of the Implicate Order in the overall evolution of organisms it is the species together with their adaptations that belong first of all in the sphere of the Explicate Order. It is the changed ecological situation of some population of a given species that triggers the projection from the Implicate Order into the Explicate Order of the reaction product of the noëtic reaction between the noëtic content of the genus-groundplan (to which the original species belongs) and the noëtic content of precisely that ecological niche, from the set of the genus' potential ecological niches, that corresponds to the new ecological situation. The higher taxa, on the other hand, above the species level, are, according to our theory, associated with groundplans, noëtically present in the Implicate Order. And they, and thus the higher taxa themselves, that is, as higher taxa (embodying those groundplans), only in a very general sense have ecological and functional significance. Their essential nature is purely formal. So in our theory the analogy between the evolution of higher taxa and the development of the individual organism does not hold].
It must be admitted that a mutually related differentiation, not only of structure but also of functions in the environment, is probable in all cases where the species-number curve follows the logistic equation of population growth [that is, where the curve indicating the number of species (of some given group) in the course of time has the same shape as the curve indicating the number of individuals of a given population in the course of time]. With simultaneous origin of differences in form among species, a species-number curve of this form would scarcely be understandable if the species differed from one another independently [So if it can be shown that the species-number curve does follow the individual-number curve (described by the logistic equation of population growth), then the species depend on one another as regards the (holomorphological) transformations they undergo, which means that the group, formed by these species, acts like a single unit in this respect]. All these questions lead far beyond the circle of problems we are discussing here. They are mentioned only because they deal with fundamental questions of evolutionary research that cannot be solved without the cooperation of the special phylogenetic systematics we are primarily concerned with here.
The laws of the development of form that were discussed briefly above are not by themselves important to special phylogenetic systematics. They are important because, following the principle of reciprocal illumination, they can help disclose the phylogenetic kinship of related groups, which is the primary task of special systematics. As already emphasized, this is possible with the aid of analysis of form only if orderly relations exist between directly recognizable alterations of form and those on which differentiation of the species -- and thus of the "groups" in general -- is based [that is, when there is some definite relationship between alteration of holomorphous structures and species cleavage, or a sequence of such cleavages]. The nature of these relationships will have to be investigated a little further. The basic fact is that there is no definite correlation between magnitude of morphological difference and degree of phylogenetic kinship, although rules with a certain range of applicability can be found. Equally important is the fact that there is no such thing as specific, generic, family, or ordinal characters. This opinion in not shared by many modern phylogenists, who believe there is an essential difference between higher and lower taxonomic groups and the attributes that characterize them. Some, for example, distinguish between constitutive and additive characters (WOLTERECK, 1940), constitutive and "nonconstitutive or functional" characters (DIELS, 1921), adaptive and organization characters (SCHINDEWOLF, 1943, STUBBE, 1942, and WETTSTEIN, 1942), or even between " type-caused fabric characters" and "adaptively caused special characters" (SCHINDEWOLF, 1943) [In especially the latter we recognize our own division between ecological-adaptive structures and the formal groundplan structures].
The type doctrine of idealistic morphology lives on in this distinction between two supposedly fundamentally different processes of evolution : aromorphosis and adaptiogenesis (SEWERTZOFF), eogenesis and evolution in the narrow sense (CLARK), micro- and macroevolution, typogenesis and adaptiogenesis (in the sense of JAECKEL, PHILIPSCHENKO, VIALLETON, OSBORN, SCHINDEWOLF, BEURLEN, SCHUH, and others, according to HEBERER, 1943), cladogenesis and anagenesis (RENSCH, see SIMPSON, 1949).
Naturally this idea is extremely important to our concept of the current state and the future of the evolutionary process. "It is not true that phylogenesis can continue indefinitely. Rather it seems to have reached its material end, since today there are no longer any unspecialized and phyletically fully active groups, as paleontological history shows (Von HUENE, 1940) [The question is whether there have ever existed unspecialized organisms. Of course certain individual characters can be unspecialized, especially those that are connected with only a very general function (in contrast to a very specific function) or with no function at all. Completely unspecialized organisms are probably not viable. Further, in Dipteran evolution there are some tendencies to develop more universal types such as there are many among calytrate flies.]. "Cladic evolution . . . ended long ago." No "new structural type" [groundplan] has appeared for more than 600 million years (CUÉNOT, 1940). WOLTERECK considers it "absolutely inconceivable how new structural plans could branch off from the presently dominant type circle of the vascular plants, the mammals, or the insects". Like CUÉNOT, he believes that "the production of new somatic [=bodily] types apparently has ended in both the plant and animal kingdoms." "There is no evidence whatever that a future origin of correspondingly different types is possible, and much speaks against it."
On the one hand this view leads to pessimistic ideas regarding the future of phylogenesis : "The axis is dead ; evolution continues, of course, but within the clades, of which only the summit is alive" (CUÉNOT, 1940). On the other hand, it necessarily leads to skepticism regarding all attempts to explain -- by genetics, for example -- evolution or the essential processes of phylogenesis from phenomena observable today. According to SCHINDEWOLF (1942), "the first representative of a new stem, at the ordinal level for example, does purely formally represent a species . . . . But this 'species' monotypically embodies at the same time the genus, family, and order, and is potentially completely different from the species that later emerge from this stem, which are the terminal members of the differentiation." Everything genetics have learned about the mechanism of speciation is said [by authors discussed here] to relate to the latter kind of species [the terminal members of the differentiation], whereas the origin of the stem species of higher taxa through the evolutionary mechanisms known to geneticists is unintelligible. Among the zoologists, particularly geneticists, who at least do not deny this idea, SCHINDEWOLF (1942) names REMANE, Von BUDDENBROCK, LUDWIG, and H.ULRICH ; GOLDSCHMIDT can be added.
[In this view a stem species, in contrast to a terminal species, already contains the 'seeds' that develop into typical features of the stem, that is, such a species is intrinsically destined to generate, during subsequent species-cleavages, one particular stem of which all members contain this stem's groundplan. This groundplan is thus supposed to be intrinsically present in the qualitative constitution of the stem species. According to our ideas this need not necessarily be so, because a noëtic trajectory can, directly after just having entered the noëtic stability field of some groundplan, leave it again.]
Representatives of an even more extreme type doctrine go so far as to deny phylogenesis altogether, or at least interpret it as of very subordinate importance within the narrowest framework. " The origin of the plant and animal kingdoms is polyphyletic. It begins with genera and is bound to genera. There is no phylogenesis in the mechanistic-monistic sense." "As monism must lead, by way of the monophyletic theory, to spontaneous generation of the primordial cell of the entire plant and animal kingdom, so must holism necessarily lead, by way of the polyphyletic idea, to genogenesis or to primordial germs of the genera" (KEMPERMANN, 1936). KLEINSCHMIDT (1926) earlier presented similar and, if possible, even more extreme views.
If it were possible to determine by objective criteria whether the daughter species arose by cladogenesis or by anagenesis [origin of new type or of merly a more or less pronounced form within the framework of the existing type], or whether at least one of them is destined to be the stem mother of a "new type", then our diagram of hologenetic relationships (Fig. 6, p.31) [depicting the general aspect of species-cleavage in detail] would in fact be incomplete or even misleading.
The diagram is reproduced here :
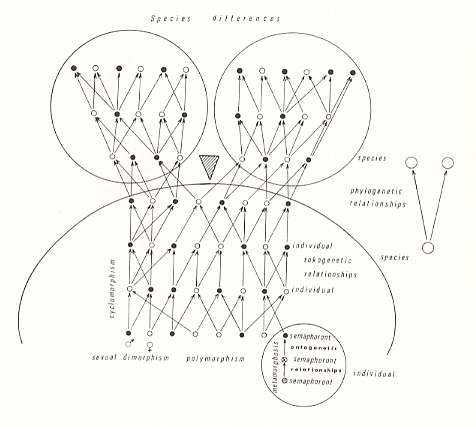
Figure 1 (= Fig. 6 in HENNIG, 1966) : The total structure of hologenetic relationships as they can be distinguished in a given species and in the process of cleavage of this species, and the differences in form associated with the individual parts of this total structure of hologenetic relationships.
These hologenetic relationships include :
(continuing with HENNIG's text :)
But even if we admit that there are groups in which the structural plan differs from the structural plans of related groups [such as that of the Birds differing from that of the Crocodylia and from the remaining reptiles], this does not mean that a special law must have governed the origin of such groups [ In the example just mentioned, we, following our own theory, would say : the noëtic trajectory, upon leaving the stability field of the groundplan of the reptiles (including that of the Crocodylia), just enters the stability field of the Birds (Aves). But it equally could not have done so, or leave it again after having just entered it. But we should realize that the noëtic trajectory cannot just go anywhere in the 'noëtic space' of (organic) groundplans. The succession of (stability fields of) groundplans and the 'space' between them visited by the noëtic trajectory must qualitatively have the nature of a true noëtic succession, that is, a noëtic (i.e. formal) derivation or a possible noëtic reaction ]. If we equate the somewhat ambiguous expression "macroevolution" with "origin of higher categories" [taxa], then this would agree with NACHTSHEIM's statement that "the laws governing macroevolution are the same as those governing microevolution". Also according to HEBERER (1958) the distinction between macro- and microevolution corresponds to "no reality". "Genetics is not in a position to support in any way typostrophism, the type-discontinuity doctrine, the systemic mutations of GOLDSCHMIDT, or the saltations of many paleontologists." Even those who insist that these views are not generally accepted must admit that the phylogenetic system is of decisive importance in clearing up the question. Its job is to refrain from using typological criteria of any kind in determining the rank of higher taxa, limiting itself to a clear expression of sister-group relations. Only after this has been done can we investigate whether or not special conditions are associated with the origin of groups that -- in comparison with their sister groups -- have the character of a "new type", a "new plan of organization", a "new anagenetic stage", or the like.
Obviously if erroneous conclusions are to be avoided in discussing the mode of origin of "higher categories" it must be sharply distinguished whether the higher categories of the phylogenetic or some typological system are meant. In the latter case we must also know the criteria used in evaluating the rank order of the categories in that particular typological system. The principles and generalizations of SIMPSON (1959) show how important this is :
" Higher categories [taxa] generally arise by acquisition of a basic general adaptive complex, which may be retained essentially unchanged in all subsequent members . . . or may be profoundly modified or lost in some . . . . The origin of a higher category may involve at one extreme (e.g. rodents, birds) a single character or adaptive complex, or at the other extreme (e.g. primates, mammals) general adaptive change or improvement in numerous ways or in virtually the whole organization."
In my opinion these generalizations say nothing about the mode of origin of the "higher categories" in the sense of phylogenetic systematics. They merely set forth how higher categories with a particularly outstanding type-connected character tend to differ from their sister groups. What about the "basic general adaptive complex" of the Archosauromorpha (Crocodiles + Birds, see Figure 54 of the previous document ), which are a higher category in the phylogenetic system (but only in this system), and in fact a higher category than the Aves [Birds], or [what about] the Theromorpha, which are a higher category than the Mammalia [mammals]? Do SIMPSON's generalizations apply to these?
[Regarding the Archosauromorpha and the Theromorpha :
One gets the impression that a few particularly high categories are characterized only by a few unimportant features of their basic plan -- as for example the Deuterostomia by a few pecularities in the ontogenetic mode of origin of the intestinal opening and mesoderm [The taxon Deuterostomia comprises all Echinodermata (star-fishes and the like) and Chordata (all vertebrates and tunicates)]. It would seem that such pecularities did not arise as an "acquisition of a basic general adaptive complex", and that they are constitutive characters of the basic plan of a higher category only because they were retained in all descendants of the stem species that evolved them (perhaps because they had little adaptive value and therefore were forgotten by evolution). On the other hand we could postulate that all higher categories must have been characterized by special adaptive qualities at their time of origin, because otherwise the characters would not have been retained for such a long time. This [mode of origin], however, can scarcely have been the sense of SIMPSON's statements.
According to phylogenetic systematics the crocodiles are phylogenetically more closely related to (some group of the) the Birds than to any other reptile. The crocodiles and birds are sister groups, and this means that the crocodiles together with the birds form a monophyletic group, the taxon Archosauromorpha. Obviously, the rank of this taxon is high, higher than that of the Birds (Aves). But does, so asks HENNIG, this "higher category" embody a "basic general adaptive complex" in the sense of SIMPSON? Does it represent a type or groundplan? This is hard to see, because the taxon Archosauromorpha is typologically heterogeneous, it comprises two types, the reptilian and the avian type or groundplan. So from this it is clear that a higher taxonomic category not necessarily means a group that embodies some particular adaptive type.
The same with the Theromorpha :
Many fossils from the Mesozoic are customarily placed among the reptiles because they do not have certain "essential" mammalian characters. Synapomorphous correspondences with the mammals show, however, that these particular fossils are more closely related to the mammals than to any group of the so-called reptiles. Consequently Von HUENE logically unites them with the mammals in a group Theromorpha. This corresponds with the basic principles of phylogenetic systematics. So also this group, the Theromorpha, is typologically heterogeneous. It contains two types, that of the reptiles and of the mammals. And also here we have to do with a higher category that does not embody a particular type or groundplan.]
In any case it seems certain, to me at least, that the only way to achieve confirmed concepts on the mode of origin of the higher categories is to compare as many cases as possible, particularly those that arose at different times and under different circumstances. Only the phylogenetic system can do this -- without preselecting them, it renders all recognizable segments of the phylogenetic tree (i.e. all higher categories) uniformly specified and comparable. The typological or syncretistic system [a syncretistic system is one that contains (and mixes) phylogenetic as well as typological elements] cannot do this -- from certain points of view it makes a choice among the higher categories and places those that are provided with type-related characters in the foreground. Investigation of the systematic "fine structure" may help to understand the development of those higher categories that at first sight seem to be sharply circumscribed, and which consequently may be suspected of owing their existence to a particular mode of origin. It is evident, for example, that an increasing number of insect groups have a form of differentiation like that shown for the Lepidoptera [moths and butterflies] in Fig.66 [see next Figures here]. The kinship (sister-group) relations shown in this diagram are probably accurate in all essential respects, even if it turns out that one group or another -- e.g. the Aplostomatoptera -- should be given a somewhat different position.
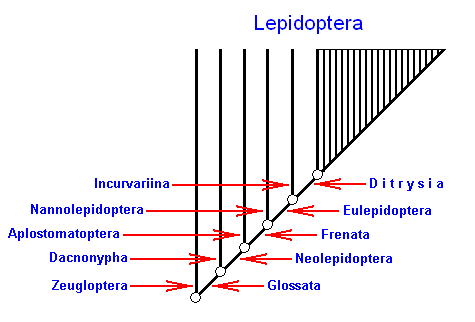
Figure 2 : Principal differentiation of the butterflies in the phylogenetic system. Because of the distribution of the species numbers in the subsidiary groups there results the impression of a directed phylogenetic development.
In each case the two members of a sister-group are indicated by their names at the same horizontal level (two opposite arrows).
Within the Ditrysia the number of subsister-groups as allegedly expressed by the vertical lines is (without doubt meant to be) arbitrary, and just symmbolizes them to be "many".
(After HENNIG, 1966)
The next Figure elucidates the expression in the previous Figure of sister-group relationships, by more explicitly depicting one of these relationships : the sister-groups Nannolepidoptera and Eulepidoptera.
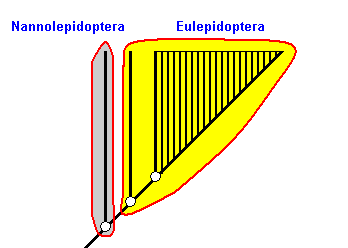
Figure 2a : Part of previous Figure, showing the sister-group relationship between the Nannolepidoptera and the Eulepidoptera. The common stem-species, indicated at the bottom of the Figure, splits into two daughter-species, one of which (not drawn) gives rise to the Nannolepidoptera while the other gives rise to the Eulepidoptera. See also Figure 2aa.
Although one would a priori expect that every monophyletic group (monophyletic in the sense of phylogenetic systematics) represents a definite groundplan, according to me only some of them do. In the present example of the Lepidoptera we can see that the typical lepidopteran groundplan is only reached in the Ditrysia. It is reached only after having passed through a series of other groundplans (all typologically having the same rank as that of the Ditrysia). Therefore, although allowing the Nannolepidoptera (see present Figure) to represent such a groundplan as well as the Incurvariina (represented by the next vertical line in the present Figure) and of course also the Ditrysia, we maintain that the (monophyletic) Eulepidoptera (yellow) (consisting of the Incurvariina + Ditrysia) do not represent a definite groundplan. They, that is the Eulepidoptera, consist of two groundplans of which one is the pre-stage of the other. In the same way the monophyletic group consisting of the Nannolepidoptera + Incurvariina + Ditrysia cannot be considered to represent a definite groundplan. And so we can go on, until -- see Figure 2 -- ending up by maintaining that the (whole of the) Lepidoptera, although being a monophyletic group, does not represent a (single) definite groundplan. During evolution the typical groundplan of the Lepidoptera, as it is expressed by all Ditrysia (representing the main mass of Lepidoptera), has been gradually developed via a series of pre-stages, where each such a pre-stage is a genuine groundplan. Therefore the Lepidoptera as a whole does not represent a single groundplan but several of them.
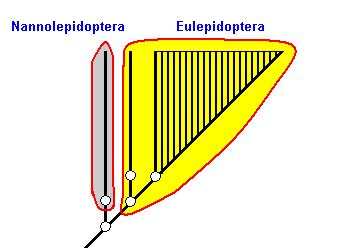
Figure 2aa : As already said, in Figure 2 we must in fact add a stem species for each group, as we have done here for the Nannolepidoptera and for the Incurvariina. Then we indeed have, for each sister-group relationship a common ancestral species that splits up into two daughter-species, of which the first one gives rise to one of the sister groups while the second gives rise to the other sister group. So this is the way we must read Figure 2.
The diagram merely shows that sister groups [in the present case] differ greatly in species number. But it is also true for the differentiation of structure, that the complex of characters present in the main mass of the Lepidoptera (Ditrysia) -- and which so strongly determine the type-related character of the group as a whole -- is reached only by stages. Each of the species-poor subgroups entered on the left in the diagram (Figure 2 ) lacks some of the characters of the total complex [. . .]. This does not completely bridge the morphological gap separating the main mass of the Lepidoptera from the sister group of this "order" (the Trichoptera [Caddis-flies] ), to be sure, but the idea that this gap did not arise by a single evolutionary jump is substantially supported by the presence of the relatively plesiomorphous [= primitive] subgroups of Lepidoptera and the graduated kinship relations between them. The limited number of species in these groups also suggests that the still more plesiomorphous sister groups needed to bridge the gap once existed, but have become extinct. The significance of everything that was said above regarding the importance of the distinction between age of origin and age of differentiation [of, for example, the Ditrysia] is also emphasized.
In terms of the noëtic trajectory visiting the noëtic stability fields of the groundplans of the groups depicted in Figure 2 things could be as follows :
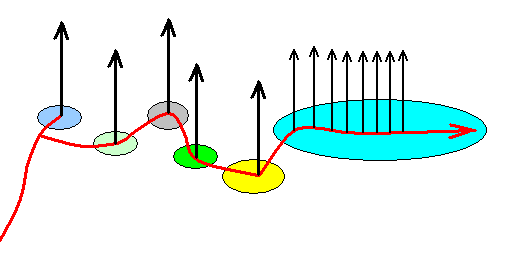
Figure 2b : One of the possible 'translations' of the situation as depicted in Figure 2 (Lepidoptera) in terms of the Implicate Order, noëtic trajectory, and projections (projections that is, from the Implicate Order into the Explicate Order). In its course, the noëtic trajectory successively passes through the stability fields of six lepidopteran groundplans (and their subordinated family- and genus-groundplans). Having at last entered the stability field of the Ditrysia-groundplan it remains there while visiting the stability fields of the subordinated Ditrysian groundplans, that is, it visits one Ditrysian family- and genus-groundplan after another, (and until now) without leaving the overall stability field of the groundplan of the Ditrysia.
The depicted noëtic situation is no more than a fictitious example -- itself in turn containing fictitious subsituations -- of how this might reflect the cladogram of Figure 2. Here the noëtic trajectory, before entering the stability field of the Zeugloptera-groundplan, splits into two branches, one of which visits the stability field of the mentioned groundplan while the other continues its course into the direction of the stability field of the Glossata-groundplan. Such a dichotomous division of the noëtic trajectory might also take place only inside the just visited groundplan-stability field as is exemplified in our diagram in the subsequent course of the trajectory. In that case it, without having split up, enters the groundplan-stability field and then does two things : (1) It projects (or, said differently, it triggers the projection of the noëtic content of a genus-groundplan -- after this content having noëtically reacted with the noëtic content of one of the genus' potential ecological niches) into the Explicate Order, and (2) continues its course directly to the stability field of the next groundplan, and then the same takes place again (In such cases the projection lines belong to the cladistic structure in spite of the fact that they do not belong to the noëtic trajectory).
It we emphasize the 'bear bones' of the above depicted noëtic state of affairs (Figure 2b) then we get :
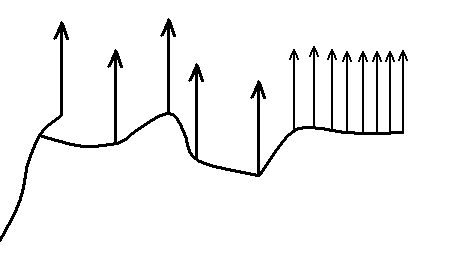
Figure 2c : The cladistic strucure of the noëtic course of things as exemplified in Figure 2b.
Although the projection lines (drawn vertically) are not part of the noëtic trajectory, they belong to the overall cladistic structure of the noëtic pattern. This cladistic structure is essentially (i.e. topologically) identical to the cladogram obtained by phylogenetic systematics (Figure 2).
And indeed we can discern the same monophyletic groups as in Figure 2 :
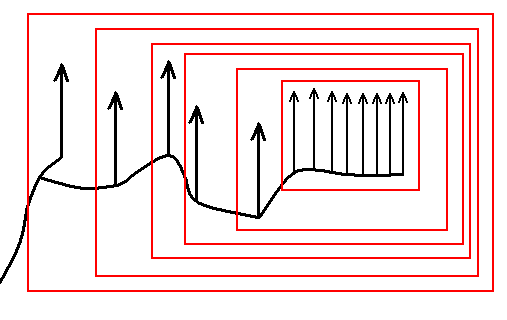
Figure 2cc : Same as previous Figure. The noëtic cladistics allow for the same monophyletic groups to be recognized as in the cladistics determined by phylogenetic systematics, see Figure 2.
Exactly the same cladistics is obtained when the following noëtic course of things is assumed to correspond to the cladistics as depicted in Figure 2 :
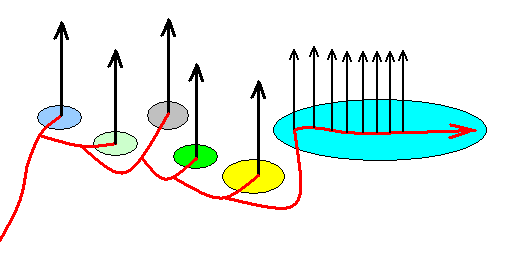
Figure 2d : Alternative course of the noëtic trajectory in the evolution of the Lepidoptera. Topologically the same cladistic structure as in Figure 2 results. And also here the same monophyletic groups can be discerned. See next Figure.
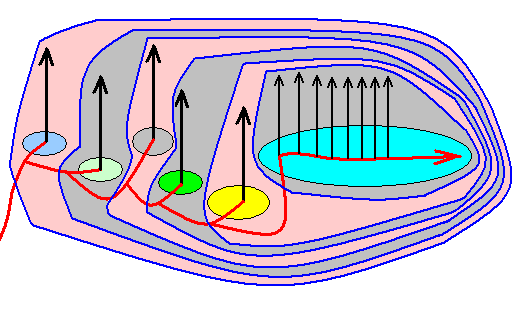
Figure 2dd : Same as previous Figure. The noëtic cladistics allow for the same monophyletic groups (encircled in the Figure) to be recognized as in the cladistics determined by phylogenetic systematics, see Figure 2.
In the previous examples of the noëtic background of the cladistics of Figure 2 it was assumed that the respective groundplans cannot be directly noëtically derived one from the other (Each time when the noëtic trajectory leaves the stability field of some given groundplan it does not directly enter that of another. Instead, upon leaving such a stability field the groundplan is noëtically disintegrated into its noëtic elements, and only upon entering the stability field of the next groundplan this groundplan has been assembled by the noëtic elements of this new groundplan among which elements there may be some elements of the previous groundplan (that is, to the elements (reactants) of the new groundplan there may belong some such elements of the previous groundplan). Such an assembling of a groundplan must be interpreted as a noëtic reaction between these elements. The latter are then noëtic reactants while the resulting groundplan is the noëtic reaction product).
On the other hand it could also be that our lepidopterous groundplans are such that they allow a direct noëtic derivation from one another, like it is in the case of mineral phases under changing thermodynamic conditions (temperature and pressure). Then our diagram of the noëtic state of affairs, (still) corresponding to Figure 2, would look like this :
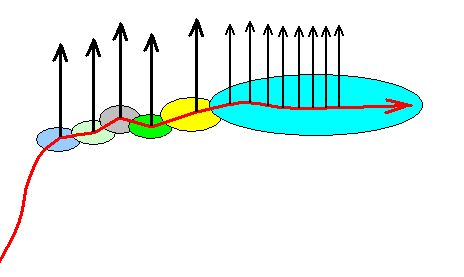
Figure 2e : Another alternative course of the noëtic trajectory in the evolution of the Lepidoptera. The groundplans can be derived directly from each other. And again, topologically the same cladistic structure appears as in Figure 2. Also here we can recognize the same monophyletic groups. See next Figure.
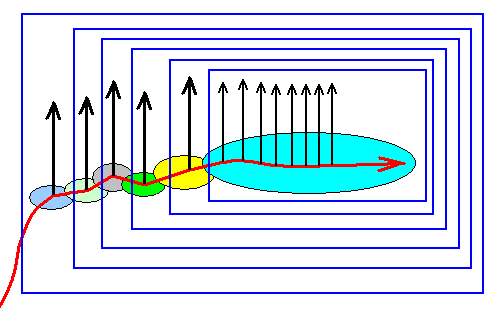
Figure 2ee : Same as previous Figure. The noëtic cladistics allow for the same monophyletic groups (bounded by rectangles in the Figure) to be recognized as in the cladistics determined by phylogenetic systematics, see Figure 2.
[Continuing with HENNIG's text :]
The theory of the "early ontogenetic origin of types" ["ontogenetic" means the course of development of an individual organism] assumes a special place in discussions of the origin of higher categories or new types. Equivalent or nearly equivalent are the concepts of "proterogenesis" (SCHINDEWOLF), paedomorphism (GARSTANG), and -- as WETTSTEIN, 1942 emphasizes -- the designations "diametagenesis" (MIJSBERG), "fetalization" (BOLK), and "neomorphosis" (BEURLEN). We must mention this theory here because, under certain circumstances, it could be important to the interpretation of the morphological methods of phylogenetic systematics. De BEER (1959) formulates the content of this theory : "Evolution is not confined to modifications of the adults, as HAECKEL thought, but can start from modifications in young stages and, when it does, its results are the most striking". He explains that the concept of paedomorphosis is designed "to account for those cases where instead of the young resembling the adult of the ancestors [like we see it in the fact that, for instance, in the human embryo gill-splits are initiated (later to be reduced again)] as HAECKEL insisted [his so-called biogenetic law], the adult of the descendant resembles the young of the ancestors".
From the examples given by De BEER it seems to me that two different phenomena are involved, and that these have a very different importance in phylogenetic systematics.
(End of the quotation of HENNIG's text in his Phylogenetic Systematics, 1966, p.219-228)
Some additional remarks on the recognition of monophyletic groups, the significance of the concept of stem-group, and on the assignment of fossils.
In drawing up these remarks we follow -- together with necessary amendments and additions -- D. SCHLEE, Die Rekonstruktion der Phylogenese mit HENNIG's Prinzip, 1971, p.39-42.
The genealogical diagram (scheme of synapomorphy) representing the result of phylogenetic reconstruction in the sense of HENNIG, and established by a study of recent forms (animals), is an ideal basis for the analysis of taxa-assignment of fossils.
The allocation of a fossil to some place in the scheme of synapomorphy, i.e. its assignment to a certain taxon of the phylogenetic system, can be performed in a well-organized (and identical to the treatment of recent forms) way : One compares every relevant character mentioned in the scheme of synapomorphy with the state of it as present in the fossil, and works one's way up in the scheme until arriving at the place where one finds, in this scheme, the synapomorphy ( = common possession of a derived state of some given character) of which the symplesiomorphous ( = primitive) alternative is present in the fossil.
[That is, one first selects that particular scheme of synapomorphy -- a known phylogenetic tree of certain recent forms -- in which the fossil probably belongs. For this to verify one tries to find in the fossil apomorphic states of certain characters and looks whether these states are also commonly possessed by all the members of our selected scheme of synapomorphy. Such characters (character states) are for instance the characters a and b in the scheme of synapomorphy of the next Figure, and have been indicated as such -- i.e. that they are commonly possessed by all members of the group -- by black dots on the first line of the scheme, that is, on the scheme's starting line. After this, one ascends in the scheme of synapomorphy and checks whether the derived states of other characters demanded there by the scheme actually are also possessed by the fossil. In this way one can establish to which sub-taxon the fossil belongs, for example on the basis of the common possession (by the fossil and by all members of the subtaxon) of the characters (character states) c and d in the next Figure. In order to more precisely determine the correct place of the fossil in the scheme of synapomorphies one works one's way further up into the scheme (now being in the mentioned subtaxon) until one finds in it the apomorphous state of a character that is present in the fossil in its plesiomorphous state, for example character e in Figure 4.]
If a given fossil X possesses the synapomorphies a, b, c, d, e (i.e. if it shares the derived states of these characters with the members of the recent group A), then it belongs in taxon (for example a family) A. See next Figure.
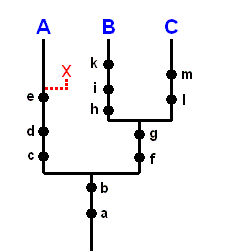
Figure 3 : Scheme of synapomorphy ( = phylogenetic tree plus its argumentation) of the taxon A+B+C. Assigment of fossil X in this taxon.
Each black dot drawn on a phyletic branch signifies the common possession of a derived state of a character by all members (synapomorphy) of this branch. For example the dot marked e placed on the left branch of the diagram means that all members of group A possess a derived state of character e . And this synapomorphy forms an indication that group A is monophyletic. The same goes, with respect to group A, for the characters d and c, for the characters h, i, k with respect to group B, and for the characters l, m with respect to group C. In the present Figure the corresponding plesiomorphous states of these characters are not drawn.
The characters c, d, e are the autapomorphies of the taxon A. The characters h, i, k are the autapomorphies of the taxon B. The characters l, m are the autapomorphies of the taxon C.
The derived state of the characters f, g is commonly possessed by the members of group B+C, while the derived state of the characters a, b is commonly possessed by the members of group A+B+C.
The characters f, g are the autapomorphies of the taxon B+C. The characters a, b are the autapomorphies of the taxon A+B+C.
The supposed fact that our fossil possesses the apomorphous states of the characters a, b, c, d and e means that the taxon *A, characterized by the complete set of synapomorphies (in HENNIG, taxon A in its proper sense), was already present at the time corresponding to the age of the fossil. As we have seen above, a "taxon *A" means all descendants of the youngest common ancestor of the recent members of taxon A, and including this youngest common ancestor itself.
If, on the other hand, the fossil X has only the synapomorphies a, b, c, d, while all other characters (e, f, g, h, i, k, l, m) are still in a plesiomorphous state, then one should allocate this fossil in that place of the scheme of synapomorphy that is indicated in the next Figure.
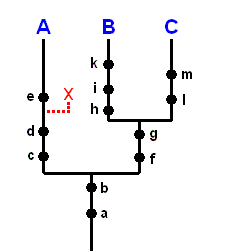
Figure 4 : Scheme of synapomorphy ( = phylogenetic tree plus its argumentation) of the taxon A+B+C. Assigment of fossil X possessing derived states of the characters a, b, c, d in this taxon.
An apomorphy of its own, the autapomorphy x, indicates that X does not represent a persisting stem-species. All this means that taxon *A (possessing the complete set of apomorphies as present in all recent members of taxon A) is not demonstrated in the time period corresponding to the age of the fossil. What is demonstrated to be present already at that time, however, is taxon A sensu lato ( = taxon a in a broad sense), a taxon that has only a part of the mentioned set of synapomorphies, a taxon that can be interpreted as the stem-group in the by HENNIG, 1969, defined sense.
The search for members of the stem-group of a taxon and the principle of their taxonomic assignment is far from an artificial or extra element of the scheme of synapomorphy. On the contrary, every scheme of synapomorphy implicitly contains statements about the 'what' and position of stem-group members :
Because we have to assume that (non-correlated) characters (such as we can assume the characters a, b, c, d, e, h, i, k, l, m to be) evolutionarily appear, not simultaneously, but one after another, meaning that a character complex has been evolved step-wise, a scheme of synapomorphy contains indirect information about still unknown taxa, be they not yet discovered recent or fossil taxa (within the overall group) to which also can belong members of stem-groups. Let us show how.
The scheme of synapomorphy as was drawn in Figure 4 can, as correctly, also be represented by the following diagram :
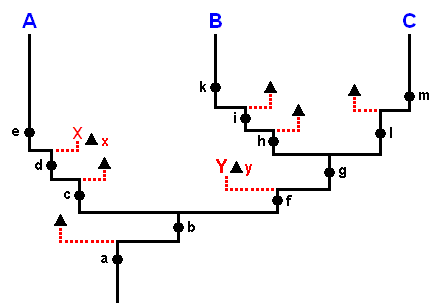
Figure 5 : Scheme of synapomorphy ( = phylogenetic tree plus its argumentation) of the taxon A+B+C of the Figures 3 and 4. The implicit information contained in the scheme of synapomorphy of these Figures is in the present Figure made more explicit. The original phylogenetic relationships are not altered as a result of this explicitation.
In this latter version the kinship relationships, that originally were known in Figure 4, appear in essentially the same form (thick black lines). By the additionally entered forks (one between every two synapomorphies) the information -- as contained in every conventionally drawn scheme of synapomorphy (as in Figure 4) -- is being cleared up, namely about the fact that after the origin of every such a synapomorphy (members of a group acquiring a derived state of some character) not only the corresponding phyletic branch is demonstrated (by that synapomorphy) but also the probable appearance of a second branch. That is to say, when, a derived state of some character is being developed, it is so developed (directly or later on) in one of the daughter-species resulting from a species cleavage. The other daughter-species generally retains the plesiomorphous state of the character (this is HENNIG's so-called deviation rule). So the daughter-species retaining that plesiomorphous state represents the extra branch. The actual existence of this extra branch is, however, still to be demonstrated by some other synapomorphy (indicated by small black triangles) among its members. Because such an extra phyletic line-segment can only branch off from certain well-defined locations in the scheme of synapomorphy, this scheme remains unequivocal. No increase of the number of possible combinations of taxa in a cladogram (phylogenetic tree) will be implied therewith.
So the fossil X, having the apomorphies a, b, c, d (Figure 4 ), allows the following statements :
Some notes on evolutionary biology in relation with phylogenetic systematics.
In addition to having followed the 1966 text of HENNIG (further above), provided with our comments and explanations, there is still much more to be said with respect to the origin of "higher categories" (higher taxa) and to the general course of organic evolution. We therefore will reproduce another text of HENNIG laid down in another book of his : "Die Stammesgeschichte der Insekten", 1969, p.41-49. In contrast to the mentioned 1966 book, this book was written in German (Hennig's native language). However, this book was later -- 1981 -- literally translated into English and revisionary notes were added ("Insect Phylogeny"). The long quotation which I present is my own translation from the German edition, provided with the revisionary notes as they are given in the English edition, and provided with my own notes. Like the previous quotation it is presented here because of (1) the high quality of its content and (2) its relevance not only to insect evolution but also to organic evolution in general, and (3) its dealing with the origin of higer taxonomic categories (taxa).
The Section from "Die Stammesgeschichte der Insekten" is called Stammesgeschichtsforschung und Evolutionsökologie, that is, phylogenetic research and evolutionary ecology, pp.41 (in the German edition). Again, our translation is provided with comments and explanations.
Today it increasingly is legitimately held that phylogenetics should further develop itself into evolutionary biology or evolutionary ecology and thus become a "causal investigation". Russian authors say that it reflects credit on MARTYNOV to have introduced this view into paleo-entomology [= study of fossil insects]. The description of each new fossil is said to have been accompanied by him by the attempt to clarify the evolutionary process. In russian works this principle is acknowledged by the fact that the description of the phylogenetic development of different groups often also contains statements that say how one can explain evolutionarily biologically the origin, preservation, and unfolding of such a group.
1. The explanation of the origin of holometabolism must be different depending on whether we consider the "Holometabola", that is, the substrates [carriers] of this feature, as a monophyletic group or as a polyphyletic group (as WEBER did). In the monophyletic case we have to do with a single one-off event, the origin and outcome of which is to be explained, while in the polyphyletic case we have to do with several independent events that could have had a different "biological significance" and therefore different causes.
2. [Also often neglected is] the distinction between the origin of a given group and the origin of the closed morpho-functional groundplan type that represents the group today. When we, as we did above, consider the "origin" of the group to have taken place at precisely that time in which the last [i.e. the youngest] stem-species common to its sister-group has been split up into daughter-species, then, from the deviation rule it follows that one of these two daughter-species, which [two daughter-species] in turn should be considered as the stem-species of the separated groups, often did not, or only little, differ from the common stem-species.
Revisionary Note 50 (English edition) [This note is given here with additions and omissions].
This view is undoubtedly correct, and without it phylogenetic research as a scientific discipline remains incomplete. It is also correct that in this context one speaks of causal investigation. If one would, however, believe that [for instance] holometabolism [that is, individual development interrupted by a pupal stage] of the Holometabolous insects [Holometabola] is causally explained by having demonstrated that in the hormonal directive system of metamorphosis in the Holometabola a change have taken place such that the corpora allata now secrete an inhibition hormone that continues to let molts for a long period be just larval molts, and that not until a certain point in time the action of pupal and imaginal molt hormones is released, then one has, it is true, in a certain sense causally explained the complete transformation (larva - pupa - imago) taking place during ontogeny [= individual development] of the single individual, but it doesn't say anything about the causes to which one could ascribe the origin of holometabolism in virtue of its "biological significance" (in the sense of BOCK U v. WAHLERT, 1965) during the course of phylogeny, and [the causes to which one could ascribe] the origin of the group of Holometabola. Because answering this question is not possible by doing experiments and because such an answer can only have the nature of a vaticinium ex eventu [prediction from the fact] in which models of the action of known evolutionary factors are connected with living-conditions, that probably have prevailed at the time of origin and unfoldment of the Holometabola, which, however, will never precisely become known, all these evolutionary biological explanations retain a markedly hypothetical nature. And that's why evolutionary biological depictions, not in the last place those of russian authors, look so much like serials [This is a rather harsh critique, that is, in my opinion, not appropriate. Russian, and also many western authors consider the world of organisms in terms of morphological or ecological types, not in terms of hennigian monophyletic groups, although they sometimes mess things up it is true. But read from their typological perspective their writings are important and stimulating.]
The unsatisfactory impression that many such evolutionary biological depictions are leaving behind is also caused by the fact that often two fundamentally important things are neglected :
The importance of these views will be clarified by some examples.
In the same way the preservation of both "paleopterous" insect groups [i.e. insects that, when at rest, cannot fold their wings backwards over their abdomen, while "neopterous" insects can do this.], the Ephemeroptera [may-flies] and the Odonata [damsel-flies and dragon-flies] all the way up into the present, must be explained differently depending on whether we consider them as a monophyletic group with their members possessing certain corresponding derived groundplan characters, or whether we assume that there do not exist closer kinship relations between them. Both hypotheses still exist today.
If one of the 'daughter-species' does not differ from the stem-species, it cannot be recognized as a 'daughter' and most people would refer to speak of a species (stem-species) which persists even after a daughter-species has separated off. The possibilies of reconstructing phylogenetic sequences containing stem-species and daughter-species, which may survive or become extinct, have been discussed by SCHLEE (1971, pp.30-37, "Rekonstruktionsmöglichkeit bei fortlebender Stammart"). In practice, none of these situations will actually give rise to any serious scientific problems, irrespective of whether there is any justification for the 'deviation rule', because a properly conducted analysis using the methods of Hennigian phylogenetic systematics makes it possible to recognize precisely the limits of conclusions that are warranted from the available facts.
The following is an example of this.
If the only result that can be obtained from analysis so far is like that shown in Figure SIX 2, rather than a sister-group relationship like that in Figure SIX 1, then concise use of Hennig's phylogenetic methodology immediately reveals several unresolved questions :
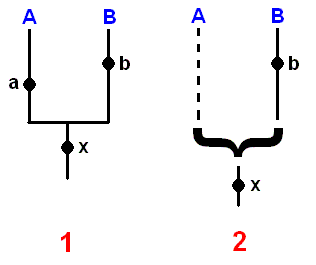
Figure 6 : Schemes of synapomorphy (= phylogenetic trees plus their argumentation) of the taxon A+B. A black dot placed on a branch indicates the synapomorphy by which the monophyly of the species group represented by this branch is substantiated.
Left scheme of synapomorphy :
A derived state of character a is commonly possessed by all members of taxon A, demonstrating its monophyly.
A derived state of character b is commonly possessed by all members of taxon B, demonstrating its monophyly.
A derived state of character x is commonly possessed by all members of taxon A+B, demonstrating its monophyly.
Right scheme of synapomorphy :
For 'taxon A' no synapomorphy is found, that is, no derived (with respect to all other members of the whole group ('A'+ B)) character, common to all members of group 'A' is found. So the monophyly of A is not demonstrated.
Now the unresolved questions implied by Figure SIX 2 :
The conclusion must be that there is no solution at present, and this fact can be seen from Figure SIX 2. Such a diagram also shows the various solutions that are possible.
[ SCHLEE, writing this revisionary note, continues it to refute an objection to all this, which I deem not necessary to reproduce here.]
(Continuing HENNIG's text)
However, in both sister-groups the additive origin of the groundplan type, characteristic to it today, will often have taken place in several steps. The precise order of these steps is not known to us, and in many cases it will remain unknown if we have to do with characters that cannot be preserved in fossils.
Of the two sister-groups of the Mecopteroidea, the Antliophora (Mecoptera + Diptera) are characterized by the following [derived] features : The presence of a sperm pump [Revisionary note 51 in English edition : I do not think that the sperm pumps of the Mecoptera and Diptera are homologous (SCHLEE)], the reduction of the legs of the larvae, and, the, probably primary, predatory nature of the adults. The other sister-group of the Mecopteroidea, the Amphiesmenoptera (Trichoptera + Lepidoptera) is characterized by the following [derived] features : Female heterogamety, and a feature of the wing-venation (loop-formation of the anal veins).
We neither know in which one of the two sister-groups the derived groundplan features, that today are characteristic to them, appeared for the first time, nor do we know in what order these features appeared in the two groups. Also the [natural] selection value, that were possessed by each one of these features or by their combination, and which must have determined their evolutionary success, is unknown to us. All evolutionarily biological statements that have been made [by authors working on these groups], but have so far not considered the internal features (sperm pump of the Antliophora [see note 51 above], female heterogamety of the Amphiesmenoptera), must therefore remain inconclusive.
In many cases also here knowledge of the genealogical relationships will help us further, that is, different assumptions about these relationships are expected to influence significantly the evolutionarily-biological explanation :
To the derived characters of the beetles [Coleoptera] among other things belong the transposition of the flight function to the hind-wings (postero-locomotion), the transformation of the fore-wings into elytra, and the prognathous [= forwardly directed] head connected with the predatory way of life. Between these characters an observable functional connection does not exist, and we do not directly know the order of origin of them.
If we now assume, with WEBER, that the sister-group of the beetles is represented by the "Blattoidea" [insects more or less like cockroaches], then this would mean that the beetles had their formation of elytra and their transposition of the flight function to the hind-wings already taken over from ancestors that are common to them and to the "Blattoidea". These features did not co-determine, or in any case did so only when additional features had appeared, the [evident] evolutionary success of the beetles [because the Blattoidea are evolutionarily less successful (judged by their smaller number of species) in spite of the fact that they possess these features too]. Contributions of the beetles themselves, contrubuting to their success, might be prognathy and holometaboly [The Blattoids are not holometabolous insects while the beetles are].
If, on the other hand we assume that the beetles are most closely related to the Neuropteroidea [ant-lions, lace-wings, and the like], then they would have taken over holometaboly and prognathy from ancestors common to other groups [i.e. these characters would then not count as the beetle's own contributions]. Own contributions of the beetles would then be, among other things, the transformation of their fore-wings into elytra, and the transposition of the main flight functions to the hind-wings [which characters are absent in Neuropteroidea].
To the difficulties in evolutionarily-biologically explaining the course and outcome of the phylogeny of monophyletic insect groups contributes the fact that in insects as being small animals their being tied up to certain definite ecological zones and niches is much harder to recognize than, for instance, in vertebrates. The ability of insects to spend an essential part of their individual life in the smallest ephemeral water-basins, in tree-holes or in thin water-films, makes it much more difficult to distinguish among them between aquatic and terrestrial ways of life than it is in the case of large animals. Also, for instance, phytophagy in insects represents a much more differentiated way of life than it is in large animals, because it can manifest itself in different ways : Their larvae externally feed on leaves or live in galls or mines in stalks, in leaf-parenchym, in roots or in flowers. Also the degree of specializing to certain definite plant species (monophagy) is in insects much larger. In many cases also their individual development is split up into several phases with fundamentally different ecological demands. Concerning these phases and their way of life, especially the eggs and their adaptive features, that might have, with markedly selection-advantages, co-determined the evolutionary success of a given group, only little is known. Even the functional significance of clearly expressed imaginal characters [i.e. characters of the adult] has been insufficiently investigated [such as most features of the wing-venation].
Between the origin of a group (e.g. the Siphonaptera [fleas] ) and the life-period of the latest stem-species common to the recent species (that is the origin of the *Siphonaptera) often a rather long time-span must be assumed. During this period the way of life, as it was in at least the line directly leading to the recent species, might have been changed stepwise. But the corresponding adaptive features must partly as pre-adaptations have formed the basis of the origin of the ecological-functional-morphological structure that is shown by the group today. This problem connects with the already posed question of in which order and in which time intervals, that is in what periods of the history of the Earth, the different derived characters, that are presently possessed by a group, have originated. These things are often almost unknown to us.
The Phasmatodea [such as stick-insects] are today tree-living, leaf-eating insects. By discovery of fossils they are with certainty known [to have existed at least] from the Tertiary [onwards]. [We can also understand this sentence as to mean that the Phasmatodea only appear -- looking upward in geological history -- as late as the Tertiary.] GANGWERE (1965) surmises that the unfolding of this group in the Jurassic might be explained by the appearance and spread of tree-like dicotyledone plants. But he assumes the Phasmatodea to have orginated already in the upper Carboniferous [note the difference between unfolding and origin of a group]. In what way the representatives of this group lived their lives and how they looked like in the Paleozoic and early Mesozoic remains unclear. Still more difficult become evolutionarily-biological explanations of the phylogeny of an extinct group. A typical example, that will be discussed more in detail in Chapter III, is formed by the Palaeodictyoptera [ancient large-winged insects from the Carboniferous].
All these difficulties have made us decide to give in the present book only modest attention to evolutionarily-biological considerations. This is thus not because we underestimate such considerations which, by the principle of reciprocal illumination, are connected with the genealogical departments of phylogeny. But founding such evolutionarily-biological considerations on an insufficient basis often has been more detrimental to it than profitable.
Purpose of the explanatory interpretation of phylogeny should be to make clear the course of the phylogeny of a given group, as a real historical process that has actually taken place, by referring to the action of known conformities to law. This explanatory interpretation experiences its coronation, however, by comparative enquiry of possibly many phylogenetic courses. In this we can recognize the closeness of phylogenetic research to investigating the course of the history of man, because both have entertained similar theories, theories that assume some sort of immanent law directing [evolutionary] development to a certain end or to generally higher forms of organization, [and further] theories that assume a cyclic course of evolutionary development, and theories that attempt to combine the two. Among the cyclic theories we can mention that of SCHINDEWOLF which, similar to already other and earlier authors, assumes a regular course and outcome in the "life" of each singular group, a course that at last can be compared with that of an individual -- birth, youth, hey-day, old age, death. To such an assumption one can object that there are monophyletic groups that exist already from pre-cambrian times. So if it is held that animal groups necessarily are sooner or later condemned to go extinct (A. H. MÜLLER), then it can only apply when these animal groups are not considered as representing genealogical units but "types", of which some are then genealogically connected with later types. By that this conception connects with other conceptions holding that [the process of] phylogeny of at least some groups runs according to a rocket scheme, such that in the course of time several "radiations" of a monophyletic group successively take place of which each younger radiation originates from a twig of the older one (see next Figure) [Revisionary note of HENNIG himself : DONOVAN (1964, p.269, Fig.6.) has published a 'rocket scheme' to illustrate the evolution of the ammonoids, showing extinctions at the end of the Permian and the end of the Triassic, and the final extinction of the group at the end of the Cretaceous.].
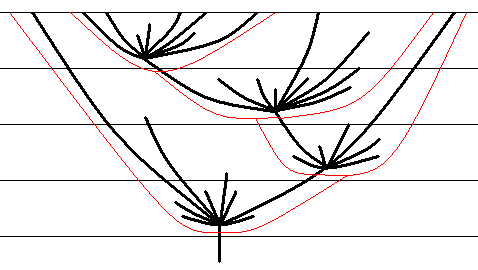
Figure 7 : Rocket scheme (thick black lines), according to which the development of animal groups supposedly takes place. The upper horizontal line represents recent times. All branches that do not reach this line have gone extinct.
(Adapted from GROSS (1964), taken from HENNIG, 1969)
Here three questions should be considered :
A special question is about the connection between phylogenesis and paleogeographic changes. That also here connections must exist (not only through the important evolutionary factor of isolation) is a priori probable. Investigation of these connections becomes utterly difficult by the fact that more extensive fossil records are known to us only from a few regions of the Earth. JEANNEL (1950), in the exposition, designed by him, of the history of insects, has attributed much significance to the fact of the separate development of different groups on the Laurentian continent, in Gondwana- and Angaraland in the Paleozoic, and then, after a period of faunal exchange, again in the Mezozoic. This exposition is, however, presently based on still too poor premises for it to be already more than an inspiring working hypothesis.
Revisionary note 56 in English edition, written by HENNIG himself :
JEANNEL (1950) discussed the history of the origin and dispersal of the insects in relation to the history of the three great continental blocks ('refugia') of Laurentia, Angaraland, and Gondwanaland. The alternating connection and separation of these continents is said to explain the origin and distribution of the insect lineages.
In Laurentia, which had a tropical climate during the Carboniferous and Permian, the Paleoptera and Polyneoptera are said to have arisen from more northern, Devonian ancestors. After the Carboniferous, Laurentia became progressively cooler.
On the other hand, Gondwanaland, which was originally close to the pole and had a correspondingly cold climate, is said to have become progressively warmer after the Carboniferous. The Paraneoptera and Holometabola are said to have arisen there, as the fauna of a cold area. Jeannel considered the 'nymphal diapause' of the Holometabola to be an adaptation to a temperate climate with cold winters.
Unlike the other two continents, Angaraland enjoyed a rather stable climate and for this reason became a faunal refugium.
At the end of the Permian, climatic assimilation took place and a retreat of the mesogaean oceans facilitated a faunal exchange. At this period, therefore, there was a mixture throughout the world of elements that had originated from Laurentia and Gondwanaland.
During the Mesozoic, development in isolation took place as Gondwanaland broke up.
The history of the Angaraland element did not begin until the Cretaceous and was restricted to Laurentia (= Holarctis).
This concludes our quotation and translation of HENNIG's exposition of evolutionarily-biological questions and the role of phylogenetic systematics.
Some general notes on ecology and on the formation of ecological niches of animal species.
There is still more to say about the role of ecological niches in evolution, especially with respect to our interpretation of evolution in terms of the Explicate and Implicate Orders.
Indeed, in order to be able to still better describe the above considerations of HENNIG concerning the evolutionarily-biological explanation of evolution in terms of the Implicate and Explicate Orders, and also in order to learn so to describe the general ecological facts that will, shortly, be taken from "Allgemeine Zoologie", 1977, by HADORN and WEHNER, it is necessary to repeat and add some crucial aspects of our noëtic theory of evolution :
Organic groundplans are among the countless noëtic patterns (qualitative contents) present in the Implicate Order. All these patterns have resulted from noëtic reactions and derivations, having taken place in that Order. They exist, in the Implicate Order, in their respective noëtic stability fields. In organic groundplans it is the genus-groundplans that are closest to the Explicate Order : And a genus-groundplan, which is, like all groundplans, originally still a noëtic entity, and as such still an inhabitant of the noëtic Order (the Implicate Order) can also exist in the Explicate Order, but only when it has acquired the appropriate existential conditions, which consist of special adaptations to a specific type of habitat in the Explicate Order. The genus-groundplan (and with it, more generally, the family-groundplan) in its form of a noëtic content implies, that is, noëtically delimits, the noëtic contents of possible conditions for the genus-groundplan to exist in the Explicate Order. These delimited contents in fact represent the set of noëtic contents of the genus' potential ecological niches, and they are turned into truly existential conditions when they, each for themselves have noëtically reacted with the genus-groundplan resulting in that groundplan now being provided with noëtic counterparts of the adaptation to one of the potential ecological niches of the genus. And when, in the Implicate Order, a genus-groundplan is provided with these truly existential conditions, it can, as a product of the mentioned noëtic reaction, be p r o j e c t e d into the Explicate Order, but only if two further conditions are satisfied : (1) Precisely those physical, chemical, and biological configurations with which the adaptations -- initially still in their noëtic form -- are geared, that is, precisely that ecological niche to which the adaptations point, must actually be present in the Explicate Order, and (2) the noëtic trajectory must have visited the noëtic stability field of the genus-groundplan (that is to be projected).
The transition from one ecological niche to another, and with it the origin of a new species of a given genus, can be described as follows in terms of the Explicate and Implicate Orders :
An animal species in the Explicate Order morphologically and physiologically consists of higher-order groundplans that are progressively partly overwritten by lower-order groundplans of which the genus-groundplan is the lowest. This genus-groundplan, provided with, or partly overwritten by, the above mentioned existential conditions, exists in the Explicate Order, and shows itself there as a species (that is, one of the species of that genus) adapted to some existing ecological niche. This existence of the adapted genus-groundplan in the Explicate Order had started with the first projection of this groundplan into this Order and is maintained by continuous alternation of projection (from the Implicate into the Explicate Order) and injection (back into the Implicate Order). This continuous projection and injection guarantees the mutual ontological immanence of the two Orders and also the mutual exchange of information.
An animal species S(A), of a given genus, existing in the Explicate Order generally consists of several populations all occupying the same ecological niche -- let us call it niche A (which is one of the genus' potential ecological niches) -- different from those of other species. And now, one such population can venture into a more or less different ecological zone, or just happens to find itself in it as a result of climatic and biological changes. This can lead to the annihilation of that population, but if it does not, then we can say that its individuals interact with elements of this (for them) new ecological zone, i.e. they materially interact with elements of some other member -- let us call it B -- of the set of the genus' potential ecological niches. Because of the mentioned constant alternation of projection and injection, information of this new environment ends up in the Implicate Order and this allows the noëtic reaction-product -- having resulted from the noëtic reaction between the noëtic content of the genus-groundplan and the noëtic content of the ecological niche B -- to be projected from the Implicate order into the Explicate Order. And there we, as observers and theoreticians, experience this as a population, initially belonging to species S, and initially occupying (along with other populations of the same species) ecological niche A, evolutionarily adapting itself to the ecological niche B, and becoming in this way species S(B). And also here the continued existence in the Explicate Order of this new species S(B) is sustained by the constant alternation of projection and injection. But if, in the Explicate Order, this ecological niche of species S(B) (and thus all corresponding habitats of the individuals of this species) is destroyed (for instance as a result of large-scale environmental changes), then the mentioned alternation of injection and projection is no longer possible, which we experience as the evolutionary extinction of that species.
We now extract some ecological facts and suppositions from the zoological textbook "Allgemeine Zoologie" (General Zoology), 1977, by HADORN and WEHNER, originally written by KÜHN as "Grundriss der Allgemeine Zoologie" (Basics of General Zoology), 1961. We take the mentioned ecological facts and suppositions from p.27-37 of volume 2 of the Dutch edition from which we translate into English, and provide with explantions and comments (in square brackets).
[Every population of animals belonging to a given species changes the density and number of its individuals as a result of several factors. In the absence of limitations the growth of a population is exponential. In reality, however, a complex of limiting factors keeps the population on some optimal value C (of the number of its individuals), in fact lets the population asymptotically approach this value. In most cases, however, the number of individuals will not so approach this optimal value, but settle itself on it by a damped oscillation. If the number of individuals is soaring too fast above the value C, then often this will result in the total collapse of the population. Exhaustion of food reserves and also a social stress as a result of too high a density of the population can be the causes of this.]
Ecological factors limiting from outside the exponential growth of a given population are first of all populations of competing species, which claim the same things to the biotope [a habitat is the type of environment in which a given species lives, and a biotope is the type of environment in which, not a single species, but a whole biocoenosis lives]. Such an interspecific competition leads to the fact that the populations of the relevant species are going to make very specific demands on their habitat, resulting in the fact that species are going to avoid each other [that is to say, in the evolutionary sense, meaning that their evolutionary development leads to a divergence of specializations and adaptations]. The concept of "ecological niche" does therefore not refer to a geographically demarcated terrain or place inhabited by a p o p u l a t i o n, but refers to the functional role that is played by a s p e c i e s, with its special demands, in an ecosystem [According to me this definition doesn't cover all essentials of an ecological niche. In fact the function of the species in the ecosystem is a characterization (of the ecological niche occupied by that species) totally seen from the overall ecosystem. The latter is compared with an organism containing functional organs. Although such a comparison makes sense in certain respects, it cannot yield a proper definition of the ecological niche of a given (animal) species. Such a definition should express the close connection of the individuals of the species with certain structures in their environment that are essential to them, not as individuals but as members of the species. This automatically means that these structures are not individual things or configurations present in the environment but qualities, that is, special qualities in the sense of special states of affairs in the environment. These qualities have mainly to do with food but also with things such as respiration, shelter, and reproduction. And in the case of being an ecological niche of a given species these special qualities together form an extension of the qualitative state ('whatness') of the individuals as members of that species. Differently expressed, certain special qualities of the environment are 'internalized' into the whatness of the species, that is, part of the external environment, external with respect to the individuals, has become internal with respect to the species. And this fact of internalization of certain aspects of the environment is known to us as a d a p t a t i o n s. So precisely that aspect of the environment that is internalized by a given species is that species' ecological niche.].
First, two species of the [what I assume to be] mammalian genus Dipodomys from North America. The geographical ranges of these species overlap, but each species occupies, as a result of a difference in the choice of food, a different ecological niche : Like many species of the genus, D. merriami feeds on seeds of cereal, while D. microps has specialized itself in feeding on salts-containing leaves of perennial salt-plants. The advantage, connected with this latter feeding habit, that this species enjoys with respect to it competitors, namely to have at its disposal the right food-plants all the year round, does, however, demand special capacities for osmotic control.
Ultimately the diversity of activities of organisms rests on this necessity for every species to form its own 'burrow' (ecological niche-formation), that is, finding a different exploitation of a given environment. In evolution, namely, following the principle that competition should be avoided as much as possible, all differentiations in a species are favored that diminish competition with other species. As a result sympatric, i.e. occurring in the same [geographical] range, species never can represent the same ecological niche. But nothing stands in the way of using the same niche by species that are geographically separated from each other (allopatric species) (for example we see the 'position' of the South American hummingbirds in Africa being occupied by the honeybirds : they occupy the same niche). This is called position-equivalence [When we hold a most strict view of the concept of "ecological niche", however, we must assume a difference between the two, if only because we have to do with two different species of organism and two different continents]. Every biotope has a characteristic spectrum of fixed relations, that lead, through the formation of niches of often different animal groups, to convergently developed ecological types (for example, planktonic marine animals, sessile marine animals, bottom-dwellers of coastal areas).
The fauna of the marine tidal zones (interstitial sand-biocoenoses) is a school example of species-differentiation by exclusion of [interspecific] competition. Many closely related species of ciliate worms (Turbellaria), roundworms, gastrotrichia, bear-animals, polychaetes, and cyclopses, together live here as 'micro-organisms' in each other's closest spatial vicinity. Because niche-formation in this biotope goes with small differences of micro-climatological and hydrographic factors, and manifests itself on a very small scale in a vertical and horizontal distribution in the [beach] sand and in the temporal spread of the periods of reproduction, relative small changes of the ecological conditions of life can already result in the origin of new species. Therefore the tidal zone forms a center of evolution.
One might wonder whether formation of ecological niches in the same geographic range can result in the origin of species or races. If one defines a species to be the total number of interbreeding individuals, that is, individuals sharing a common gene-pool and reproductively isolated from individuals of other species, then in the animal world sympatric speciation [i.e. new species being formed in the same territory, and thus in the absence of physical or geographic barriers] would be rare : Formation of niches is not sufficient to prevent the exchange of genes. Generally a spatial separation of the relevant populations must precede the development of a mechanism of isolation, that is, the obstruction of gene-exchange (allopatric speciation). Some sympatrically living species of birds that look quite the same, are the result of separation [of populations of the stem-species] during the Ice-age, and only after that their geographic ranges began to overlap, so that now the different species occur together. A species may consist of a number of geographically separated races (subspecies), that is, of populations that significantly differ from other populations of the same species. In the zones of overlap of the ranges of these subspecies these subspecies will interbreed. As a result of this in one and the same region no 'ecological races' can occur [that is, only geographical races will occur, and they do as long as there exist populations that are geographically isolated (but not necessarily ecologically isolated) from other populations of the same species]. The fact that formation of ecological niches in a given biotope does not suffice for the origin of races and species on a large scale, but that geographical isolation is necessary, is impressively demonstrated by the wealth of species of island faunas. On the Hawaii-islands a quarter of all Drosophila species [Diptera, acalyprate flies] (namely 500 species) of the world lives [This might be the result, not so much of separation of populations of the same original species, but of the fact that relatively few animal species have reached the islands so that many potential ecological niches initially were not occupied. And the presence of such an array of free niches makes possible a r a d i a t i o n of some one original species, initially present on the islands, say a Drosophila species, finally resulting in a great many new species.]. [And when races have originated by geographical isolation, and not necessarily by ecological isolation, then it would be no surprise that] as a result of this members of the same species often occupy the same ecological niche. Therefore, intraspecific competition can only manifest itself in the spatial separation of the individuals that belong to the reproductive community (formation of territoria). In interspecific competition, on the other hand, where the danger of genetic exchange does no longer exist, the possibilities of ecological specialization will be fully unfolded.
Different species cannot only be each other competitors with respect to the same factors of the biotope, they also can represent each other's food, stand in a host-parasite relationship to each other, or mutually benefit from living together (symbiosis). Because parasitism is often derived from a prey-predator relationship (episition), one cannot, in certain marginal cases, precisely determine whether one has to do with parasitism or with a prey-predator relationship. Generally, a predator kills its prey, while a parasite uses its host only for obtaining food, and living, to suit this purpose, temporarily or permanently on or in its body. But one can observe many transitional forms : The larvae of Tachinidae (Diptera) initially parasitize in the fat-bodies of caterpillars, which is not particularly injurious to the host, but towards the end of their larval period they penetrate also the vital organs of the caterpillar, causing the demise of the host. So initially they behave as mere parasites, but shortly before pupation they become predators. This is why one calls such organisms "parasitoïds". The motto of a really successful parasitism, however, is : live and let live. This rule is followed by the close relatives of the Tachinidae, the warble flies (Oestridae), which spend their larval live in the skin of mammals, not threatening in this way the life of the host.
All this can clearly be illustrated by considering some examples.
Often two species inhabit different preferential biotopes, but penetrate in that of the other species [better: penetrate in some individual instance of the biotope of the other species] if [individuals of] that other species happen[s] not to be there, or is experimentally removed by investigators. If one places two species of mealbeetle (Tribolium castaneum and T. confusum) into a nutritive substrate of wheat twaddle, in which temperature and relative humidity can be adjusted independently of each other (respectively varying in the range of 24-34 C0 and 30-70 percent, then, after some time, in the humid-warm experimental set-up, only T. castaneum survives, while in the cool-dry set-up , on the other hand, it is T. confusum that survives. So when both species occur in a given range, each one of them has its own ecological niche in it and completely dominates there over the competing species. In intermediate conditions of temperature and humidity in which each of the two species can thrive optimally, always only one of the competitors (with equal probability) survives [I can imagine this when with "competitors" is meant "two individuals, one of one species, and the other of the other", but I cannot imagine that so well when with "competitors" is meant "two competing species". These two species must in nature (contrasting this with an experimental set-up) occupy two different ecological niches which, however, might overlap with respect to their temperature and humidity ranges].
The formation of a definite ecological niche is sometimes limited to certain stages of development of the developmental cycle of a population : In the marine barnacles (Cirripedia) only the sessile adults in the domain of the high-tide mark are each other's competitors, while the planktonic larvae live mixed up [in the same localities] freely without impeding each other.
Even a whole phylum, which apparently is distributed over the whole Earth (Gnathostomulida) and at present counting about 50 species of small parenchymatic worms mostly less than 0.5 mm, has [evolutionarily] developed from such tidal forms.
True parasites differ from predators in a series of essential population-features. To a parasite the host not only is a source of energy [by way of the food that it supplies], but also its biotope. As a result much closer functional relationships will be established between parasite and host than between a predator and its prey. In parasites these close relationships often manifest themselves as fundamental alterations of their groundplan [we would say, by the appearance of metatypic characters that are added to, or partly overwrite, the prototypic characters constituting the groundplan]. In this way whole taxonomic groups, that had shifted to a parasitic way of life -- such as sporozoa among Protozoa, and sucking-worms (Trematoda) and tapeworms (Cestoda) among Flatworms -- have been subjected to group-specific alterations of the general groundplan. Many parasites are so specialized in their morphology that to find out what they really are [that is, to what (higher) taxon they really belong, or, said differently, what their groundplan really is] can only be done by considering their early developmental stages : The 'crab sac' Sacculina carcini which lives in beach-crabs as internal parasite, only consists of a branched network of little sacs that encapsulates all internal organs of the host. The nauplius larva of Sacculina, on the other hand, freely swimming in the open, possesses all the features of a small crustacean [such as, for instance, a shrimp]. See next Figure.
Figure 8 : Crab-sac (Sacculina carcini), living as an endoparasite in the beach-crab (Carcinus maenas) in the form of a network of threads (Sacculina interna). Ventral view of the host. To the right : larva.
se = bladder-shaped Sacculina externa, containing ovaries and testes.
Individual development is through free-swimming larval stages (Nauplius larva).
(After KAESTNER, from HADORN and WEHNER, 1977)
[From a form like this it is clear that its evolutionary development -- from some ordinary completely free-living crustacean such as a shrimp -- in no way could have been realized by the mechanism of random genetic mutation + natural selection.]
Another difference between parasites and predators lies in the high density of the population of the former. While the number of predators always lags far behind that of the prey animals, the parasites in most cases substantially outstrip their hosts in this respect.
Also the outstanding host-specifity, in other words the occupation of a very precise -- that is, very narrow -- ecological niche, is a typical feature of parasites, contrasting them with predators. Usually, ectoparasites occur only in very definite animal species [host species], and may even be limited to determined regions of the body. Examples are the Mallophaga [Insecta] in the plumage of birds, see next Figure,
Figure 9 : Various species of feather-lice (Mallophaga) that often are specialized in living on definite parts of the plumage.
(After DUBININ, from HADORN and WEHNER, 1977)
the hair-lice in humans (genera Pediculus and Phtirius [Phtirius]), or the larvae of Drosophila carcinophila, which exclusively develop in the grooves of the third jaw-leg of the land-crab Geocarcinus ruricolis (a species limited to certain islands in the Caribian). [The origin of this peculiar parasite-host relationship is almost impossible to understand.]
Figure 10 : Left (a) : Offering food by a Formica worker-ant to a beetle-larva (Atemeles pubicollis) that parasitically lives in the ant nest. Right (b) : Atemeles-larva with position of the cutaneus glands (k) producing baits for the host ants. (After HÖLLDOBLER, from HADORN and WEHNER, 1977)
Phylogenetically viewed [here is merely meant : evolutionarily viewed] the connection of parasites with such extreme [ that is, narrow] ecological niches brings, however, with it some significant disadvantages that have far-reaching consequences : With respect to their way of life parasites are in such a strong degree dependent on a definite species of host, and therefore being so specialized, that they hardly possess any evolutionary potential anymore. Indeed, no group of parasites is known from which phylogenetically new species have been evolved that are again free-living. Because, moreover, the host-environment of the parasite evolutionarily changes more slowly than do the environmental factors to which the host itself is exposed, the evolutionary development of the parasites always lags behind that of their hosts. Thus many lice, from the family Echinophthiriidae, now living on seals (Pinnipedia), have (evolutionarily) changed only little since their hosts, originally land predators, switched over to a life in the sea. The whales, which in contrast to the seals, do not even go ashore anymore to reproduce, have, in their (evolutionary) transition to an aquatic life, lost all ectoparasites of their terrestrial ancestors [So these parasites also did not evolve further, but here not by remaining the same, but by abandoning their hosts]. The, as a result of this, vacant niches have then become occupied by small crustaceans from the group of the amphipods (Cyamidae). And they have their functional anatomy as well as their individual development -- they are the only parasitic crustaceans without free-swimming larval stages -- completely adapted to a living as lice do.
Because in parasites in the course of their evolutionary development, even when far-reaching radiation of their hosts takes place, only small evolutionary shifts are possible, they can be indicators of the degree of kinship of their hosts. Flamingos and duck-birds possess precisely the same feather lice (Mallophaga), while in herons, to which the flamingos are often allocated by systematists, based on so-called kinship, they are completely absent.
Even in dromedaries and llamas, which today live on two different continents, their parasites indicate common ancestors.
Evolutionarily, parasites have not necessarily evolved via a predator-prey relationship from free-living forms. Often one sees s a p r o z o i c s p e c i e s -- for instance in Nematode worms -- appear as being predestined to parasitism. Also commensals ('eating from the same table', not harmful to the host) sometimes develop into parasites. Departing from commensalism, the relevant species may, however, also switch to a positive interaction and ultimately establish such a close mutual relation of dependency that a s y m b i o s i s results. In the classical example, the symbiosis of a hermit crab with a sea anemone, one can observe different phases of increasing bond between the partners. In all cases the crab benefits from the protecting action of the nettle cells of the anemone, while the anemone in turn benefits from the food catch and the mobility of the crab. At the English coast one sees the anemome Calliactis parasitica climbing the snail's shell [in which (empty shell) the crab lives] without help of the hermit crab (Eupagurus bernhardus). In the Mediterranian, on the other hand, the same species of sea anemone can climb the shell only with the help of the crab (Pagurus arrosor). While in both cases crab and anemone only come together facultatively, in other organic species the adult partners never again occur separate from each other : The symbiosis has become an obligatory community of two species. Such close relationships between two species especially apply to those symbioses in which the partners supply each other with essential nutrients. This is, for instance the case in a large number of protozoans, sponges, coelenterates [such as polyps, for example those of corals], and Turbellaria, which obtain oxygen and nitrogen-containing products from intracellularly-living algae. It also occurs in symbioses of animals with bacteria, which, as in wood-eating insects, decompose cellulose, while the hostcells do not possess the enzyme cellulase [that decomposes the cellulose]. As a matter of fact, obligatory symbioses occurs in all extreme food-specialists to which especially belong plantsap-sucking, blood-sucking, and keratine-eating insects. In them, bacteria and fungi that live in special organs (mycetomes), produce vitamins, which cannot in any other way be obtained by the insects. Loss of the symbionts as a result of sterile cultivation, heat, application of antbiotics, or removal of the mycetomes, can only be compensated for by administering of vitamins from the B-group [. . .]. In some fishes and cephalopods symbionts play a role in the production of light, beause they give off metabolic energy in the form of photons (bioluminescence). These symbionts occur in special photogenic organs, which may possess characteristic reflecting epithelia and even pre-set lenses.
With the help of unicellular algal symbionts the foraminifer Heterostegina (Nummulitidae), measuring a few millimeters, is even provided with its complete energy need. Through its symbionts this unicellular organism feeds therefore autotrophically as [green] plants do, and achieves -- because it does not obtain organic substances from its envirnment, the benthos of shallow tropical seas -- much greater densities than other foraminifers do.
Very often, symbioses, like parasitic relationships, only become possible thanks to the development of specific behavioral patterns in both partners. The especially in many coral-fish occurring characteristic grooming symbioses, for example, are connected with special asking postures (bowing down the head, spreading of the gill lids, or even changing color). Also here it is again certain behavioral patterns that trigger a selecting influence in the direction of interspecific dependency relations, and as a result make possible the occupation of new ecological niches.
( End of quote from HADORN and WEHNER, 1977, p.27-37 ).
Will be continued if necessary.
Before we continue with the systematic treatment of the wing-venation in Nematocera (we had done it up to the Rhyphidea and will continue with the Fungivoridea) we add some more phenomena that have a bearing on the theory of organic groundplans : In some epizoic dipterous families (families whose representatives live on the body of animals) the family-groundplan, and even the order-groundplan (the groundplan "Diptera"), has been largely over-written by metatypic characters most of which are adaptations to the epizoic way of life. The next document will consider four of such epizoic dipterous families, viz., the Braulidae, Hippoboscidae, Nycteribiidae, and the Streblidae.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXVI.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXIV-A
Back to Evolutionary Part XXIV-B