

In the long series of previous documents (parts XXXI-LXV ) we have, following Malyshev, 1966, studied the evolutionary development of instincts in Hymenoptera. And, of course, in studying instincts of Hymenoptera one had to rely on observations of the behavior of active living wasps, ants, and bees, that is, recent Hymenoptera.
Most fossil insects have come to us as impressions in sedimental rocks of wings. But some sites where such fossils are found may contain impressions of whole insects (especially of insects from the Mesozoic and Tertiary). But even then the morphological features of body parts are hard to reconstruct. The wings are always best preserved, including their venation, and we are lucky that precisely the latter constitutes a set of important clues as to the identity of the fossil remains. Nevertheless much uncertainty will remain. The fossils must be interpreted by comparing them with their living desendants. And, of course, things like behavior cannot directly be seen in fossils. Individual developmental stages of Hymenoptera as well as nests or cells are not found in fossil form, so we have to rely on the study of adult forms only. Ecological relationships in fossil Hymenoptera, as in all fossil insects, can only be roughly assessed indirectly, in fact they are depicted as mere guesses. But if we combine the features of fossil Hymenoptera, as far as they have been found at all, with what we know about recent Hymenoptera, we might be able to present a crude picture of their origin and of the successive appearance of Hymenoptera groups in the geologic history of planet Earth.
Earlier we have established that each organismic species (and each higher taxon as well) represents, with all its features, morphological as well as behavioral, strategies, strategies, that is, of how to exist and persist in the Explicate Order. At first glance we would expect that every feature of a given species plays a role in its strategy, that is, every feature seems to have a functional significance. But maybe this is not entirely the case : In addition to the majority of features that do indeed have such a significance, there seem to be features, especially among the morphological ones, that do not have any functional significance. And precisely they are the expression of the supposed fact that the evolution of organisms has a partly formal (in contrast to biological) aspect. As was expounded in earlier documents (especially in parts LXc and LXd), we assume -- in our "noëtic theory of evolution" -- that strategies are first of all present in the Implicate Order in the form of 'descriptions' or 'prescriptions'. And these descriptions turn out to be related to each other according to some system of formal derivations, derivations that is, from higher to lower strategies. And upon projection of these noëtic strategies (descriptions) into the Explicate Order, the order of Time and Space, they will appear in reverse order : Of some given group of organisms, say the Order Hymenoptera, the lower forms (lower strategies) appear first, followed by higher and higher forms (strategies). It would be interesting to compare the successive apearance of Hymenoptera-groups, as revealed by fossils, with the earlier established evolution of Hymenoptera based on the progressive development of their instincts. But, unfortunately, there are by far not enough fossils found, and from many geological periods we have no fossils at all. Nevertheless we will do our best, by setting up hypothetical forms and relationships. We will do this by studying Rasnitsyn's book on the paleontology of the Lower Hymenoptera (that is, the Suborder Symphyta, containing saw-flies and their relatives), published (in Russian) in 1969, and later by studying his next book on the paleontology of the Higher Hymenoptera, published (also in Russian) in 1975. In studying all this we hope to supplement our noëtic theory of evolution with details and extensions. Of course Rasnitsyn's views on the general nature and mechanism of organic evolution will differ from ours in many respects, but that does not at all diminish the value of his works.
Let us, then begin with the Suborder Symphyta.
Orientation
Before we study the evolution of the Suborder Symphyta of the Order Hymenoptera, following Rasnitsyn, 1969, it is necessary to provide some orientation in the sytem of Symphyta, and in the way of life of its recent and fossil representatives. To begin with the way of life of the immediate ancestors of the Hymenoptera and of their direct and further descendants, we can refer to three earlier documents :
Evolution, Part XXXI : Archaic Soil-dwelling Phase (at the end of the document).
Evolution, Part XXXII : Ectophytic Sawfly Phase .
Evolution, Part XXXIII : Endophytic Sawfly (Cephoid) Phase .
As to the classificatory System of recent Symphyta we may give the arrangement as it is adopted in RICHARDS and DAVIES in Imms' General Textbook of Entomology, 1977 :
Superfamily Xyeloidea (Xyelidea) :
Family Xyelidae
Superfamily Megalodontoidea (Megalodontidea) :
Family Pamphiliidae
Family Megalodontidae
Superfamily Siricoidea (Siricidea) :
Family Siricidae (Wood-wasps or Horn-tails)
Superfamily Orussoidea (Orussidea) :
Family Orussidae
Superfamily Cephoidea (Cephidea) :
Family Cephidae (Stem Saw-flies)
Superfamily Tenthredinoidea (Tenthredinidea) :
Family Argidae
Family Blasticotomidae
Family Cimbicidae
Family Diprionidae
Family Pergidae
Family Tenthredinidae
In addition to these (recent) taxa, we will meet with fossil taxa, established on the basis of fossil Symphyta found in Mesozoic deposits (and described by Rasnitsyn, 1969).
On the character and evolution of Symphyta
Reconstruction of the most recent common ancestor of the Symphyta
Morphological analysis of the Hymenoptera of the Suborder Symphyta has revealed that almost all known plesiomorphic characters [= primitive, original, (condition of a given) character] in them can be found in the representatatives of the family Xyelidae.
See next Figure.
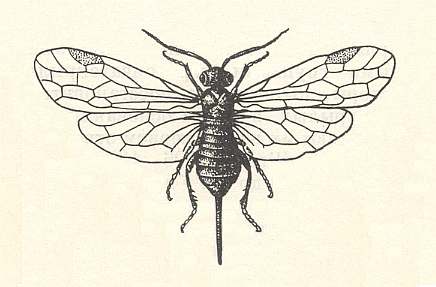
Therefore, the common ancestor of the Suborder, possessing all plesiomorphous characters, is expected to differ so little from the common ancestor of the Xyelidae, that this very ancestor may naturally belong to this family itself [that is, as a member of this family], not, thereby, changing the family's diagnosis. In other words, one or another until now not more closely known representative of the Xyelidae was the common ancestor of all Symphyta (and through them, evidently, of all remaining Hymenoptera). In line with this supposition is the fact of the ancientry of the family Xyelidae and the fact of its great diversity in the Mesozoic.
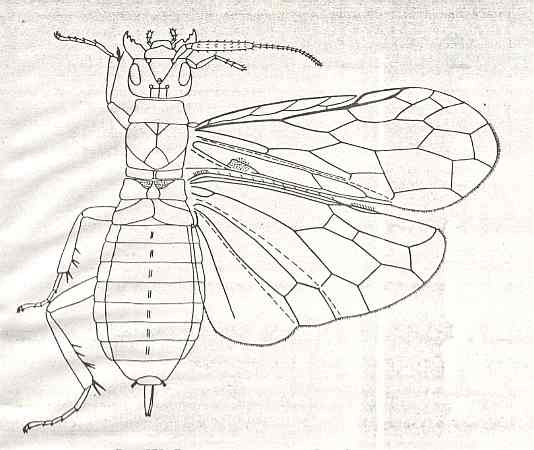
Its larva, insofar as we can judge from available statements, was morphologically similar to the larva of Macroxyelini [a tribe of the family Xyelidae]. See next Figure.
More complicated is the question about the ecology of the ancestral form. The larvae of the Macroxyelini feed on leaves of angiosperm plants (Angiospermae, flower-plants) which did not exist in the Triassic and Jurassic from which periods representatives of various groups of Symphyta are already known. In addition, the larvae of Macroxyelini are relatively badly adapted to an open way of life on leaves of plants and to feed upon them : Their mandibles are, as to their structure, more similar to the mandibles of schizophaga and predators, than to those of typical phyllophaga [leaf-eaters] as are the larvae of the higher Tenthredinidea and Lepidoptera [butterflies and moths]. The legs of the larvae of Xyelidae, apparently, are [too] weak for a firm grip on such a loose substrate as rocked-by-the-wind leaves and thin twigs of plants, because many of them, similar to the larvae of Pamphiliidae [Symphyta], complementarily adhere themselves to the substrate with the help of a light cobweb. It is possible that such a wholly imperfect method of fixation really was the first stage of adaptation to an ectophytic way of life, and that the larvae of the Xyelidae originally possessed the ability to use the secretion from their salivary glands not only for constructing a cocoon, but also for fixation onto the substrate.
On the evolutionary potential of Symphyta (and generally of Hymenoptera), and the evolutionary significance of the genetics of Hymenoptera determining their reactions to a changing environment.
The following (long) account, also taken from RASNITSYN 1969 -- and considered to be instructive for understanding some aspects of hymenopterous evolution -- is about the relation between fertility, genetics, individual stability, the environment, and the evolutionary potential, in Hymenoptera, especially in Symphyta. Things are then compared to those in Lepidoptera (butterflies and moths, which are ecologically similar to Symphyta).
More or less halfway this account we will encounter the following more or less central statement :
"Because in all organisms in each generation all offspring except two individuals, perishes, the fertility first of all expresses the average mortality of the species, that is, the ability of its individuals to withstand external influences. In conditions comparable to the level of complexity of the environment the fertility, consequently, expresses individual stability of the organism, its ability to counteract environmental factors, whereby fertility is higher the lesser the individual stability is [Here a high mortality rate is compensated for by a high fertility, as it is the case in many animals and plants]. The individual stability is determined by the degree of independency of the environment, that is the autonomy, of the organism with respect to the environment. The concept of autonomy with respect to the environment is, as is known, very close to morphofunctional progress. Thus, the fertility in conditions of an equivalent environment in a certain degree expresses the level of morphofunctional progress."
All this will be applied to the special case of Hymenoptera, which differ from all insects, and from most higher animals for that matter, by the fact of their males being haploid, meaning that they develop from unfertilized eggs, and having thus only one set of chromosomes, implying that there can be no dominance of one gene over another homologous one, in turn meaning that a given genetic mutation cannot 'hide' itself in a recessive gene and thus not getting expressed. Also important is the fact that in Symphyta only 1/3-1/4 of the individuals of a population are males. The result of all this will be that in Symphyta (and all Hymenoptera) there is not always an evolutionary answer to a changing environment, because such an answer is not necessary. Hymenopterous species are relatively insensitive to environmental changes because of their complex and flexible b e h a v i o r. [The haploid condition of the males in Hymenoptera is also a condition, albeit not a necessary one, to develop social life. And indeed, the social Hymenoptera (but also termites) largely create their own environment, making them in a high degree independent of the [already] existing environment.]
Lepidoptera (butterflies and moths) and Symphyta (lower Hymenoptera, here, mainly, Tenthredinidea) are very similar ecologically. Both are chiefly living-in-the-open phyllophags (leaf-eaters), and in both groups forms appear that [as larvae] make mines in the tissues of a leaf, roll-up leaves, making cobwebs, galls, feed inside stalks and fruits, etc.
Larvae of Tenthredinidea and caterpillars of Lepidoptera are habitually very similar. The origin and bloom of both groups is strongly connected with the appearance of the angiosperm plants. The Lepidoptera, are, it is true, represented by a significantly large number of species -- in the order of 100000, whereas of Tenthredinidea there are about 5000 of the about 5500 species of Symphyta. The general structural variability of Lepidoptera (adults as well as larvae) is, apparently, lower than the morphological diversity of the Tenthredinidea. It is true, the ecological plasticity of the Lepidoptera is greater -- among them there are fairly many inhabitants of the soil, there are predatory and aquatic forms, not present in the larvae of Symphyta, and, finally, they are frequent in all climatic zones, whereas the Symphyta are chiefly connected with the northern moderate zone. But the numbers of both groups, at least in the moderate zone, seem to be comparable. To this bear witness, for instance, the results of the around-the-clock mowings, done in the years 1965-1967 by Tjernov and colleagues. According to the data, which Tjernov kindly handed over to the author, larvae of Tenthredinidea are, in number and biomass, several times more abundant than caterpillars of Lepidoptera in various biotopes of the tundra of Taimyr [northern Siberia] and in open living-stations around Moscow. Not far from the forest belt and around Moscow, and in the Strjeletsky steppe, the quantity of both groups is about the same, and only in the open steppe caterpillars turn out to be several times more numerous than are the larvae of sawflies. Thus, in the moderate zone these groups reached a comparable level of biological progression, by, however, different methods, although they occupy practically the same ecological niche. The difference consists first of all of the number of species inhabiting one and the same territory, and of fertility. Sharp (1910), for instance, gives for England 2000 species of Lepidoptera, and Benson (1951-1958) gives just over 400 species of Tenthredinidea (460 species of all Symphyta). In the Moscow region are, according to verbal communication with Antonova, found about 1400-1500 species of Lepidoptera, whereas, according to data of Prochorova, also not published, about 370 species of Symphyta [are found], of them about 320 species of Tenthredinidea, that is, also 4-5 times less than Lepidoptera.
Fertility in Symphyta is estimated by Benson to be on average 60 eggs, with a maximum of 1000 eggs in Urocerus (Siricidae) and a minimum of 16 in Acantholyda (Pamphiliidae). Similar data are obtained by Iwata, 1958, in the autopsy of a large number of representatives of the Suborder. Here the fertility, being over 200 eggs, is characteristic only of Siricidae and Xiphydridae, wherby an especially high number of eggs of the former, close to 1000, is clearly connected with their 2-3 yearly life-cycle. It is, moreover, interesting that among the remaining Symphyta a higher fertility (up to 100-150 eggs) and a regular development of several generations in the course of the year are characteristic chiefly of the higher representatives of the Suborder -- Tenthredinidea (some Tenthredinidae, Diprionidae) and the Siricidea. On the contrary, the more primitive Symphyta (Xyelidae, Megalodontidea, Cephidae, Blasticotomidae) usually possess a very low fertility in the order of 20-50 eggs, and only one generation in the year. The author found 30-40 eggs as a result of autopsy of three females of Xyela julii Bréb. In the extremely specialized Orussus the fertility is also very low.
In the Lepidoptera the picture is, generally, turned round -- the highest typical number of eggs is apparently of the order of 500-1000, whereby the highest fertility is characteristic, according to Mell, 1940, of the more primitive ones (especially Hepialidae), and the lowest fertility of the higher ones (Rhopalocera and others).
Clearly, the Micropterygidae form an exception to this rule. They lay in total about 150 eggs. Anyway, these are completely special insects, not seldom placed in a separate insect Order Zeugloptera.
In addition, it is, apparently, more characteristic of Lepidoptera to develop several generations a year, than it is of Symphyta, which still more emphasizes the difference between them in this respect.
As regards the large number of species of Lepidoptera, it may, in addition to explaining it with their greater ecological plasticity, be explained by the presence of an effective and unstable apparatus of reproductive isolation in the form of diverse and often clearly colored wings of the butterflies, and of a strongly developed system of odorous hairs producing sex-attractants -- volatile species-specific substances, guaranteeing the meeting of the sexes. Indeed, let us consider two populations for some time having been geographically isolated and here having diverged such that in the next contact [of these two populations] caused by the destruction of the geographic barriers, interbreeding between them turned out to be possible, but the viability of the hybrids had decreased somewhat. As a result of the contact of the populations, exchange of genes must begin and, consequently, their assimulation [i.e. the two populations becoming more similar], decreasing the negative effect of interbreeding. However, because this effect is not yet over, also a selection of adaptations will take place that impede interbreeding, a selection gradually weakening with the degree of assimilation of the [two] populations. [We see, how lightly the neodarwinian mechanism of genetic mutation and natural selection is taken for granted. As if it were clear as to what precisely these "adaptations impeding interbreeding" actually are, and how they become selected for. At least much time would be needed for evolutionarily developing such (genetically determined) adaptations, if they can be so developed at all.]. The result, with equal initial viability of the hybrids, depends on the ease of the appearance of effective isolating mechanisms. In cases of the latter's quick origination, the exchange of genes may slow down - such that the assimilation of the populations [involved], caused by it [i.e. by the exchange of genes], will have been fallen by their [i.e. the populations'] divergence evoked by initial differences of ecology and by genetic-automatic processes [genetic drift]. [When there are no effective mechanisms that reproductively isolate, say, two, populations from each other, the latter will interbreed resulting in the populations to become more similar to each other. When, on the other hand, such isolating mechanisms do arise, the populations will diverge from one another (by genetic drift and other causes) ultimately resulting in their becoming two different species]. This leads to a complete separation of species. In a more slowly proceeding development of mechanisms of reproductive isolation, on the other hand, the exchange of genes may succeed in eliminating the difference between populations and increase the viability of hybrids up to the norm before isolating mechanisms become somehow effective. As a result the selection against interbreeding vanishes, the isolating mechanisms become eliminated, and the populations become united. It is clear that in these conditions in which populations of Lepidoptera succeed to split up, a merging of populations may for the Symphyta be more probable. It is possible that with this is partly connected the higher diversity of Lepidoptera. As another cause may turn out certain features of the genetics of the Hymenoptera making a large population size especially fortunate to them (see below).
A very important characteristic of the organism is its fertility. It expresses the mortality of the offspring because it is known that the average many-year significance of the number of individuals in populations compared with rates of multiplication, changes slowly and may be reckoned as approximately constant. This means that from the number of offspring of each pair of organisms, independent of their fertility, on average only one pair succeeds in leaving behind offspring. Because in all organisms in each generation all offspring perishes except for two individuals, the fertility first of all expresses the average mortality of the species, that is, the ability of its individuals to withstand external influences. In conditions comparable to the level of complexity of the environment, the fertility, consequently, expresses individual stability of the organism, its ability to counteract environmental factors, whereby the fertility is higher the lower the individual stability. The latter is determined by the degree of independence of, or [equivalently] the autonomy of the organism with respect to, the environment. The concept of autonomy with respect to the environment, is, as is known, very close to that of morpho-functional progress.
We call this form of progress "morpho-functional" because morpho-physiological progress was much too closely connected by Sewertsov with energetic processes ("energy-level of life-activity"), which, apparently, are weaker correlated with progressive evolution than is the degree of autonomy of the organism from the environment. The most essential method of reaching such autonomy is, as is known, the development of the nervous system -- one of the most evident features of progress -- and other paths of perfecting of regulative abilities of the organism.
Thus, the fertility, in conditions of an equivalent environment, expresses in a certain degree the level of morphofunctional progress. At the same time, different ecological niches have the tendency to become equivalent in this sense. Indeed, when one or another niche is more favorable and guarantees an easier survival as compared with a neighboring one, then the ability of organisms to migrate and to change leads, after sufficient time, to the intensive colonization of that niche. As a result, the enlargement of the biotic environment, consisting of factors, compensates for the insufficient stability and tension of the earlier having existed connections of the members of the coenosis [living community] with each other and with the abiotic environment, and the niche becomes equivalent to others, [and] consequently, inaccessible to an intensive colonization [because many members of the initial colonization will spill over to other equivalent niches]. Thus, ecological niches-and-biocoenoses as a whole, may be represented by a system of communicating vessels, in which the role of the connective tubes is played by the ability of the organisms to evolve and migrate, while the role of gravity is played by natural selection. Because the conditions on the Earth uninterruptedly change, and because migration, and especially evolution, demand a significant amount of time (the volume -- contents -- of the "vessels" is variable, and the let-through capacity of the channels connecting them is low), in individual niches significant temporary deviations from the average level are possible sometimes. Specialized inhabitants of such not long lasting (measured in terms of evolution) niches may have a low fertility, [such inhabitants] apparently not being progressive, but also the living-span of such groups will be short. If, on the other hand, we orientate to relatively long-living groups, especially to taxa of higher rank than a species, then the ecological niches may be reckoned as being approximately equivalent, and fertility may be reckoned as to be an expression of the level of morphofunctional progress.
It is interesting that decrease of fertility is not only a result of morpho-functional progress, but also may contribute to biological progress. As was shown by Victorov (1967), a decrease of fertility eases the regulation of the number of individuals, making possible to reach the necessary change in density of the population, and [eases the establishment] of a lower variability of mortality [i.e. keep it more constant].
In comparing fertility of different organisms we should take into account that to this purpose one must calculate the number of offspring not of one female, but of one individual in the parental population, or, what is the same, the number of females in the offspring of a single female. For this overall number of offspring it is necessary to multiply it with the portion of females in the population. In taking account of this factor, the difference in fertility between Lepidoptera and Symphyta becomes a little less : In Lepidoptera males usually are not less numerous than females. In Symphyta, on the other hand, they, on average, make out about 1/3-1/4 of the population. Further, fertility must be determined per unit of time, and not per generation, such that in the presence of a number of generations in a year the fertility of each one of them must be added. Lepidoptera, apparently, more often give several generations a year than Symphyta do, such that taking this factor into account rather enlarges the difference in fertility.
What has been said above, allows us to conclude that in comparable levels of biological progress of Lepidoptera and Tenthredinidea, in the latter this progress is reached as a result of increase of morpho-functional progressivity. Therefore it is relevant to ask the question of the concrete progressive adaptations. Some of these adaptations guarantee, apparently, a relatively low mortality of eggs. Indeed, many authors have found this mortality of eggs of Tenthredinidea to be within 0-25 percent, whereby in some of these cases a low mortality of eggs is observed even with a decrease of the overall number of individuals. On the other hand, in Lepidoptera, in the egg-stage, often more than half of the offspring perishes. Known are, it is true, individual indications also of negligible mortality of eggs of some Lepidoptera, and of increased mortality of eggs of Tenthredinidea. However, much data about significant mortality of eggs of the latter belong to moments of sharp drop of the number of individuals in the population : The calculations, given by, for example, Geri, Dusaussoy, 1966, and Dahlsten, 1967, bear witness to the fact that in these cases in the offspring of each female survives up to the adult stage on average significantly less than one female. In this, the data of the last mentioned author testify that mortality of eggs in the highest degree correlates with the intensity of decrease of the number of individuals : With mortality of eggs of 14.5, 18, and 34 percent, the egg-production of the next generation was respectively 31, 23, and 18 percent of the total fertility of the previous generation. Other analogous cases of high mortality of eggs, also, apparently, may be connected with a drop in the number of individuals in the population. Therefore, it is very likely that in Tenthredinidea, in conditions of the population in equilibrium, mortality in the egg-stage is on average less than 20-30 percent of the total mortality. Such a low mortality of eggs may be partly caused by the [albeit still] primitive form of care for the offspring in these insects, expressing itself in oviposition inside the tissues of plants, in conditions of constantly maximal moisture, in the total protection of the eggs at least against predators, and in the possibility of growth of the germ in the egg, in addition to using the yolk, by sucking-in liquid from around the egg. It is true, that this does not explain the causes of the often insignificant (up to 5, more rarely 15-20 percent) mortality of eggs in Pamphiliidae. This family is ecologically close to the Tenthredinidea, and their eggs also are able to increase their size at the expense of saps of plants. However, they are oviposited in the open on the surface of plants. Therefore it seems totally unexplained how the Pamphiliidae manage not only to have a low mortality of the eggs laid in the open, but also to have the very possibility of their existence with an extremely low fertility (on average in the order of 20-40 eggs), and with a two-three annual generation in a part of the population in many species.
In many Tenthredinidea the mortality is relatively low also of their young larvae, often not more than it is of fully-grown larvae, and especially of pre-pupae and pupae in the cocoons. In Lepidoptera, on the other hand, the mortality of young caterpillars not seldom often is very high. An increased stability of young larvae of Tenthredinidea, also being characteristic of higher organized animals, may be explained by the possibility of increasing the size of their eggs by having their number decreased [that is, at the expense of their number]. Very important is the fact that here it was succeeded to accomplish a more significant decrease of their number [the number of eggs produced] (and the increase of their size) than it would have been possible merely as a result of decreasing the effective fertility (that is, the fertility, calculated by taking into account the interrelations between the two sexes [meant is, apparently, the ratio of the two sexes] and the number of generations. Indeed, in Hymenoptera the necessary number of males in the population is strongly decreased, that is, the effective fertility (the number of females in the offspring per unit of time) is decreased significantly less than the absolute fertility. Decreasing the necessary number of eggs that develop into males, made it possible for the Hymenoptera to supplementarily enlarge the portion of the remaining eggs.
The change of the sex ratio took place here totally in line with the hypothesis about the function of males [of Hymenoptera, but also of males generally], proposed by Geodakjan (1965). According to the latter the males are engaged in the function of being the main channel of the connection between selection and genotype [natural selection and genome] of the next generation : being more variable than females, they take up the main strike of the selection, because the latter most strongly eliminates extreme variants of the individual variability. Therefore, whatever change of the environment, first of all has its effect on the more variable males, rearranging in this or that direction the genes in their genomes. In the process of mating and of subsequent multiplication the genotypical changes of the males are passed on to the offspring and the selection regulates the genome of the latter, almost not affecting the females and not decreasing with this the overall fertility of the population.
The mechanisms of increased variability of males is, in the majority of cases not known. However, the Hymenoptera are in this respect a lucky exception. In contrast to the majority of other insects, their males develop from non-fertilized gametes and are [thus] haploid. Thanks to this, in them the effect of dominance is absent : Any mutation fully expresses itself, not choked by the action of the other genes homological to it. Therefore, all mutations are expressed in the beginning in males of the Hymenoptera, and only in the very case when selection allows here their significant concentration, these mutations may express themselves in females too (we are here speaking of recessive and semi-dominant mutations, making up, as is known, the majority of mutations). Even within the range of those variants that have survived selection, males are more diverse than females, of which a significant part of the genotypical variability is hidden by dominance, and the males first of all become subjected to the selective elimination in the case of change of living-conditions. Thanks to this, the males of the Hymenoptera with great intensity carry out their function of a channel of connection between environment and genome of the next generation, at the same time being a powerful barrier, protecting the females against the action of selection, and, consequently, strongly decreasing their mortality. Precisely therefore, the number of males could be lowered without impeding their functions.
In Lepidoptera things are the other way around. In them the males are not only diploid but also homochromosomic, that is, the sex-chromosomes of the males are equal, whereas in females they are different, in contrast to the majority of other animals. As a result, not the males, but the females are 'haploid' in two chromosomes (X and Y), and, consequently, more diverse in features controlled by them.
In addition to the ability to change into the proper direction the sex ratio, the haploidity of males gives the Hymenoptera still more advantages. First of all it automatically creates a negative reverse connection between the sex ratio of coming generations, keeping it at its optimum. Indeed, a deficiency of the number of males leads to the fact that the females in large quantity begin to lay unfertilized eggs from which males come forth. In the next generation the number of the latter increases, but when this increase becomes excessive, the females receive an abundacy of sperm and the number of unfertilized females strongly diminishes. As a result, the number of unfertilized eggs will decrease, and, consequently, the number of males.
Being a powerful filter against unfavorable mutations, the haploidity of the males of Hymenoptera also effectively keeps their variability within the limits of the optimum, lowering the mortality of the chief part of the population -- the females -- by preventing the isolation of unfavorable mutations in them. However, this effect of the haploidity of the males is useful only in stable conditions : The powerful filter, eliminating any unfavorable mutation whatsoever, strongly diminishes the stock of variability of the population. In a normal diploid population the equilibrium frequency of an unfavorable recessive mutation to occur is signified by 1-q , which is known to be equal to Square Root [u / s] , where s is the coefficient of selection, that is, the difference between unity and the ratio of mortality of mutants to the mortality of normal individuals, and u is the frequency of appearance of the given mutation, [that is,] the frequency of mutation of the gene into the given direction. In a completely haploid population, where the sex process is absent or where the diploid phase is brief, 1-q = u / s , that is, usually 2-3 orders lower, than in diploids. In Hymenoptera with their haploid males 1-q = u / s . 1 / k , where k is the proportion of males in the population. Consequently, the frequency of a recessive mutation in them is in total 3-4 times greater than in haploids (because k = 1/3 to 1/4), which only insignificantly brings the Hymenoptera closer to normal diploid organisms. Such an impoverishment of the hidden stock of variability in a population of Hymenoptera must decrease their evolutionary plasticity, hamper in them the process of adaptation to changing conditions. Constant haploids, usually belonging to more primitive groups (unicellular organisms, lower plants, etc.), in such conditions compensate their small store of variability by accelerating the circulation of genes -- by increasing the rate of reproduction (fertility and the number of generations), resulting in the fact that the number of mutations appearing per unit of time increases. The fertility of Symphyta is, as we saw, relatively very low, and it can hardly be compensated for by a certain increase, as compared to Lepidoptera, of the population size (a lower number of species with a comparable overall frequency of individuals). If in such conditions the Tenthredinidea may turn out to be a blooming group [which it is], then this bears witness of their high individual stability even in a variable environment, that is, it shows the high degree of autonomy with respect to the environment of these insects. It is hard to imagine that such a stability, such a "reliability", of the organism, could have been guaranteed merely by the enumerated forms of primitive care of the offspring, especially by the concealed development of the eggs, by the possibility of their growth, and so on. We here have, apparently, to do with some other, until now not sufficiently known, factors, perhaps with the great diversity of adaptive b e h a v i o r, with the high organization of the nervous-system, the development of which had such a great significance in the evolution of the higher Hymenoptera of the Suborder Apocrita [that is, Terebrants (= parasitic Hymenoptera) + Aculeata (= sting bearers, such as (higher) solitary true wasps, social wasps, ants, solitary and social bees). Indeed, among the Tenthredinidea there exist forms that not only lay their eggs into a protected place, but also guard over them and over the larvae hatched from them, for example Perga, Dielocerus, Pachylota, and others. With this, concerning Perga affinis Kby, Carne, 1962, describes extremely complex forms of behavior of the adult insect and its colonial larvae (meticulous preparation of the place of oviposition, mutually corresponding actions of the larvae with an uninterrupted exchange of information between them, a highly developed ability of visual and other orientation, the presence of constant "leaders" in columns of migrating larvae, and so on). Some less clear, but essentially similar adaptations are demonstrated, apparently, also in other Tenthredinidea. Thus, for many of them, coloniality of the larvae, the diversity of active ways of protection all the way up to ejecting over a significant distance of various poisonous liquids, and so on, are characteristic. It is possible that an advanced development of the nervous-system indeed is typical of the representatives of the superfamily. But for solving this question special investigations are needed.
Above, we spoke largely about Tenthredinidea. The Megalodontidea are ecologically very close to them, and all what had been said about the first superfamily possibly also applies to the second. The ecology of the larvae of the remaining Symphyta is close to the larval ecology of beetles (Coleoptera). Of course, these forms are relics and cannot be compared with the level of biological progress of beetles. However, it is interesting that as to the methods of adaptation, guaranteeing the Xyelidae, Cephidae, and the Siricidea the ability to persist in the presence of such severe concurrents, to these Hymenoptera correspond only the higher Coleoptera. Thus, the Ipidae and Platypodidae, similarly to Siricidea, specially sow the substrate, on which their larvae will develop, with symbiotic fungi. The Attelabidae, similarly to Cephidae, process in a determined way, living plants, significantly changing their features into a direction favorable for the development of the larvae of these insects. In male pine cones together with larvae of Xyela the author found only larvae of the snout-beetle Anthonomus varians Pk.
As to the most specialized representatives of the Symphyta, the Orussidea, their ecological analogues can only be found among such highly organized insects as are the parasitic Hymenoptera.
In this way, the Symphyta have reached a relatively high level of biological progress as a result of morpho-functional progress. In this, the great individual stability of these insects is, apparently, caused by the diversity and complexity of adaptive behavior, making it possible for each individual organism to survive in wide ranges of variation of the environment. As a reult, the population may not be subjected to selective annihilation, and, consequently, may not evolve in answering to such changes.
Intensive increase of the diversity of methods of individual adaptation is, apparently, characteristic already of the first evolutionary stages of the Hymenoptera. This adaptation, allowing for lowering the necessary rate of the evolutionary reply to changed conditions, possibly is a protective reaction against the impoverishment of the hidden stock of variability and [against] the decrease of the evolutionary instability [= decrease of the evolutionary plasticity] of the group, caused, as we saw, by the haploidity of the males of the Hymenoptera.
All what has been said about the Symphyta still more applies to the Apocrita, the higher Hymenoptera, which have remarkedly further progressed along the path begun by the representatives of the first Suborder. The individual stability of these insects not seldom is remarkedly higher (and the fertility lower) than it is in Symphyta, in connection with the extraordinary complexification of their behavior, surpassing all what has been accomplished by invertebrates and lower vertebrates. With this problem many, often substantial, works deal.
Rasnitsyn here concludes that the Hymenoptera possess less evolutionary plasticity than most other insects do. Any given hymenopterous species of the Symphyta, and especially of the Apocrita, is less sensitive to environmental changes because of the high development of their nervous system. But, of course also the nervous system has developed evolutionarily, becoming more and more versatile and complex during the evolution of the Hymenoptera (as we saw in our treatment of Malyshev's view of the developmental evolution of their instincts). So it is evident that Rasnitsyn mainly refers to the decreased morphological plasticity of the Hymenoptera.
So we have now prepared sufficiently the study of fossil Symphyta (lower Hymenoptera), of which many are found in mesozoic sediments. As it is the case with almost all fossil insects, those parts of the body which are best preserved in fossils are the wings (wing impressions), especially the forewings. Therefore, we will concentrate on these organs, and especially on their venation, in order to understand the evolutionary processes, and we will see whether we can find support for our noëtic theory of evolution. And for fossil Symphyta we will again rely entirely on the data published by Rasnitsyn (1969).
Evolution of the wing-venation in the lower Hymenoptera (Symphyta)
Theoretic priliminaries
Toward the middle of Part LXd (end of noëtic intermezzo) , from the Section "Projection into the Explicate Order" onwards, we had found out that the wing-venation in insects is largely a non-functional structure, meaning that it is a 'formal' morphological pattern in insects, not associated with the execution of one or more functions or biological tasks. Of course things as the relative size of the wings, their shape, their number, the coupling of hind- to forewings, the existent folds and hings in the wing-membrane, and also the gross-features of their venation, do have functional significance, that is, do have bearing on the particular flight-regime of the insect, but the details of the venation such as the precise course of the main veins, the number of their branches, the presence or absence of cross-veins, etc. do not have such significance. Therefore, these details do not belong to the strategy-proper of a given species. And whereas true strategies do come directly from the Implicate Order by the phenomenon of projection, these venational details, or, better, their transformations, originate in the Explicate Order. Therefore these details may change at a high rate (but not necessarily so) because even a relatively great change of them will not affect the biological functions of the insect. But, as we must conclude from the study of the wing-venation in insects, these changes must somehow remain within the venational type set by the given insect Order.
Let us reproduce two diagrams given in Part LXd of the present Series (there, Figures 7 and 8) depicting, in a general way, venational change in the Explicate Order in the course of time :

Figure 4 : Diagram depicting the consecutive first projections (first red vertical line in the one, and first red vertical line in the other species), and the continued alternation of projections and injections, of two different but related insect strategies A and B.
The one venational character v (as such not belonging to the strategy sensu stricto) changes its state in both species as time goes by, resulting in character sequences symbolized by consecutive numbers. So we get two transformation sequences : v1, v2, v3, v4, ..., v41, ... and v1, v2, v3, v4, ..., v16, v17, ... .
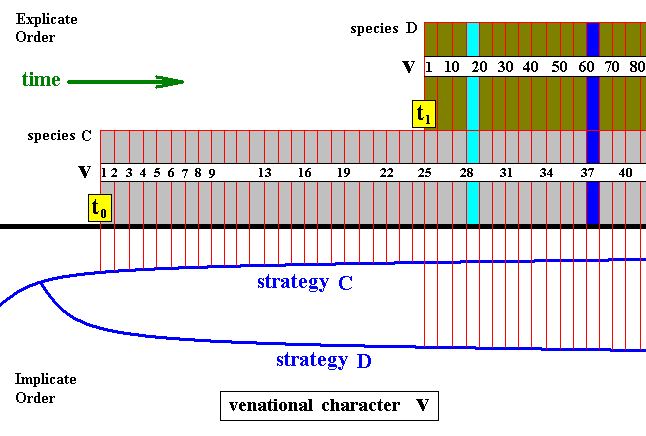
Figure 5 : Diagram, as the above diagram, depicting the consecutive first projections, and the continued alternation of projections and injections, of two different but related insect strategies C and D.
The one venational character v (as such not belonging to the strategy sensu stricto) changes its state in both species as time goes by, resulting in character sequences symbolized by consecutive numbers. So we get two transformation sequences : v1, v2, v3, v4, ..., v41, ... and v1, ... v10, ... v20, ... v30, ..., v40, ... v50, ... v60, ... v70, ... v80, ... .
If we now consider these two species at the time indicated by the l i g h t blue column, we see that, with respect to the development of venational character v , the later-projected species D still lags behind the earlier-projected species C . But when we consider them at the later time indicated by the d a r k blue column, we see that species D has catched up with species C , and even has progressed beyond it. So when looking at these two species at this later point in time, we see that, with respect to venational character v , species D is more advanced than species C and thus is the "derived species". And this is compatible with its being younger (if we think of the conventional view that the younger species is expected to be more advanced in its characters than is the older species).
In order for the reader to recall the general structure of the wing-venation in insects, that is, the general scheme of the eight main-vein systems (of which the last one, the Jugal system, has often vanished, leaving seven main-vein systems), traceable in all (fossil and recent) winged insects, we here reproduce Figure 1 + subscript from Part II of the present Series on evolution :
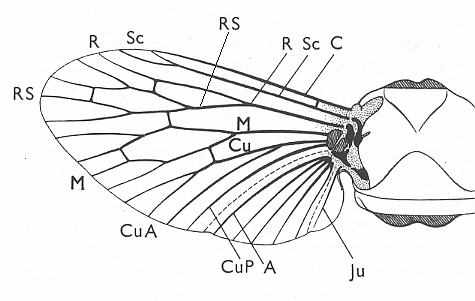
Diagram above :
Generalized wing-venation of insects, showing the eight venational systems :
The wing itself is membranous, while its veins are hollow thickenings supporting the flexible wing-membrane. The external outline of the wing (its shape), its absolute size, its relative size, its [if it is a fore wing] coupling to the hindwing [if present], the presence of hairs on its membrane, veins or margins, and certain structural details of its venation, are supposed to determine the aerodynamic properties of the wing. As to the majority of the structural details of the wing-venation, this is not so sure : These venational details might be totally non-functional.
Within certain taxonomical groups the venational pattern is remarkably constant.
The main veins generally connect with the insect's thorax mediated by certain sclerites that form something like a hinge. The wing is moved by the flight muscles of the thorax. Often, that is, in certain groups of insects, the wing-beat frequency is very high, up to 500 or more wing-beats per second.
Something more must be said about the wing-venation in general in the context of our noëtic theory of evolution. With this, the reader should bear in mind that this theory was developed by us step by step in the Series on the evolution of insects, that is, from Part II all the way up to the present document (Part LXVI). And its development is still far from being concluded. This means that in earlier Parts of the mentioned Series [Part II-LXVI] things had been proposed that might -- in the light of the newest findings of ours in the later Parts of this Series -- have become more or less obsolete. But because our noëtic theory is still in its 'experimental state', those earlier propositions still do retain their theoretical value. All this is especially the case with the general status of the wing-venation in insects. Let us consider this general status.
In our noëtic theory of evolution we have said that all noëtic patterns (pure Forms), existing in the Implicate Order, have an inherent tendency to acquire ontological completion, meaning that they become individual material beings in time and space. They acquire this ontological completion by being "projected" from the Implicate Order into the Explicate Order. Many such patterns are already such that they indeed can as such exist in the Explicate Order, and so they are spontaneously projected into that Order. However, many other noëtic patterns, especially the complex ones, can only exist in the Explicate Order when they themselves are strategies that prescribe how to exist in the Explicate Order. And upon projection they appear in the latter Order as organismic species, in fact as a multitude of individuals of each such a species. So any such organismic species appears in the Explicate Order as a materialized strategy. Its physiology, morphology, behavior, and reproduction are all part of such a materialized strategy. And this implies that all the characters of such a strategy are truly functional characters. And indeed, these characters ultimately come from the Implicate Order. And upon projection they constitute, what we had called, the "strategy sensu stricto" of an organism in the Explicate Order. But this further implies that all intrinsically non-functional characters do not come from the Implicate Order. They are the result of actions and influences prevailing in the Explicate Order. And because they are non-functional they follow laws of transformation of their own, that is, they can evolutionarily change according to their own laws. In the case of the mentioned multitude of non-functional details of insect wing-venation, they can evolutionarily change freely, in contrast to truly functional characters whose change may quickly lead to the destruction of their function. But, as has been said, these non-functional details of the wing-venation may change only within the limits of the venational type of the Order to which the given insect species belongs. It must remain morphologically compatible with this type. And, further, it may not be such that it will damage or destroy the overall function of the wing. But within these limits the venation can and does change, following its own rules of transformation (which may be different, as to their details, in the different special Oder-dependent types of wing-venation). In this way we must understand the above diagrams of Figure 4 and 5 of venational change . And because the flexible membrane of a medium or large insect-wing must be strengthened by at least a small number of longitidinal veins, in order for it to function properly as an organ of flight, the presence of at least a primitive venation is itself a functional character and as such belongs to the strategy-proper. So when a given insect strategy is projected into the Explicate Order, appearing there as a particular insect species, and when the individuals have medium or large wings at all, these wings possess a venation. This venation first of all complies with the general and original venational scheme of the subclass Pterygota (winged insects), consisting of the seven or eight main-vein systems [where "complies with" here also includes all cases of wings with only a few veins, a state interpreted as being the result of extensive reductions of the original venational scheme]. Secondly, it complies with the typical venational plan of the particular Order to which the given insect belongs. With this, all the functional structures of the wings and their venation are thus given from the outset : Winged insects, continually appearing as individuals in the Explicate Order, from the outset possess the full set of functional characters and structures of their wings (including any devices to fold and stabilize the wings when at rest). All subsequent venational changes, on the other hand, insofar as they are truly non-functional, take place in the Explicate Order. We have diagrammized this in the above diagrams . There, concentrating on venational characters and their transformation, we said that upon the first projection of the strategy (resulting in an insect species) the original venation of that strategy co-appears, and that then, as time progresses, that is, as the alternation of projections and injections progresses, the venational characters are more or less slowly transformed. And upon projection of a second, related, insect species, the latter again starts with the venational scheme belonging to the (its) strategy (and, as in the first species, compatible with the typical venational plan of the insect Order), and again this venation is gradually transforming as time progresses. We have expounded that such cases, where they happen, explain the fact that sometimes a later [as to its origin] species cannot, as to its wing-venation, be derived from an earlier [as to its origin] species. But often, later species can, as to their wing-venation, perfectly well be derived from earlier ones. And this is the case where the transformational series of the later species catches up with that of the earlier one (because it happens to change faster in this respect). However, this is not the only cause of the fact that later species can, as to their venation, be derived from earlier ones : When the original wing-venation of a first projected insect species has been transformed during a certain period of alternating projections and injections, the end-result [that is, the result-achieved-up-to-then] may be directly taken over by the wing-venation of a related species that is for the first time being projected just at the time when the mentiond end-result of the first species was achieved (and, subsequently, injected). This is possible thanks to the phenomenon of injection (always following projection) : The end-result of the transformed wing-venation of the first species is, following upon the continuous series of projections and injections, injected, and may then noëtically influence the original venational structure of the second species bound to be projected. If we horizontally stretch the diagram of Figure 5 a little (separating everywhere the injection from the previous projection) we can depict what we had just said, by giving a small but relevant section of it (and taking different strategies -- E and F -- that branch a little differently than C and D in Figure 5) :
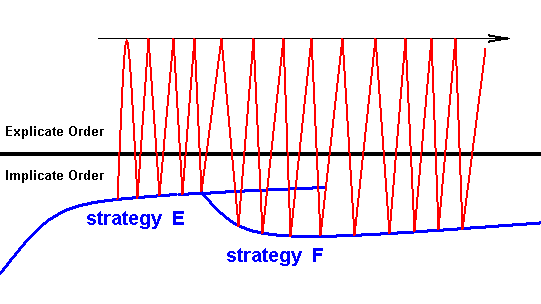
Diagram above : The continuance of evolutionary transformation of the wing-venation from one insect species to the next.
An insect strategy (E) has been projected (and then emerging as an insect species), followed by its continual projection and injection (red zig-zag lines). Later, a second, related, strategy (F) is being projected (emerging as a second -- later -- insect species).
Now, the 'last' I N J E C T I O N of the first strategy (carrying with it, among other things, the last-obtained result of the venational transformation in this strategy) noëtically interferes with the starting-venation of the second strategy-to-be-projected, resulting in the continuance of the series of venational transformations begun by the first species.
We maintain that such a 'take-over' can only occur between closely related strategies. If the second strategy is not so closely related this will not happen, And even when they are closely related, a take-over will not necessarily follow.
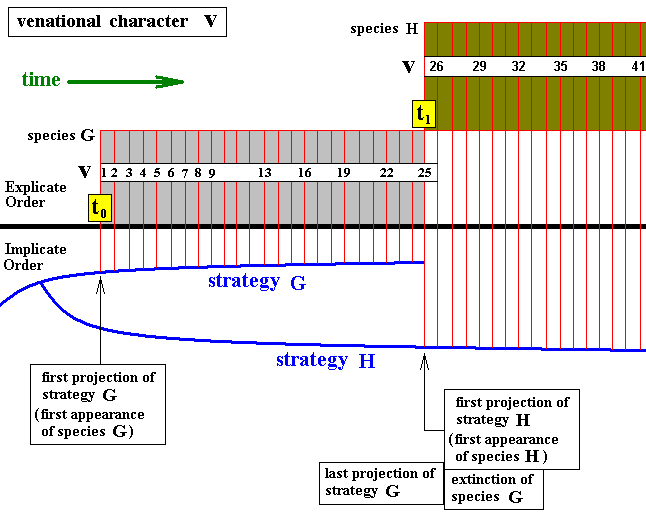
Diagram above : The continuity of evolutionary transformation of the wing-venation from one insect species to the next, in terms of Figure 5 above.
The series of incremental venational character transformations, symbolized by a number-sequence, begun in the first species (G), is continued in the second species (H), resulting in the single transformational series 1, 2, 3, 4, ... 36, 37, 38, 39, 40, 41. (or, if one whishes, v1, v2, v3, etc.)
Here it is supposed that, upon the emergence of the second species, the first species became extinct.
It is also possible that a given insect species splits up into two new species, both of which remaining in existence (until the next branching), and where in one of them the original series of venational transformations is continued into the same (morphological) direction (as we had it in the previous case), whereas in the other (new species) the subsequent series of venational transformations heads into another (morphological) direction. In the next diagram we indicate the fact of one transformational series heading into a new direction by coloring the band of consecutive numbers symbolizing these venational transformations :
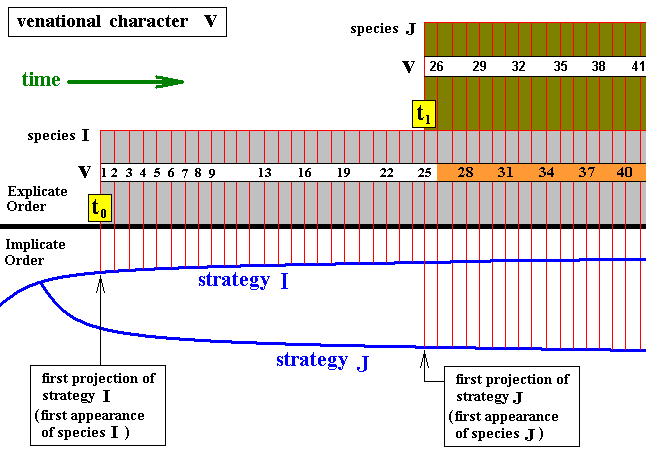
Diagram above : The continuity of evolutionary transformation of the wing-venation from one insect species to the next, in terms of Figure 5 above.
The series of incremental venational character transformations, symbolized by a number-sequence, begun in the first species (I), is continued in the second species (J). The species I remains existing (alongside species J), but the series of venational transformations takes, in I, another (morphological) direction (symbolized by the orange color of the band of numbers).
Possibility of re-appearance of an extinct species.
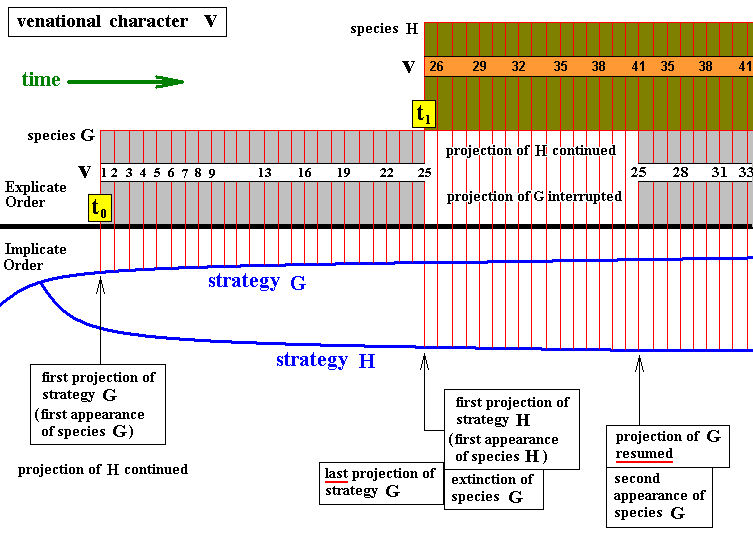
Diagram above : Things as in the previous diagram, but now with species G re-appearing in geological history, after having been extinct for some longer or shorter period. Upon resumption of the projection-injection series, the sequel to the series of venational transformations in this species picks things up where they were left when the projection-injection series was terminated.
So, on the basis of these suppositions we can expect an evolution of the wing-venation in insects that is more or less synchronous (but branched) with the successive (but also branched) appearance of new insect species as geological time progresses. Not excluded, however, is the re-appearance (in the Explicate Order) of (very little changed) extinct species ( The conventional interpretation, on the other hand, is that the species [or some higher taxon] in question had continually persisted all the time).
The evolution of the wing-venation in Symphyta (lower Hymenoptera).
In the light of all this we will now consider the details of the wing-venation as it is found in Symphyta from the upper Triassic up to the present time. In all this we should realize that the number of fossils found are negligible as compared to the number of Symphyta actually living at the time. Further, small venational details often can be seen only with great difficulty or not at all. So small errors in the drawings of these fossils are not expected to have been avoided.
In order to see what actually has happened with the venational structure in the Symphyta in the course of time, we must depart from a venational structure that is considered to be the starting-condition in this Suborder (which condition itself is already a much derived state with respect to the starting-condition of the venation of all insects (that is, the wing-venation of the first winged insects)). I am sure that we cannot precisely know the starting venational condition in Symphyta. Therefore, we do not pin it down to one particular, allegedly most primitive, venational scheme, but mix things a little up. For this we will depict some, allegedly primitive, venational schemes given in the literature :
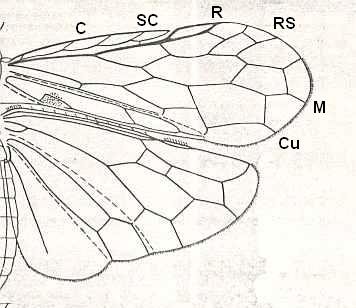
Figure 6 : Wings of the reconstructed ancestor of the Symphyta (see Figure 2, above). As to the forewing : The vein, running along the anterior part of the wing-margin until the break, is the Costa (C). Directly posterior to it lies the Subcosta (SC), also running until the break and giving off several anterior and one posterior branch. The strong vein after it is the Radius (R), terminating well before the wing tip. From the Radius branches off the Radial Sector (RS), itself giving off some branches or cross-veins. It and its branches also terminate before the wing tip. Posterior to RS lies the Media (M), which is a single vein, terminating just after the wing tip. And then after it runs the Cubitus (Cu), in fact CuA, the anterior cubitus, terminating at the posterior wing margin, well after the wing tip. After the Cubitus (CuA) lies a weak vein or fold, the posterior Cubitus (CuP). The veins posterior to the CuP are the anal veins (A), of which there are three. But only the first analis is a strong straight vein, while the other two are partly reduced and difficult to identify.
The venation of the hindwing is more or less the same as in the forewing, but different in the anterior, anal and jugal regions [the Jugal region comes after the Anal region]
The next Figure is the same as the previous one, but now taken from RASNITSYN, 1980, in which he has added the interpretation (naming) of veins and cells.
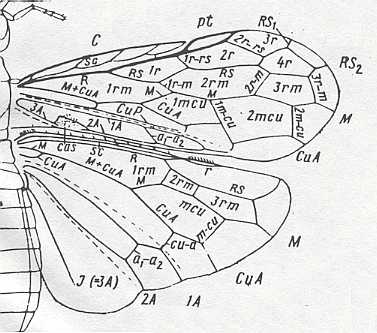
Figure 6a : Wings of the reconstructed ancestor of the Symphyta (see Figure 2, above). (After RASNITSYN, 1980)
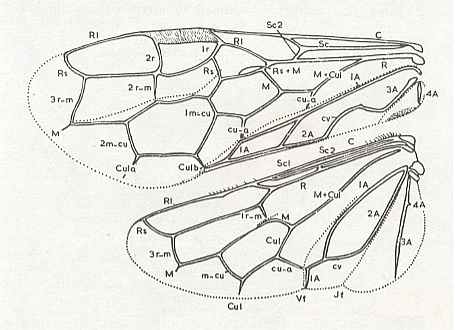
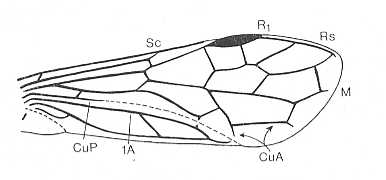
Figure 7 : Left wings of Pamphilius ( Pamphiliidae, Symphyta, recent), with the veins lettered. cv - anal cross-vein, vf - vannal fold, jf - jugal fold.
Interpretation of venation according to Ross, 1936. The family Pamphiliidae is considered to be a primitive (generalized) family of Symphyta. The sclerotized cell at the anterior margin of the forewing is the wing-spot (pterostigma). It is present in almost all Hymenoptera.
(From Imms' General Textbook of Entomology, (RICHARDS and DAVIES), 1977)
Although in different authors (Rasnitsyn, Ross, and perhaps others) the interpretation of the wing-venation in Hymenoptera differs somewhat, they largely agree on the course of the main veins. Therefore we will not discuss these differences.
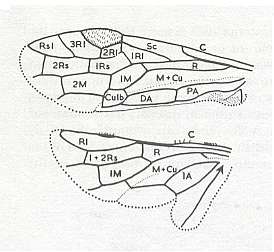
Figure 8 : Left wings of Xyela ( Xyelidae, Symphyta, recent), with the cells lettered. DA, PA, distal and proximal anal.
Interpretation of cells according to Ross, 1936. Also the family Xyelidae is considered to be a primitive (generalized) family of Symphyta. The sclerotized cell at the anterior margin of the forewing is the wing-spot (pterostigma). It is present in almost all Hymenoptera.
(From Imms' General Textbook of Entomology, (RICHARDS and DAVIES), 1977)
The next Figure depicts the forewing of Figure 8 (Xyela, Xyelidae, Recent), now without the letterings of the cells, but with letterings of main veins added.
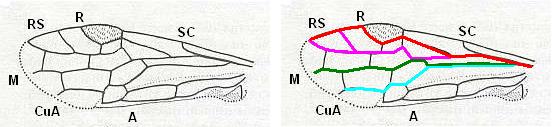
Figure 8a : Left forewing of Xyela (Xyelidae, Symphyta, Recent). Letterings of main veins added. The stem of the Subcosta (SC) has merged with the Radius (R). More distally, the Radial Sector (RS) branches off from the Radius, and finally produces two branches. The Radius continues around the pterostigma and beyond (along the wing-margin) until it meets the branches of the Radial Sector. The Media (M) originates from or close to the trunk of the Radius, gives off the anterior Cubitus (CuA), transiently merges with RS, and independently proceeds to the sub-apical wing-margin. Also CuA proceeds to the wing-margin. In the right-hand image some main longitudinal veins are colored : Radius -- red, Radial Sector -- purple, Media -- green, and (anterior) Cubitus -- light blue.
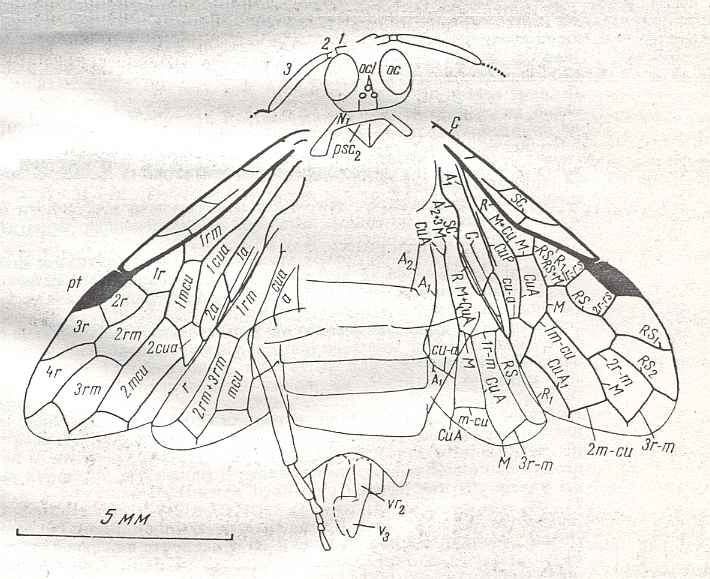
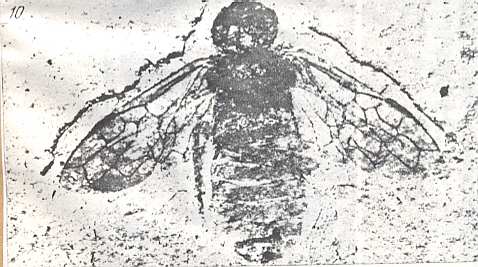
Figure 9 : Gigantoxyela quadrifurcata Rasn., family Xyelidae. Combined drawing after holotype PIN No. 1989/2591 and specimen PIN No. 1989/2613. Lower Cretaceous (?), Zabaikalj (Baisa), USSR.
oc - complex eye, ocl - ocellus, N1 - pronotum, psc2 - middle prescutum, vr2 - valvifer, v3 - leaf of ovipositor.
(After RASNITSYN, 1969)
The next Figure is a diagram of the right and left forewing of a primitive symphytum (Hymenoptera, Symphyta). It illustrates the seven main-vein systems C, SC, R, RS, M, Cu, and A. We will use it (either the left or the right wing) when interpreting fossil and recent representatives of Symphyta.
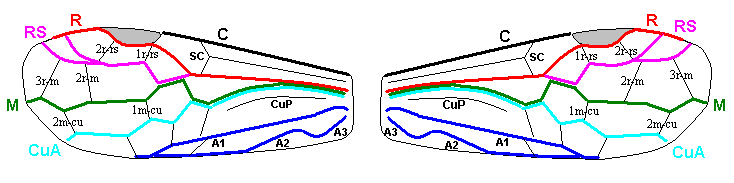
Figure 10 : Diagram of left and right forewing of a primitive representative of the Suborder Symphyta. We have roughly indicated the seven main-vein systems : Costa (C, black, bold), Subcosta (SC, black, thin), Radius (R, red), Radial Sector (RS, violet), Media (M, green), Cubitus (CuA, light blue, CuP, black, thin), Analis (A1, dark blue, A2, dark blue, A3, dark blue). Further we have -- also roughly -- indicated where the chief cross-veins lie and how they are named :
Cross-veins between R and RS (from proximal to distal) : 1r-rs, 2r-rs.
Cross-veins between RS and M (from proximal to distal) : 2r-m (1r-m suppressed), 3r-m.
Cross-veins between M and CuA (from proximal to distal) : 1m-cu, 2m-cu.
This diagram of the wing-venation in the right and left forewing of a primitive saw-fly (Symphyta), as [this diagram] being placed here, and also as accompanying (right or left wing) the Figures of real wings (fossil or recent)(see below), is not meant to depict a precise reconstruction of the original wing-venation in the first Hymenoptera. It only serves to provide a general orientation in this original venation.
As to the wing-venation in the Order Hymenoptera RASNITSYN, a decade later, writes in "Origin and evolution of the Hymenoptera", 1980, p. 4, the following :
Finally, it is instructive to reproduce yet another proposed hymenopterous starting-venation from which all remaining venational patterns in Hymenoptera are said to be derivable :
Transformations of the individual main-vein systems in the forewings of Symphyta in the course of their history
Before we describe the venational changes in Symphyta, we will give a c o n c i s e s u m m a r y of our theoretical suppositions with respect to the evolution of wing-venation in insects :
The original venation of especially the forewing in insects gives us -- when we subtract from it all its functional aspects (folds and hinges in the wing-membrane, structures in the anal and jugal region connected with wing-folding, etc.) -- a formal aspect of the morphological whatness (essence) of the given insect Order (and often already of the suborder or family), that is, every insect Order in the Pterygota starts with a wing-venation (the 'original venation') which expresses an aspect of the very essence ('whatness') of that Order (as being a higher taxon).
Much of the hindwing often is largely (but not always, for example not in the Odonata, dragonflies) determined by functional demands (especially in Orthoptera (grasshoppers and the like)).
But let us stick to the forewings of insects.
In more or less hardened (sclerotized) forewings (Orthoptera, Coleoptera [beetles], Dermaptera [earwigs], Hemiptera [bugs] ) many aspects of their venation are functionally determined, and as such reflect an aspect of the strategy sensu stricto. And of course also the very base of the insect-wing is functionally determined because it integrates with the hing of the wing, that is, with the mobile connection of the wing with the thorax of the insect.
But although in the evolution of (i.e. within) an insect Order some transformations in the venation may be functionally determined, and so do belong to the strategy sensu stricto, and thus come from the Implicate Order, most venational transformations taking place within a given insect Order are not functionally determined (and thus cannot be explained by random genetic mutation followed by natural selection) and so do not belong to the strategy sensu stricto, and thus do not originate in, and not come from, the Implicate Order, but originate in the Explicate Order.
But, as has been said earlier, these non-functional changes remain, first of all, within the morphological scheme of the seven or eight main-vein systems, and, secondly, remain within the venational type intrinsic to the given insect Order. Further, these changes should not interfere with existing functional structures in the wing. All this means that these changes cannot, and will not, be purely random. The result always remains a definite pattern of veins, if they do not, but one [the Radial vein], vanish in the first place. So in the evolution of the wing-venation in insects functional and non-functional features evolve together in harmony, resulting in a coherent venational pattern.
Within a given species-strategy the non-functional transformations (as all hereditary [= definitive, persistent] transformations), take place through, that is, mediated by, the Implicate Order, whereby these non-functional transformations originate, first of all, in the Explicate Order but are then passed on through (the medium of) the Implicate Order (as explained above) :
A given morphological result, obtained by individuals of a given species (strategy sensu lato) through the action of factors in the Explicate Order, is being injected back into the Implicate Order (together with the whole strategy-pattern), and so alters the original venational aspect of that [same] given strategy, without, however, changing the strategy sensu stricto ( So although a noëtic strategy is a pure functional strategy, as such without non-functional elements -- because this is sufficient for it to be able to exist in the Explicate Order and so obtain ontological completion -- non-functional features may nevertheless exist in the Implicate Order in a (noëtic) strategy, either by being just a necessary implication or corollary of the essence of the strategy sensu stricto, or by having come from the Explicate Order (by injection)). Upon projection (of this same strategy but now with the alteration mentioned), a further alteration takes place, again caused by factors in the Explicate Order, which alteration, when injected, transforms the original venational pattern still further, and so on, and so on.
Together with causing changes in the given species, it can also cause, upon injection, similar changes in other more or less closely related species (strategies), as described earlier. And moreover, a change in just a single main-vein system may, because it is often morphologically more or less insignificant, not only pass over to a newly projected closely related species, but also, and at the same time, to one (or more) more-distantly related species (of the same higher taxonomical unit) bound to be projected. So these changes can be followed through a whole phylogenetic tree of, say, several families or even superfamilies.
Upon projection into the Explicate Order of a next insect species, that is, a next strategy (s.str.), the latter is not the product, nor is it altered by the ongoing non-functional venational changes. But the venation of this new strategy may already be different from the outset namely when this new strategy necessarily and intrinsically implies a new venational pattern, which is then as such present from the outset, functionally or not functionally (co)determined.
And upon projection, finally, of the first representatives of a new insect Order (or a new suborder, and sometimes of even already a new family) an entirely new type of wing-venation will appear in the Explicate Order (not necessarily because of functional reasons). And from now on the subsequent venational changes will remain within the confines of this new venational type, set up by the new Order, until a representative of yet another new Order appears.
Let us diagrammize things (in the next four Figures), that is, graphically explain (1) the nature and way of depicting of projections and injections, and (2) the passing-over, through these projections and injections and in the Implicate Order, of a noëtic specification (of the course and form of a given main-vein system) from one strategy to another.
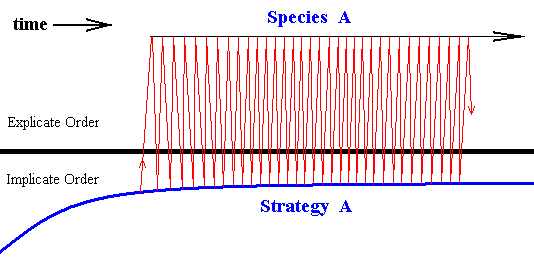
Diagram above : The series of projections and injections of strategy A. Each projection drawn as a slightly oblique upwardly directed line segment, while each injection is given by a slightly oblique downwardly directed line segment.
In fact, each projection of the qualitative content of the strategy is immediately followed by an injection of this content, and this, then, immediately followed by the projection of this content, and so on, and so on, resulting the series to be virtually continuous (as long as the series keeps on going). That's why we have, in earlier diagrams, depicted a projection and subsequent injection as a single (vertical) line.
A projection, and also the injection, always is a projection or injection of the whole qualitative content of a given strategy (a strategy embodied by a given insect species). To this content belongs, among many other features, the somehow noëtically specified initial venation in the form of the venational type of the Order to which the species belongs. And this initial venation itself consists of elements, namely, first of all, of the (specification of the) course and shape of each individual main-vein system extending over the surface of the wing, and secondly, the (specification of the) position and number of cross-veins. We will concentrate here on the course and shape of each individual main-vein system. So when we magnify a projection and subsequent injection of a strategy of an insect species (belonging to the Pterygota) we get, diagrammatically, the following :
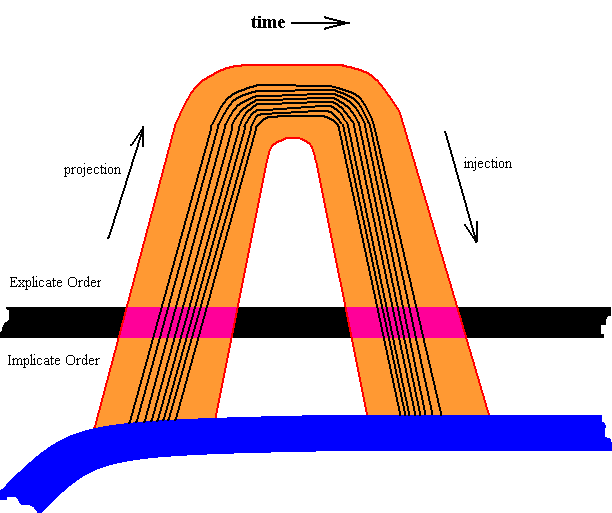
Diagram above : A magnification of a projection and subsequent injection of the (noëtic specification of the) qualitative content of a given insect strategy (appearing in the Explicate Order as an insect species). Belonging to the elements of this strategy (and carried by the latter) is the (specification of the) initial wing-venational pattern consisting of the (specifications of the) eight main-vain systems symbolized by the eight curved line segments.
In the course of projections and injections the details of the main-vein systems may be changed by the action of explicate-order-factors, and upon injection these changes are added to the noëtic specification of the insect species' wing-venation.
Injected strategies can, in the Implicate Order, noëtically react (interfere) with one or several other, more or less related strategies, in such a way that a given evolutionary state of some given main-vein-system may be passed over to these other strategies. That is to say, the evolutionary trend, started in some given species, may continue its course not only in that species itself but also in a number of other species. And in some of these other species this trend could be altered (changed direction), depending on the very essence (qualitative content) of such species. Diagrammatically we may depict this as in the following Figure. The blue branched curved line is the "noëtic trajectory" connecting related strategies (their noëtic specifications) according to their formal derivability. The zig-zag line segments, running up and down, symbolize the projections and injections of the changing noëtic specification of some given main-vein system (for example the Subcostal (SC) system), that is, specifications co-projected and co-injected together with the noëtic specification of the whole strategy containing these specifications. :
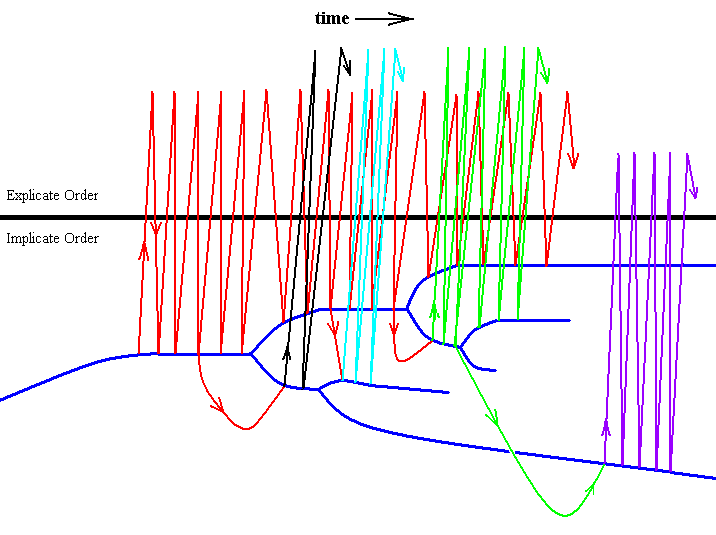
Diagram above : Projections and injections of a changing specification of the course and form (in the insect-wing, when in the Explicate Order) of some given main-vein system. The changed specification may, in the Implicate Order pass over also to specifications of other more or less related strategies, resulting, upon projection (of the whole strategy), in wings with corresponding courses and shapes of that given main-vein system. Also the other main-vein systems of the wing may behave in about the same way together or not together with the first main-vein system considered, resulting in the evolution of the wing-venation as a whole in the given insect group or taxon.
A simplified version of the pattern, in this diagram, of projections and injections is given in the next diagram

Diagram above : Simplified version of the previous diagram. Projections and injections of the noëtic specification of different morphological states of a given main-vein system in a number of related insect species (insect strategies). These projections and injections are carried by the corresponding projections and injections of the (specification of the) strategy as a whole.
We spoke about the possible passing-on of a given existing evolutionary trend in the wing-venation -- this trend going on in a given insect species, and thus going on in the Explicate Order -- from one species (strategy) to another species, where precisely this "passing-on" takes place in the Implicate Order as a result of noëtic interaction between (1) the injected [noëtic specification of the] qualitative content of the given insect strategy sensu lato (and thus co-containing the specification of the corresponding state of the venation following this trend), and (2) the noëtic specification of the qualitative content of another, but closely related, strategy, residing in the Implicate Order (the content of this other strategy co-containing the specification of its own state of the venation). And further we said that this passing-on can only take place between qualitatively related strategies. And if the feature to be passed on is morphologically and functionally insignificant it may be passed on even to more distantly related strategies. And all this, that is, all these changes, take place, so we said, not only definitely within the confines of the general type of wing-venation of insects (this general type being in the form of, or departing from, the seven [or eight] main-vein systems), but also within the venational type set by the insect Order to which the given species belong. And this means that although a "passing-on", as described above, may not take place (because it is impossible) between representatives of different insect Orders (beause the corresponding strategies stand qualitatively too far away from each other), it [the passing-on] may easily take place between representatives of one and the same insect Order. Well, the latter may be true for most insect Orders, each one of them indeed having its representatives adorned with a special type of wing-venation, typical and unique for the Order, as we see it so clearly in the Order Hymenoptera (and certainly also in the Orders Odonata [dragonflies], Mecoptera [scorpionflies], Lepidoptera [butterflies and moths], and others). But there are insect Orders which do not completely satisfy that what has just been said. Indeed, as we have seen in the first half of the present Series on Evolution, the Order Diptera [midges, mosquitoes, and true flies], although complying with the general venational scheme of [the forewings of] insects, contains many significantly different types of wing-venation even within the single Suborder Nematocera ( we need only to compare the wings of, say, the psychodoid midges (Psychodidae [moth-flies] and allies) with those of the tipuloids (crane-flies)). We may call these venational types "subtypes", and we may assume that a "passing-on" of some given venational evolutionary trend from one such subtype to another -- although remaining within the confines of a single insect Order -- is impossible. Indeed we see how diversely diverse the insects are.
Evolution of the main-vein systems of the wing-venation in Symphyta
We will now describe -- following RASNITSYN, 1969 -- the transformation of the wing-venation in Symphyta, as it has taken place during their history, departing from the supposed original venation (starting-venation) of Symphyta, and consequently of the Order Hymenoptera.
The fact of the persistence of the general scheme of the seven (or eight) main-vein systems C, SC, R, RS, M, Cu, A, and, if present, J, not only in Hymenoptera but in virtually all insects, allows us to assume that, except for all cases where functional transformations of the venation take place, each main-vein system evolves virtually independently of the other main-vein systems. Therefore, we may follow the fate of each main-vein system in Symphyta separately, and so we do, following RASNITSYN, 1969, pp.120. Thereby we will, as often as we can, refer to Figures of wings listed below in geologic order.
Uncomparably more important [than is the consideration of wing-structures determined by flight-mechanics] for the study of the phylogeny of the Hymenoptera are the more subtle details of wing-structure, namely the nature and features of the wing-venation.
It is true, we almost in no case can give a functional explanation of differences in the wing-venation, but even a purely morphological analysis of the venational system of the wing gives extremely much to reconstruct the evolution of the group, and more so by sassthe facat especially the wings most often and best of all are preserved in the fossil state.
The general plan of the wing-venation is fairly equal in the fore- and hindwing of Hymenoptera, which aspect strongly differs from the wing-venation in other insects as a result of sharp bends, of the many coalescences of veins, the loss of the morphological differentiation between longitudinal and cross veins. As a result of this, the nomenclature of the veins in Hymenoptera for a long time did not correspond to the venational scheme generally accepted in entomology, and only in 1936 Ross, and a little later, but independently of him, Marynov (1937) gave a real rational nomenclature, which today is widely distributed in the hymenopterological literature, especially abroad [that is, outside the Soviet Union], and, with insignificant alterations, accepted in the present work [that is, of RASNITSYN, 1969].
The evolution of the main-vein systems (in the forewing) separately considered. [In fact in what follows is merely a description of the state of each main-vein system (considered separately) as it is found in the families (fossil and recent) of Symphyta. And from this the evolution of these systems can more or less be deduced.]
The Costal vein.
The anterior margin of the forewing in its proximal and middle part is formed by the Costal vein (C), of which the apex [the end] is separated from the pterostigma [wing-spot] by a fracture (see, for example, Figure 9 ).
To recall the systematics of recent Symphyta, see the above LIST .
The Subcostal vein.
Posteriorly of C there is the weak Subcostal vein (SC) of a very variable structure. The probably most constant feature of it is the triangular widening of its base, which is preserved even in the case of the vein's complete reduction, and also very constant is the presence of the short humeral cross-vein (h) between SC and C a little distally from their bases. In Hymenoptera SC is the only vein that is concave (i.e. lying in a groove of the wing-membrane) [ In the wings of most other insects there exists a certain alternation of convex and concave longitudinal veins].
Most primitive is the form of SC in the Upper Triassic Archexyelinae ( Figure 29 , Figure 30 , Figure 31 ), of the Family Xyelidae, where the vein [the SC], while giving off several anterior branches (in the impressions 1-2 branches are visible, but it is possible that their number was large, see also the reconstuction Figure 6a ), reaches the apex of C before the pterostigma.
The next evolutionary stage is shown us by, apparently, the Upper Jurassic Pararchexyela ( Figure 39 ) (Pararchexyelidae), where the base of the apical branch of SC, while bending, unifies with the Radial vein (R) lying behind it, but from there departs from it again and ends up at the apex of C, as in Archexyelinae. At the place of unification of SC and R originally there was, probably, a cross-vein, which was secondarily lost in Archxyelinae : To coalescence of parallel veins usually precedes the shortening of the vein connecting them (more often of a cross-vein). Veins, not connected to each other by anything, rarely coalesce.
Further evolution of the subcostal system is characterized by the extension of the area of coalescence of SC and R, such that of the distal part [the Russian text says "branch", but this is certainly wrong] of SC only remains the base, often looking like a cross-vein between SC and R, and the apex, distally closing the costal field (which name in Hymenoptera conditionally stands for the region between C and R).
The apical part of SC very often altogether disappears. It is well developed, often even widened, only in Macroxyelinae (Xyelidae), see Figure 9 , and, apparently, in Tenthredinidea, see Figures 119-120 . In this, the apex of SC with its base tightly lies against the pterostigma in such a way that the fracture in the forewing at the base of the pterostigma seems to run through it. See especially Figure 120 .
As a result of the described processes the -- of the Symphyta considered typical -- straight SC appeared with a fork at its end. See Figure 127. In this, in the majority of forms also the extra anterior branches disappeared, being preserved only in some Xyelidae, see Figure 9 and Figure 105 . In Caenolyda (Pamphiliidae), moreover, the anterior branch of SC was strongly lengthened and secondarily reached the apex of C. However, also the typical two-branched SC is far from being preserved in all Symphyta, and is characteristic only of a part of the Xyelidae (Pinicolites graciosus (oligocene [lower tertiary] ) as opposed to Xyela (recent) and Archexyela crosbyi (upper triassic) ), of Pseudoxyela (Xyelotomidae?) ( Figure 60 ), Pamphiliidae (Cephalea caplani (oligocene [lower tertiary] ), Xyelydidae (Figure 42 ), Gigasiricidae (Figure 35 and Figure 66 ), some Siricidae, and, perhaps, Myrmiciidae.
[We have then, within the family Xyelidae, a primitive Subcosta in the upper triassic Madygella, the jurassic Liadoxyela and in the lower tertiary (oligocene) Pinicolites, and an advanced (derived) state of it in the recent Xyela, while we also have an advanced state of it in the upper triassic Archexyela, the upper triassic Ferganoxyela, and the upper triassic Leioxyela (?).
Summarizing the states of Subcosta gives :
Recent : advanced.
Tertiary : primitive.
Jurassic : primitive.
Triassic : primitive and advanced.
So here we have, within a single family (Xyelidae), with respect to the evolution of the Subcosta, not to do with later (in geological time) species being, without exception, more advanced than earlier species. This brings with it that in this respect (that is, with respect to the structure of the Subcosta) the later species cannot formally be derived from certain earlier species (if one does not want to violate Dollo's law of irreversibility), for example the mentioned tertiary form, Pinicolites cannot formally be derived from the mentioned upper triassic Archexyela , because the latter is more advanced than the former. And it is our noëtic theory of evolution which explains such facts. See for this, among other things, Figure 4, above , where species B, although a more recent species, is nevertheless more primitive than the older species A.]
The further evolution of SC is in many cases characterized by a coalescence with R of also its basal part, such that of SC remains, in the form of a cross-vein, only its anterior branch (in many Tenthredinidea, in Sepulcidae ( Figure 46 ), Xiphydriidae ( Figure 126 ), Anaxyelidae ( Figure 80 ), some Siricidae), or even this cross-vein disappears. Apart from that, the latter result -- total absence of a free SC -- may be accomplished also through a direct reduction of it, without coalescence with R. This process took place in certain Xyelidae, see Figure 33 and, probably, also in Megalodontidae (Figure 121), Parapamphiliidae ( Figure 41 ), Cephidae (Figure 122), Orussidae (Figure 123), and in some recent Siricidae [that is, not all of them, see, for instance Urocerus ].
The Radius
The Radial vein (R) runs more or less parallel with C and SC. Usually not beyond the middle [on the line connecting wing-base to wing-tip] of the wing, downwards from R [i.e. branching off from R) runs its main branch, the Radial Sector (RS), while the vein (Radius) itself runs further under the name R1. The latter, far beyond the middle of the wing, runs toward the anterior margin, posteriorly and apically delimiting a thickened (sometimes weakly so) cell, the pterostigma. After that, R1 runs along the wing-margin usually until it meets with the posterior branch of RS, this branch ending up at the wing-apex or anteriorly of it. The next diagram helps us to orientate.
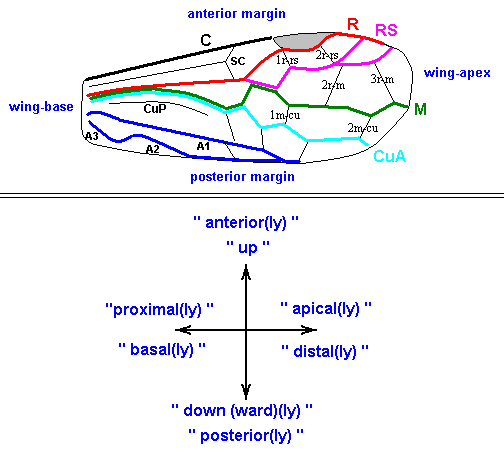
Diagram above :
Top : Generalized course and position of main-veins and cross-veins in the forewing of Symphyta.
Bottom : Generally used expressions of the main directions in the wings of insects.
Sometimes R1 ends without reaching RS ( Figure 111 ), or runs down inside the wing before meeting it ( Figure 130 ).
Radial Sector (RS)

or with R1 proximal of this base. The cross-vein 1r-rs is one of the most stable [i.e. constant] cross-veins of the wing, only rarely not reaching the pterostigma (in some Cephidae) or completely reduced (in Orussidae ( Figure 123 )). Sometimes the segment of RS between RS+M and 1r-rs disappears, and then the cells 1r and 2rm normally divided by it merge, see Figure 112 , Figure 118 , and Figure 119 .
Beyond the cross-vein 1r-rs RS is approximately parallel to the anterior wing-margin. Here, onto it, as a rule, originally falls the cross-vein 2r-rs, which usually goes off from the middle or end of the pterostigma, and only in certain Cephidae, Karatavitidae, and the majority of Orussidea it is displaced closer to the base of the pterostigma. In Tenthredinidea the cross-vein 2r-rs is directed significantly more oblique toward the wing-apex than it is in other Symphyta, and runs into RS distally from 2r-m, whereas in the other superfamilies the relationship of the cross-veins is almost always the other way around. Not seldom (principally among those very Tenthredinidea) 2r-rs is absent ( Figure 130 ).
In the majority of Xyelidae RS branches a little proximally or distally of 2r-rs. Usually two branches are present, in Pleroneura sometimes more. Between the branches of RS there may be found a cross-vein ( Figure 29 , there we see RS branching off from R, merging for a short distance with M, then slightly curving upwards, running past the two cross-veins 1r-rs and 2r-rs, then branching into two branches, which are, near their ends, connected with a cross-vein.)
In Pseudoxyela (Xyelotomidae? Figure60 ) also two branches of RS are developed, but their fork is displaced almost to the wing-apex, and its anterior branch is not obliquely directed to the wing-apex as in Xyelidae but directly to the anterior margin of the wing.
The posterior branch of RS before the wing-apex where the cross-vein 3r-m falls onto it, may be more or less sharply curved forwardly (apparently this is the more primitive state).
The Media
The medial vein is in its basal segment merged with CuA over a large distance (where CuA is the anterior branch of the Cubital vein). Moreover, it is, at its very base merged with R. From the fork M+CuA, about in the middle of the wing, M runs obliquely up, until the cross-vein 1r-m or up to RS, after which it sharply bends toward the distal margin of the wing. From there M is almost straight, lightly diverging from RS. From above, the cross-veins 2r-m and 3r-m fall onto M, which fairly often are not developed. From below, the cross-veins 1m-cu and 2m-cu, which are almost constantly present, fall onto M ( 2m-cu is absent in Myrmiciidae and Orussidea, 1m-cu is absent in certain Orussidea).
1m-cu falls onto M more proximally of 2r-m ( Figure 11 ), sometimes even at the end of RS+M ( in Microryssus, Paroryssidae, see Figure 94 , and in Syntexis, Anaxyelidae, see Figure 125 ). 2m-cu falls onto M between 2r-m and 3r-m, and more rarely, in certain Tenthredinidea, before 2r-m. Branches of M are not known in Hymenoptera.
The branches of M, drawn by Sharov (1957) in Cretavus (Cretavidae, according to us [Rasnitsyn] Apocrita incertae sedis), probably are, as is the anterior branch of the two extra branches of RS (his RSa), simply usual folds of the membrane.
The Anterior Cubitus.
CuA apparently goes off, together with M, from R. However, in certain Xyelidae, Argidae, Pergidae, Pamphiliidae, Siricidae, Sepulcidae, and in Parapamphilius ( Figure 41 ), in addition to the usual cross-vein cu-a, lying distally from the fork M and CuA, there is developed an extra proximal "cross-vein", and in a series of cases it below is bent into the proximal direction and connects with the posterior cubital vein (CuP) which is a weak vein, often not developed at all in Symphyta. Maybe, this "cross-vein" is the real base of CuA, the more so by the fact that an analogous, but more proximal, vein of the hindwing of Xyelidae ( Figure 8 ) and Gigasiricidae surely is the free base of CuA ( Figure 9 , Figure 66 ).
Distally, see Figure 7 (forewing), with respect to the fork M and CuA the latter often (not always) is slightly bent down and here unifies with cu-a (the latter) falling with its other end directly onto the anterior anal vein (A1). Further, sometimes after a light forward bend, CuA takes the cross-vein 1m-cu, then sharply bends down, takes the forwardly bent apex of A1, again sharply bends parallel to the anterior margin of the wing, and then often lightly bends down at the place of falling onto it 2m-cu. In Hymenoptera CuA is also unbranched.
Posterior Cubitus and the Anal veins.
CuP, as already mentioned, is a weak vein, [as vein] not reaching the cross-vein cu-a. Often it isn't expressed at all. CuP is not bent. Its base is independent of other veins and lies closer to A1 than to R+M. CuP and A1 lie closely together, and between them runs the thin anal suture, delimiting the anal region in front and guarantees it a certain mobility with respect to the remaining part of the wing. The anal suture interrupts the cross-vein cu-a and the vein A1 before the latter falling onto CuA and proceeds to the posterior wing-margin about at the level of the pterostigma. A1 is also almost straight, but a very strong, always well developed, vein, strongly bent upward only at its very base and apex (before CuA) and with a weak downward bend distally of cu-a. Proximally of cu-a, from A1 runs downwardly (often obliquely) the cross-vein a1-a2. See Figure 7 (forewing).
A2 and A3, both being not complete, form a curved vein characteristic of Symphyta, posteriorly closing the anal cell. In line with Ross (1936) and Benson (1951), A2 is not developed for a short distance from the base up to the cross-vein a2-a3. A3, on the other hand, is usually not developed distally from the cross-vein a2-a3 up to the place of its unification with A2 (see again Figure 7 ), whereby here, in a series of cases (in certain Xyelidae and Siricidae) are developed its rudiments at the beginning, and sometimes also at the end, of this segment ( Figure 24, lower image ). As a result of these reductions and small changes of direction of the veins, evening out the angles at their meeting points, A2 and A3 unify into one single vein, at its base bent upwardly into the anal cell and falling onto A1 close to the tip of the latter. In the concavity of the anal cell, [the concavity] formed by the just described bend, lies a roughened field ( Figure 127 ) which, during the state of wings-folded, becomes fixed on the cenchri of the metathorax.
This is thus the typical structure of the anal system (of veins) in Symphyta. In a series of cases its simplification takes place. Thus, in Cephidae ( Figure 122 ) and Karatavitidae ( Figure 85 ) in connection with the reduction of the cenchri and the roughened field, A2+A3 is straightened out and the concavity [the upward bend in it] disappears. In Megalodontidae ( Figure 121 ) the concavity has also disappeared, but the roughened field is preserved, displaced inside the cell (a partly forward transposition of the field above A2 is observed already in certain Xyelidae).
In many Tenthredinidea the cross-vein a1-a2+3 has moved proximally and gradually shortens such that A1 and A2+3 merge in their middle part, and the anal cels remain far away from each other. In Orussidea and certain Tenthredinidea A2+3 is more or less reduced, such that one or both cells are open ( Figure 118 and Figure 123 ).
Jugal region (forewing).
Behind the anal veins at the base of the wing runs the short jugal suture, about which takes place the movement [folding back] of the jugal part -- lying behind it -- of the wing when it is folded [back onto the abdomen]. Inside the jugal region the elastic axillary cord runs -- a thickening of the membrane, similar to a vein, but in contrast to it not completely sclerotized, but strengthened by a spiral chitinous thread and therefore elastic, not stiff. It is possible that the cord is a desclerotized rudiment of the base of the anal fan. Running to the posterior margin of the jugal lobe, the axillary cord runs along it up until the posterior margin of the scutellum. Sometimes that part of the cord that is inside the jugal lobe remains sclerotized (in Xyelidae, Pamphiliidae, Xiphydriidae, some Tenthredinidae). Rarely (in certain Tenthredinidae) close to the wing-margin it gives off a short forward outgrowth, apparently a rudiment of the Jugal vein (J). The latter is developed in many Oligoneoptera [insects with complete metamorphosis], but in them usually lying directly behind the jugal suture. Therefore, the just described rudimentary vein of the Symphyta is more similar, not to the jugal (J), but to the remains of the anterior branch -- usual of many Oligoneoptera -- of the axillary cord, this branch fringing the distal part of the margin of the jugal lobe. Here this part of the cord had only lightly to be moved forwardly in this case.
Distally of the jugal region, the posterior margin of the wing is thickened and folded downward and forward. During flight the hooks at the anterior margin of the hindwing lock into this fold of the forewing, fastening both wings into one single whole. The thickening of the posterior margin extends up until the outlet there of the anal suture. After it the wing-margin is normal, thin.
Folds in the forewing.
The veins of the forewing are cut-through by a system of folds -- thinnings of the membrane, which run through more or less desclerotized segments of veins ( Figure 119 , and Figure 127 and others). One of these folds starts from the interruption of the Costal vein before the base of the pterostigma, cuts through R below this interruption, and splits up into two at the posterior outer angle of the cell 1r. The anterior branch, often also split up, runs between RS and M to the wing-tip, and the posterior branch runs through the desclerotized piece of the unification of RS+M and the cross-vein 1m-cu into the cell 2mcu. From there often go out already three branches -- one inside the cell 1mcu, a second through the cross-vein 2m-cu to the outer margin of the wing, and a third through the internal angle of the cell 2mcu to the end of the straight hind-margin of the wing. Above CuA, from this third fold often goes out still one outer branch, also cutting through 2m-cu. In addition, above A1, from the wing-base up to the end of its posterior margin, runs the anal fold, cutting through cu-a and the end of A1, and behind the base of A2+3 runs the short oblique jugal fold.
We will not describe the hindwing of Symphyta. It is largely similar to the forewing and is seldomly well preserved in fossils.
Distribution and (first) appearance in Time (Upper Triassic - Recent) of some venational features in the forewings of Symphyta ( The LINKS in the tables below refer to figures of wings of fossil and recent Symphyta [ Further below, in the present document, these figures are listed in geologic order, together with descriptions of the sites where the fossils were found. To see it, click HERE )
Branched (primitive) Subcosta.
|
Upper Triassic : Figure 13 Figure24 Figure 26 Figure 29 Figure 31 Figure 32
|
Lower Jurassic : Figure 35
|
Middle Jurassic : Figure 37 Figure 38
|
Upper Jurassic : Figure 39 Figure 42 Figure 55 Figure 59 Figure 60 Figure 61 Figure 62 Figure 63 |
Lower Cretaceous : Figure 96 Figure 98 Figure 100 Figure 101 Figure 102 Figure 103 Figure 104 Figure 105 Figure 106 |
Lower Tertiary : Figure 114 Figure 115 Figure 116 Figure 117 |
Recent : Figure 124 Figure 127 Figure 7 Figure 117A |
Unbranched Subcosta, partly or wholly reduced Subcosta, or merged with Radius.
|
Upper Triassic : Figure 11 Figure 15 Figure 16 Figure 22 |
Lower Jurassic : Figure 33 Figure 36a |
Middle Jurassic : ---- |
Upper Jurassic : Figure 47 Figure 56 Figure 67 Figure 69 Figure 73 Figure 78 Figure 85 |
Lower Cretaceous : Figure 107 Figure 108 Figure 109 |
Lower Tertiary : Figure 111 Figure 112 Figure 113 |
Recent : Figure 118 Figure 119 Figure 120 Figure 121 Figure 122 Figure 123 Figure 125 Figure 126 Figure 128 Figure 129 Figure 130 Figure 131 Figure 132 Figure 8 Figure 117B Figure 122A Figure 122B Figure 124A |
From the above table it is clear that all the various morphological states or stages of the Subcostal vein (in the forewing of Symphyta) occur in all the mentioned geological periods. Especially, its primitive state is still present in the Tertiary and even in recent forms. This might be due either to its constancy (stability) in certain taxa, or to its repeated re-appearance in the course of time. So we cannot say that there has been a general rectilinear evolution of the Subcosta, except maybe within certain individual taxa (of course, we must take into account that many taxa, especially the fossil ones, or recent taxa recognized in fossils, are largely based on the wing-venation, including the state of the Subcosta).
Branched Radial Sector (two branches).
If the Radial Sector (RS) is branched in Symphyta, it is always two-branched.
|
Upper Triassic : Figure 11 Figure 12 Figure 13 Figure 14 Figure 15 Figure 16 Figure 17 Figure 18 Figure 19 Figure 21 Figure 22 Figure 23 Figure 24 Figure 25 Figure 26 Figure 27 Figure 28 Figure 29 Figure 31 |
Lower Jurassic : Figure 33 Figure 34 |
Middle Jurassic : Figure 37 |
Upper Jurassic : Figure 41 Figure 52 Figure 53 Figure 55 Figure 59 Figure 60 |
Lower Cretaceous : Figure 96 Figure 97 Figure 98 Figure 100 Figure 101 Figure 102 Figure 103 Figure 104 Figure 105 Figure 106 |
Lower Teriary : Figure 115 Figure 117 |
Recent : Figure 8 Figure 117A Figure 117B |
Unbranched (or in some way specialized) Radial Sector.
|
Upper Triassic : -- |
Lower Jurassic : Figure 35 Figure 36 Figure 36a |
Middle Jurassic : -- |
Upper Jurassic : Figure 42 Figure 44 Figure 45 Figure 46 Figure 47 Figure 48 (Xyelidae!)
Figure 49
Figure 61 |
Lower Cretaceous : Figure 107 Figure 109 |
Lower Tertiary : Figure 111 (?) Figure 112 Figure 113 |
Recent : Figure 118 Figure 119 Figure 120 Figure 121 Figure 122 Figure 123 Figure 124 Figure 125 Figure 126 Figure 127 Figure 128 Figure 129 Figure 130 Figure 131 Figure 132 Figure 7 Figure 122A Figure 122B Figure 124A |
A branched Radial Sector is the primitive state of this vein with respect to an unbranched Radial Sector.
Symphyta with an unbranched Radial Sector are not found in the Upper Triassic. All Symphyta found from this period possess a branched Radial Sector (2-branched). This complies well with such a Radial Sector being interpreted as primitive. In the Lower Cretaceous there are still many Symphyta with a branched Radial Sector. And even there is at least one recent form with such a Radial Sector.
So there is, it is true, some tendency of the branched Radial Sector to become unbranched, but it is not a universal process.
In the forewings of some recent Symphyta, for example in those of Urocerus (Siricidae) , the basal segment of the Radial Sector branches off from the Radius at a reverse angle, that is, it is directed obliquely to the base of the wing instead of to its apex (or simply downwardly). Later, in the higher Hymenoptera, we will encounter this feature quite often. In fossil Symphyta (that is, at least in the list of drawings below [taken from RASNITSYN, 1969] ) such a state of the basal segment of the Radial Sector is not found.
At the end of the present document we shall have to say something more about the evolutionary transition from Symphyta (saw-flies and allies) to Apocrita (parasitic wasps, true wasps, ants, and bees) from the point of view of their wing-venation. If the reader wants to consult it now she or he may click HERE .
List of fossil Hymenoptera-Symphyta (Triassic-Recent)
Let us now list the wings of fossil (and recent) Symphyta through the Mesozoic and Cenozoic up to the present time. As we have said, with the fossils found, we do not have at our disposal a 'complete report or record' of these wings and their changes, but only a few snap-shots of them here and there along the line or bundle of venational transformations in Symphyta. For each relevant geological period we describe (following Rasnitsyn, 1969) the sites, that is, the locations where symphyta fossils are found.
D j a i l j a u c h o. The settlement Djailjaucho is in the Batkensk district of the Oshk region, of the Kirgizen Republic (central Asia), 30 km to the west of the town Shurab. The shales with impressions of insects belong to the upper part of the Madigen suite and is dated to be of lower Triassic. [Later it was found that this dating was not correct. It must be Upper Triassic, according to Rasnitsyn, 1980, and Rohdendorf and Rasnitsyn, 1980.]. Sedimentation of the insect-bearing layer took place, apparently, in the conditions of a vally or even of a delta of a large river. Of this bears witness the large plain taken by the insect-bearing layers (insect beds) and the vicinity of parts with intensive grading of material as to size and weight (for instance in-between-layers literally filled up with isolated elytra of beetles) [alternated] with parts where sedimentation took place in calm conditions and where impressions of large wings and whole insects are found.
As Sykstel (1962, 1967) indicates, the climate of sout,hern Fergana in the early Triassic was dry and hot, continental, with strong seasonal changes. The prevailing type of relief consisted of plains and mountain plateaus. The xeromorphous florae, with numerically prevailing pine trees, pteridosperms, cicas trees, and cordaites, were connected mainly with "oases".
At the site of Djailjaucho, have been, by the expeditions of the Paleontological Institute, but also by Sykstel in the years 1956-1964, collected about 10000 impressions of insects, including 37 remains of Symphyta. On the basis of this material 12 genera and 25 species of teh family Xyeledae were described, belonging to two subfamilies -- Madygellinae (one species) and Archxyelinae. However, these data do not express the real picture of the taxonomic diversity of the Hymenoptera of Djailjaucho, because the described taxa, as a result of strong warping of the impressions, and also of their fragmentational condition and bad preservation are essentially parataxa.
C r o s b y. Site of late Triassic age (the base of the Ipswich series) at Mount Crosby in Queensland, Australia. Here is found one hymenopterous fossil, Archexyela crosbyi Riek, belonging to the same subfamily Archexyelinae, to which the majority of Xyelidae from the site Djailjaucho belongs.
All the Figures of wings of fossil (and recent as the case may be) Symphyta will be accompanied by our colored drawing of the supposed starting-venation (only as regards the main-vein systems) of the Suborder.


Figure 11 : Archexyela crosbyi Riek. Upper Triassic, Australia (Crosby). Family Xyelidae, subfamily Archexyelinae.
R goes around the area which normally is the pterostigma. RS, branching off from R at the level of the apex of SC, passes along the cross-veins 1r-rs and 2r-rs, and a little before the latter forks into two branches. M+CuA forks into CuA and M, and the latter merges for some part of its course with RS and then independently heads off to the apex of the wing.
Compare with Xyela (recent) . (After RIEK, 1955)

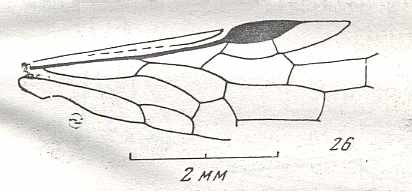
Figure 12 : Xyelinus angustiradius Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

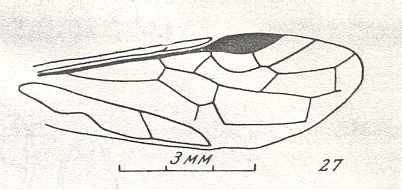
Figure 13 : Xyelinus majus Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

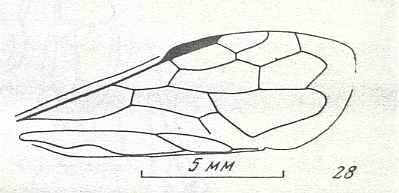
Figure 14 : Oryctoxyela anomala Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

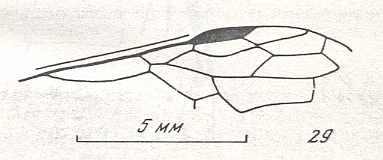
Figure 15 : Oryctoxyela triassica Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

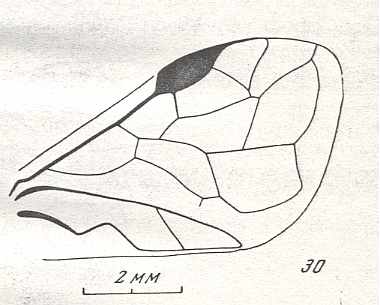
Figure 16 : Ferganoxyela sogdiana Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

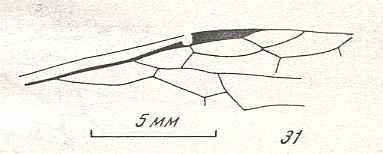
Figure 17 : Ferganoxyela destructa Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

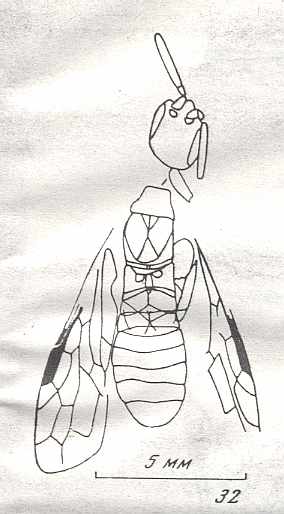
Figure 18 : Triassoxyela foveolata Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

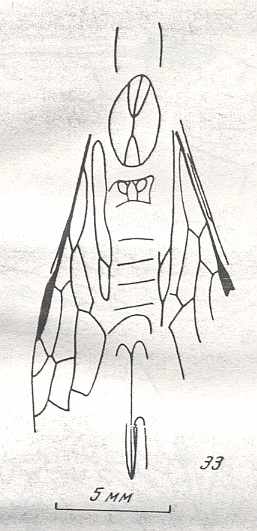
Figure 19 : Triassoxyela orycta Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)


Figure 20 : Asioxyela smilodon Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

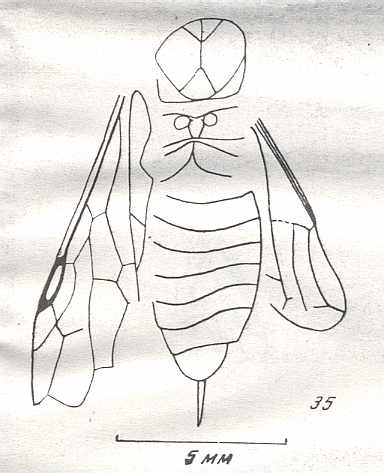
Figure 21 : Asioxyela paurura Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae. Combined drawing based on two specimens. Compare with Xyela (recent) . (After RASNITSYN, 1969)


Figure 22 : Asioxyela grandipennis Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

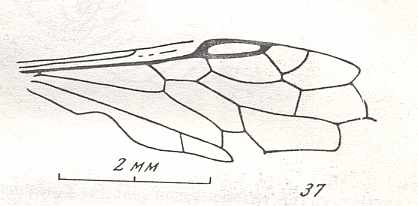
Figure 23 : Asioxyela parvula Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

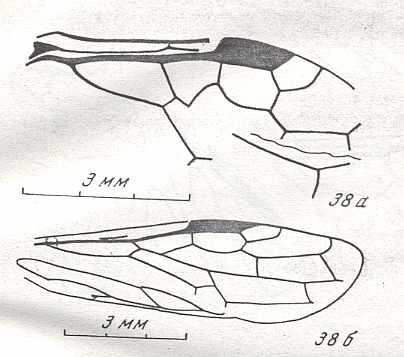
Figure 24 : Leioxyela (?) lata Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 25 : Leioxyela mollis Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

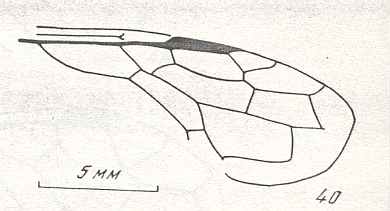
Figure 26 : Leioxyela (?) grandis Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 27 : Leioxyela mitis Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

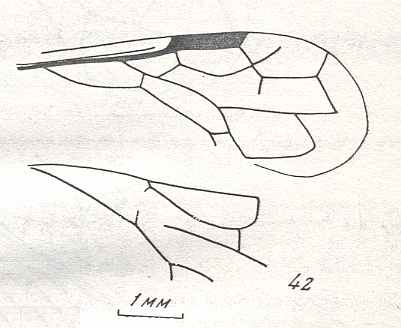
Figure 28 : Leioxyela (?) antiqua Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 29 : Madygenius extraradius Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Here we see RS branching off from R (halfway the wing), merging for a short distance with M, then slightly curving upwards, running past the two cross-veins 1r-rs and 2r-rs, then branching into two branches, which are, near their ends, connected with a cross-vein.
Compare, if needed, with Xyela (recent) .
(After RASNITSYN, 1969)

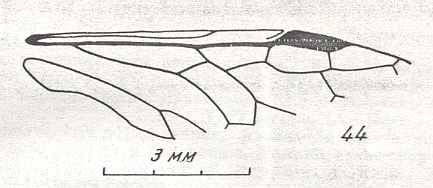
Figure 30 : Madygenius primitivus Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

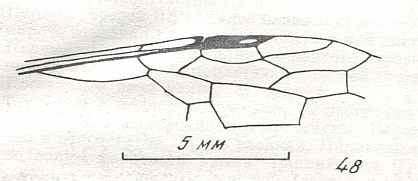
Figure 31 : Lithoxyela fenestralis Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Archexyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

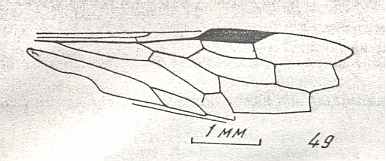
Figure 32 : Madygella analoga Rasn. Upper Triassic, Central Asia, Djailjaucho. Family Xyelidae, subfamily Madygellinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)
I s s i c - K u l. The site, where insect fossils were found, is at the settlement Sogjuta in the Tonsk district of the Kirgizen Republic, at the shore of lake Issic-Kul. Insects were collected by the expedition of the Paleontological Institute in 1942 in deposits of the dzylsk suite, which Henkyna (1966) determines as belonging to the lower Lias [The Jurassic period consists of three sub-periods (where "lower" means older, and "upper" means later), viz., Lias (lower Jurassic), Dogger (Middle Jurassic), and Malm (Upper Jurassic)]. To the early Jurassic age of the insects of this site also points Martynova (1948). The early Liassic age of the insect-bearing deposits is also confirmed by the discovery here of diverse Kazacharthra, identified by Novojilov as Almatium Novojilov (supposedly A. gusevi Chernyshev). As is known, Kazacharthra are, until now, only found in early Liassic deposits of Asia. On the other hand, Rohdendorf (1964), basing himself on the extraordinary archaic nature of the Diptera of Issic-Kul, determines these deposits to be of Upper Triassic (Rhaet) age.
Of Symphyta is found here only Kirghizoxyelea mirabilis Rasn. (Xyelidae, Liadoxyelini), much more closely related to the Jurassic Asian Liadoxyela Mart., than to the late Triassic Australian Archexyela. However, this discovery cannot claim to be a weighty argument in favor of the Liassic age of these deposits -- there exists too little material of late Triassic and early Jurassic Symphyta, and the sites of discovery of the few of them are much too far apart from each other.
The relief in the district of lake Issic-Kul in late Triassic (Rhaet) and early Jurassic times, was elevated, the forests were chiefly of ginkho-sago(palm) content, the zoogeographic region (set by marine fauna) is tropical, the climate was humid, equable over large distances (Sinitsyn, 1962). The paleofloristic region was, according to data of Wachrameev (1964), Indo-European (Central Asiatic Subregion).
K i z i l - K i a. The site where fossil insects have been found is close to the town Kizil-Kia in the Osk region of the Kirgizen Republic. Insects were mainly collected in deposits of suite H, for which Martynov (1937) indicated an early Liassic age, whereas Kuzitskina, Repman, and Sikstel (1958) determine them to be of middle-upper Liassic age.
Of Symphyta is only found Liadoxyela praecox Mart. (Xyelidae, Liadoxyelini). As Sinitsin (1962) indicates, the paleogeographic site of southern Fergana in early Jurassic times was approximately as it was in the district of lake Issic-Kul, with the only difference that here a more intensive aggregation of coal has taken place, meaning that humidity was greater, and the vegetation more abundant.
S h u r a b. This site of fossil insects (sai Sagul) lies in the Batkensk district of the Osk region in the Kirgizen Republic, 12 km SW of the town Shurab, and is approximateley of the same age with the site Kizil-Kia. Paleogeographic conditions in it probably were also equal, and entirely similar were also the insects of some groups, for instance Coleoptera Archostemata.
The Symphyta, found here by the expedition of the Paleontological Institute (1964), are totally different -- three impressions belong to representatives of three families of the superfamily Siricidea -- Liasirex sogdianus Rasn. (Gigasiricidae), Sepulenia syricia Rasn. (Sepulcidae), and Shurabisca liassica Rasn. (Myrmiciidae). However, this difference could be merely accidental.
All the Figures of wings of fossil (and recent as the case may be) Symphyta again will be accompanied by our colored drawing of the supposed starting-venation (only as regards the main-vein systems) of the Suborder. The lower [i.e. early] Jurassic is called the Liassic, or simply Lias.

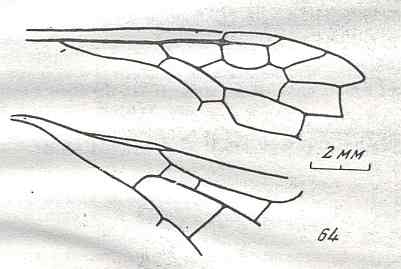
Figure 33 : Kirghizoxyela mirabilis Rasn. Lias, Central Asia, Issic-Kul. Family Xyelidae, subfamily Xyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

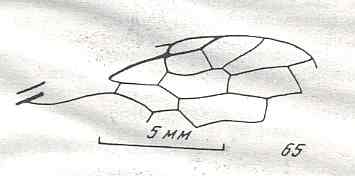
Figure 34 : Liadoxyela praecox Mart.(1937) Lias, Central Asia, Kizil-Kia. Family Xyelidae, subfamily Xyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

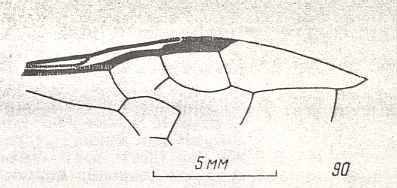
Figure 35 : Liasirex sogdianus Rasn. Lias, Central Asia, Shurab. Family Gigasiricidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

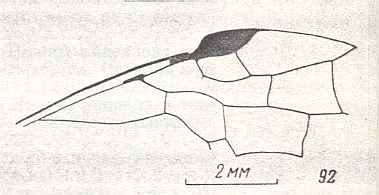
Figure 36 : Sepulenia syricta Rasn. Lias, Central Asia, Shurab. Family Sepulcidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

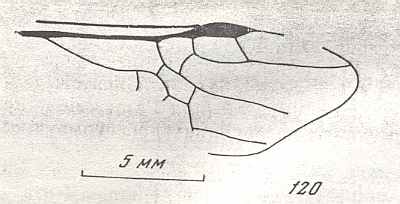
Figure 36a : Shurabisca liassica Rasn. Lias of Central Asia, Shurab. Family Myrmiciidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)


Figure 37 : Liadoxyela iensis Rasn. Lower or Middle Jura, Pri-baikalja (Iya, Irkutsk region, Siberia). Family Xyelidae, subfamily Xyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

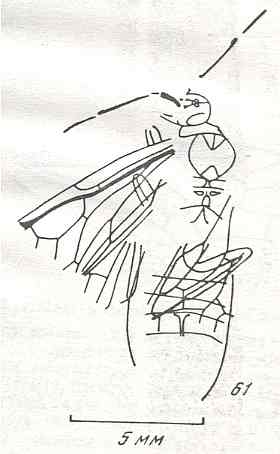
Figure 38 : Xyelisca leptopoda Rasn. Dogger or Upper Lias of Zabaikalj [Byrjatskaya Republic, Siberia]. Family Xyelidae, subfamily Xyelinae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)
K a r a t a u. Fossil insects were collected at several sites of the southern part of of the mountain-chain Karatau (Kazachskaya Republic [Kazachstan], Tchimkentsky region, Algabasky district). At two of these sites were found also Symphyta -- near the settlement Galkino at the foot of the mountain Buckuï-Tau (mountain range Kulbastau -- eastern end of Karatau), and at the settlement Michailovka (urotchitsche Aulije). The age of the karabastau suite is, by the majority of authors, determined to be of late Jurassic (Callovian [upper Dogger] - Tithonian (Portlandian) [Upper Malm] ). Rohdendorf, 1964, increases the age up to middle Jurassic based on the similarity of certain Diptera [found there] with early Jurassic Diptera from Europe. Panphilov, 1968, on the other hand, determines the Karatau deposits with insects as to belong to the very top of the upper Jurassic, basing it on the appearance of cryptogenic forms, having migrated from the ancient pacific land-mass. However, the supposition of the sudden appearance of those or other insects in late Jurassic times is not well-founded because of the almost total non-existence of treatises about insects undoubtedly having a middle Jurassic age, not only of Central Asia, but also of other regions in the world. As to Hymenoptera, the number of their remains in deposits intermediate between those studied in Djailjaucho (upper Triassic) and Karatau, is simply negligibly low to draw similar such conclusions. Therefore, we can only speak of a real significant change of the fauna when comparing karatau-Symphyta with later forms, those from Baïsa.
The insects of Karatau were buried in a shallow lake, with water rich in ions of Ca [Calcium] and Mg [Magnesium] and thereore with an impoverished fauna and flora. Panphilov explains the poverty of the aquatic entomofauna of Karatau as a result of predatory activities of fish. The climate was hot and dry, only in places the conditions were more humid. According to Panphilov (1968) this was maintained in mountains not far from the lake, whereas the authors of "The Karatau Jurassic lake" (1968) speak of sparce shoreline marshes. The paleofloristic province, is, according to data of Bachrameev (1964), Indo-European, in the middle zone of it.
By A.W. Martynov, A.I. Turutanova-Ketova, and N.W. Shabarov, in the years 1924-1930, and later by the expeditions of the Paleontological Institute, in the years 1962-1967, in Karatau were collected 70 impressions of Symphyta, belonging to 60 species, 35 genera, and 11 families of the Suborder.
In the subscripts of the Figures below, the phrase "upper Jurassic of southern Kazachstan" always refers to the site Karatau there, either near the settlement Michailovka, or near the settlement Galkino.
S o l n h o f e n. The lithographic layers in the district of Solnhofen -- Eichstadt (Bavaria, Germany) were deposited in late Jurassic times in a lagoon of the southern coast of Fennoscandia, covered chiefly with fern and sagopalm forests. The zoogeographic region is, according to data of Sinitsyn (1962), tropical, and the paleofloristic region is, according to Wachrameev (1964) Indo-European (middle zone).
Of Hymenoptera are found here many impressions of Myrmiciidae, belonging to a single species of the genus Myrmicium Westw. However, the bad preservation of this material does not allow us to be convinced that under the name of M. schroeteri (Germ.) are collected representatives of just one, and not several, albeit closely related, species of Myrmicium.
D u r d l e s t o n B a y. Deposits of the lower Purbeck (upper Jurassic) in Sussex, England. Climate and vegetation, apparently the same as of Solnhofen. Two impressions of Symphyta are found here : Myrmicium heeri Westw. and Formicium brodiei Westw. (Myrmiciidae).
All the Figures of wings of fossil (and recent as the case may be) Symphyta again will be accompanied by our colored drawing of the supposed starting-venation (only as regards the main-vein systems) of the Suborder. The upper [i.e. late] Jurassic is called Malm.

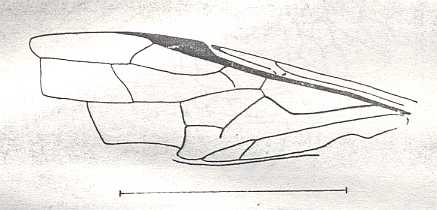
Figure 39 : Pararchexyela macroptera Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Pararchexyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

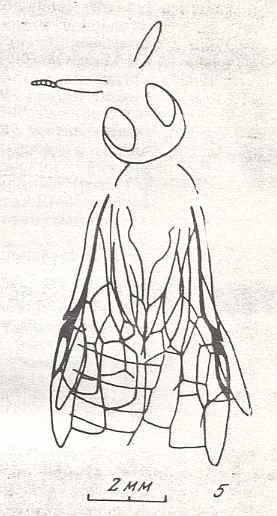
Figure 40 : Xyelocerus admirandus Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelotomidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

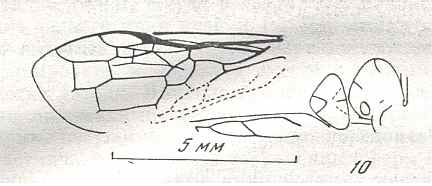
Figure 41 : Parapamphilius confusus Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Parapamphiliidae.
Here we see M+CuA branching off near the wing-base from R. It first gives off a short "cross-vein" to A1, and a little thereafter splits up into M and CuA. And little after that we see the cross-vein cu-a (not clearly preserved) connecting CuA with A1. The first mentioned "cross-vein" is perhaps the real base of CuA the proximal part of which is apparently merged with A1. From the posterior margin of the wing to its anterior margin we see, in succession, A1 (apical part not preserved) (with the cross-vein a1-a2 (also not preserved), the mentioned short "cross-vein" connecting A1 with the Cubitus, which, after having given off M, runs obliquely down to the posterior wing-margin. Above CuA we see the Media which, after having touched RS [or merged with it for a short distance], runs to about the apex of the wing. Above the Media we see the forked RS.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

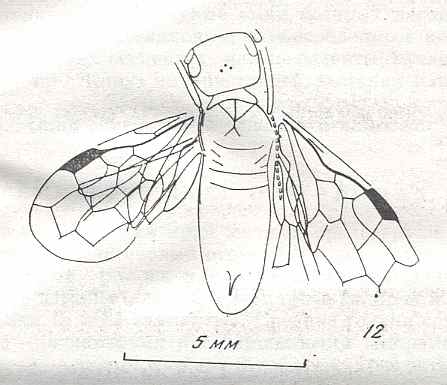
Figure 42 : Prolyda karatavica Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelydidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

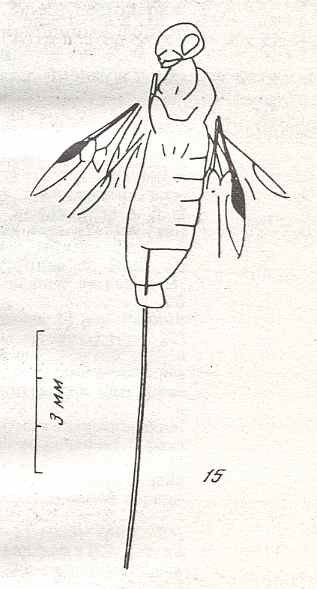
Figure 43 : Paroryssus extensus Mart. Upper Jurassic of southern Kazachstan (Galkino). Family Paroryssidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

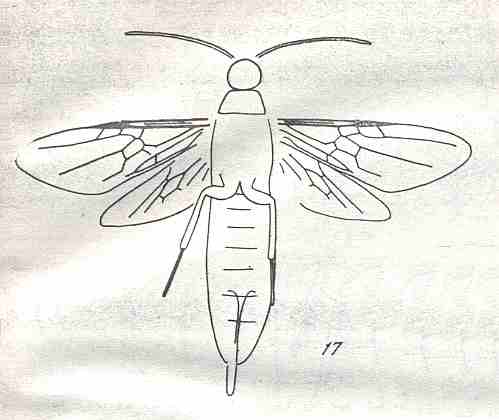
Figure 44 : Myrmicium schroeteri Germar. Upper Jurassic of Germany (Solnhofen). Family Myrmiciidae. Composed drawing chiefly from two specimens.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

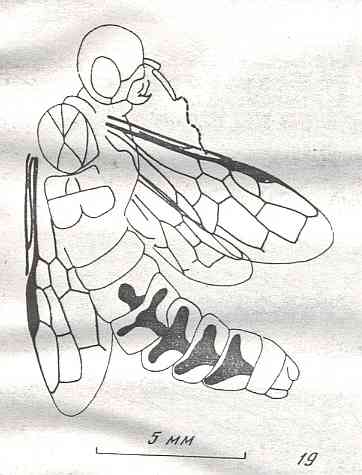
Figure 45 : Protosirex xyelopterus Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Gigasiricidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

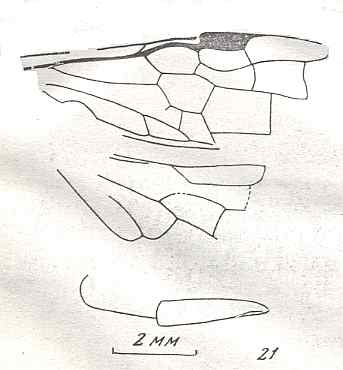
Figure 46 : Sepulca mirabilis Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Sepulcidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

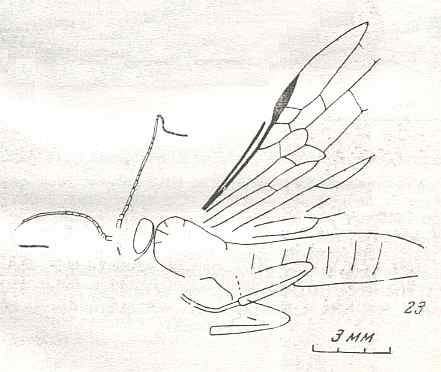
Figure 47 : Karatavites angustus Rasn. Upper Jurassic of southern Kazachstan (Galkino). Family Karatavitidae. See for yet another individual of this species Figure 86 .
Compare with Xyela (recent) . (After RASNITSYN, 1969)

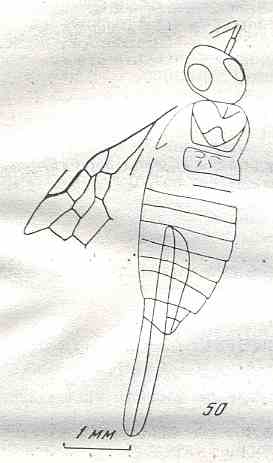
Figure 48 : Sirecomima xiphophora Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

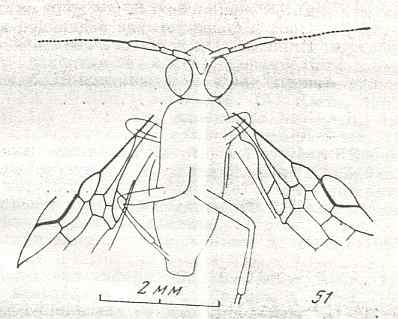
Figure 49 : Xyelula hybrida Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 50 : Enneoxyela (?) karatavica Rasn. Upper Jurassic of southern Kazachstan. Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 51 : Enneoxyela crassicauda Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

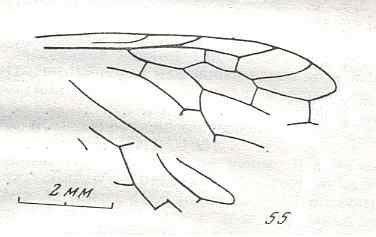
Figure 52 : Enneoxyela compressicauda Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 53 : Eoxyela scoliura Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
psc - prescutum of thorax, sc - scutum of thorax, scl - scutellum of thorax, aest - anepisternite of thorax, prest - preepisternite of thorax.
Compare with Xyela (recent) . (After RASNITSYN, 1969)
Figure 54 : Eoxyela atra Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
ba1 - basalar 1, aest - anepisternite of thorax, prest - preepisternite of thorax, cx2 - middle coxa. . (After RASNITSYN, 1969)

Figure 55 : Anomoxyela incerta Rasn. Upper Jurassic of southern Kazachstan (Galkino). Family Xyelidae.
pmx - maxilla, hy - hypostomes, psc - prescutum of thorax, N1 - notum of prothorax, ba1 - basalar 1, prest - preepisternite of thorax.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

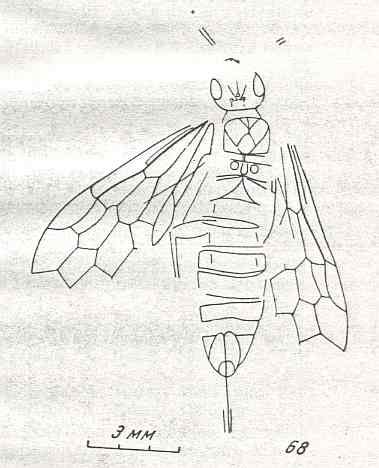
Figure 56 : Lydoxyela excellens Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

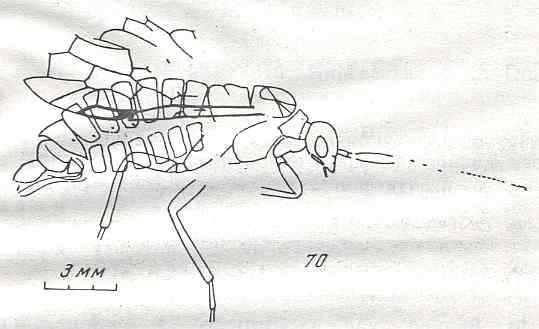
Figure 57 : Nigrimonticola longicornis Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

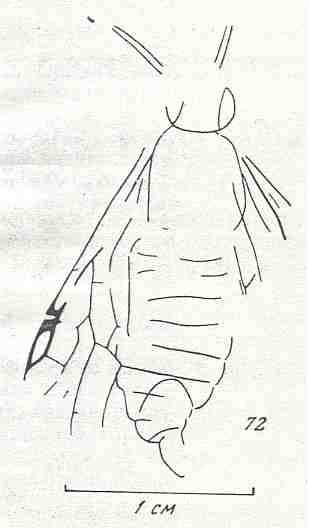
Figure 58 : Ophthalmoxyela brachyura Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

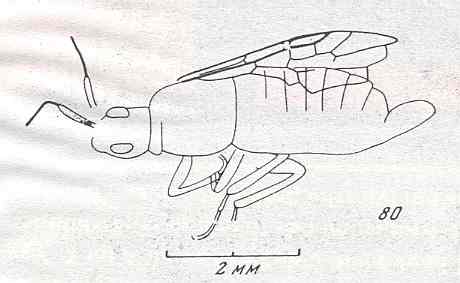
Figure 59 : Microxyelecia brachycera Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

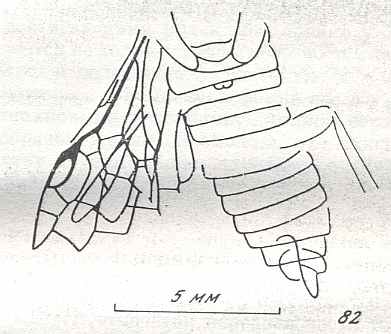
Figure 60 : Pseudoxyela heteroclita Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelotomidae.
Here we see M+CuA branching off from R close to the wing-base. Then it forks into M and CuA. M, after having curving upward, merges for a short distance with RS and then runs to the wing-apex. RS, leaving M, curves upward and downward, thereby passing along the cross-veins 1r-rs and 2r-rs, and then forks into two short branches, of which one runs directly to the anterior wing-margin.
Compare, if needed, with Xyela (recent) . (After RASNITSYN, 1969)

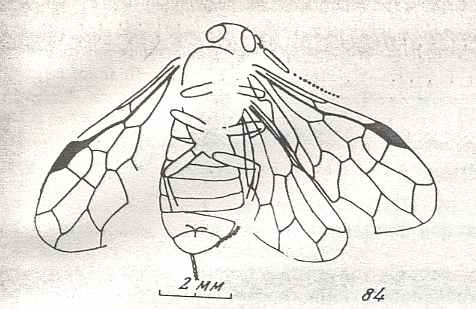
Figure 61 : Xyelyda excellens Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelydidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

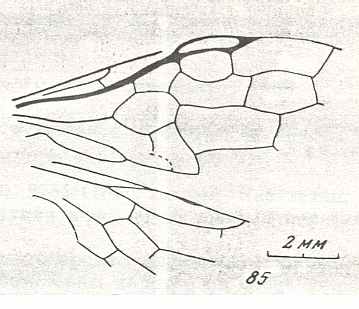
Figure 62 : Mesolyda jurassica Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelydidae.
Compare with Xyela (recent) . Here the base of the Radial Sector is oriented rather steeply : It runs off from R at about a right angle, perhaps foreshadowing the obliquely backward course in later groups (See Figure 121 ) (After RASNITSYN, 1969)

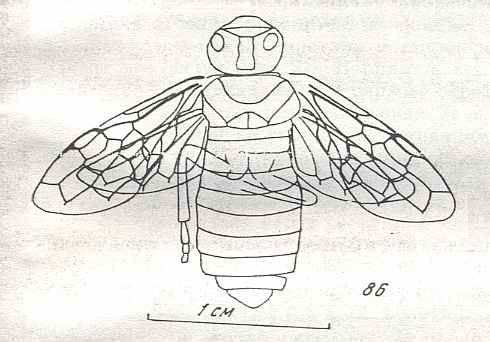
Figure 63 : Mesolyda depressa Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelydidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

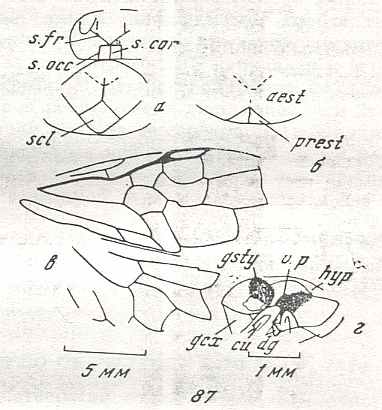
Figure 64 : Strophandria grossa Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelydidae.
Upper left image : head and middle dorsum. Upper right image : mesothorax. Center image : wings. Bottom right image : genitals.
s.fr - frontal suture, s. cor - coronar suture, s. occ - suture of occiput, scl - scutellum of thorax, aest - anepisternite of thorax, prest - preepisternite of thorax, gsty - gonostylus, v. p - valve of penis, hyp - hypopygium, gcx - gonocoxa, cu - cuspis, dg - digitus.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 65 : Prolyda xyelocera Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Xyelydidae.
est1 - episternite of prothorax, cx - coxa, aest - anepisternite of thorax, prest - preepisternite of thorax.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 66 : Gigasirex longipes Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Gigasiricidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 67 : Sphenosyntexis antonovi Rasn. Upper Jurassic of southern Kazachstan (Galkino). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

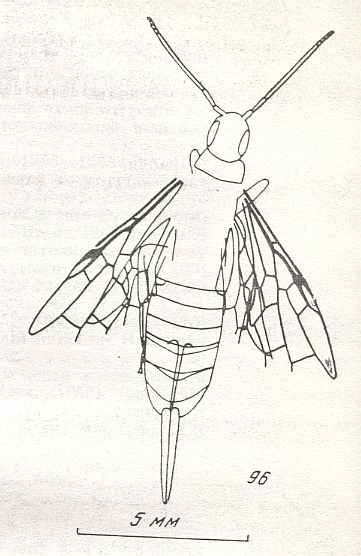
Figure 68 : Sphenosyntexis pallicornis Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

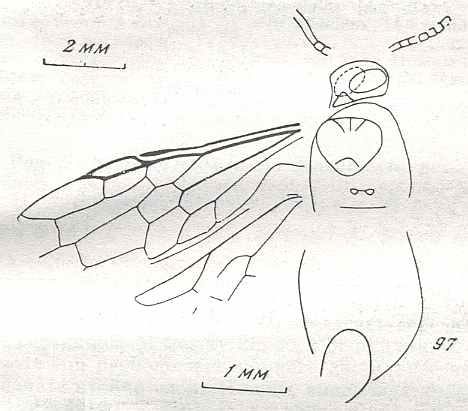
Figure 69 : Anaxyela parvula Rasn. Upper Jurassic of southern Kazachstan (Galkino). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

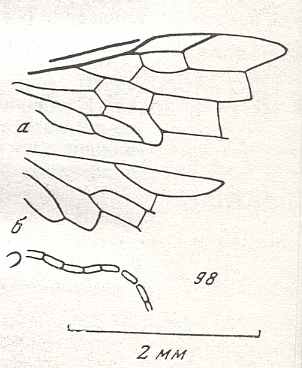
Figure 70 : Anaxyela nana Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Below : antenna.
Compare with Xyela (recent) . (After RASNITSYN, 1969)


Figure 71 : Anaxyela gracilis Mart. Upper Jurassic of southern Kazachstan (Galkino). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

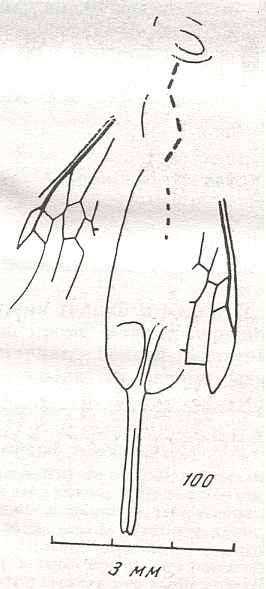
Figure 72 : Anaxyela destructa Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

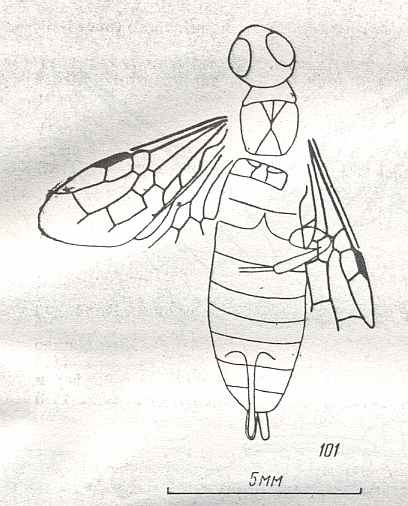
Figure 73 : Brachysyntexis nova Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

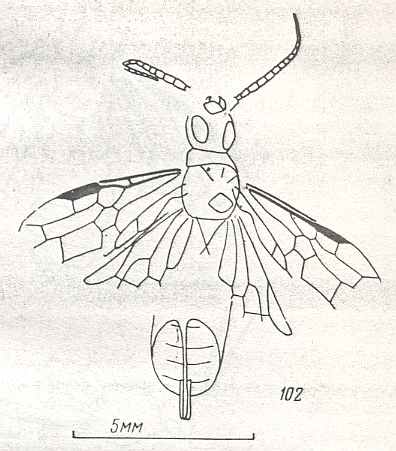
Figure 74 : Brachysyntexis brachyura Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

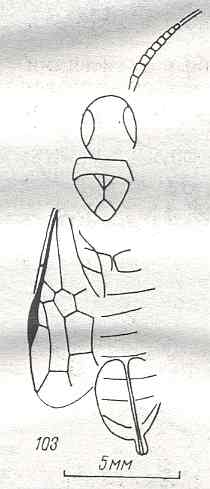
Figure 75 : Brachysyntexis micrura Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

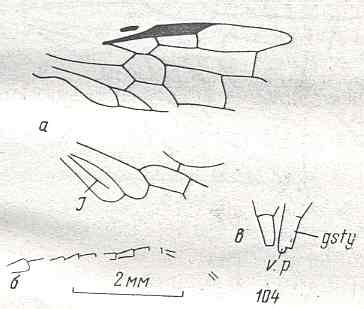
Figure 76 : Anasyntexis strophandra Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Bottom left : antenna. Bottom right : genitals.
gsty - gonostylus, v. p - valve of penis.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

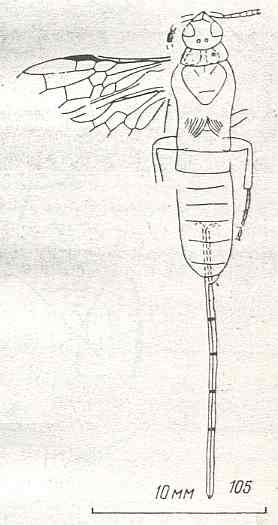
Figure 77 : Kulbastavia macrura Rasn. Upper Jurassic of southern Kazachstan (Galkino). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

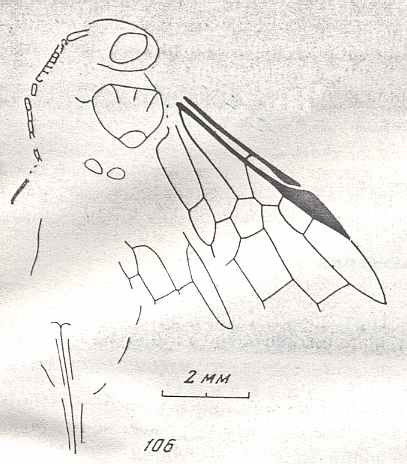
Figure 78 : Syntexyela media Rasn. Upper Jurassic of southern Kazachstan (Galkino). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

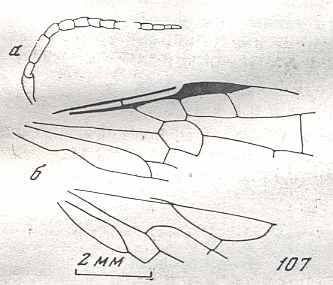
Figure 79 : Syntexyela asiatica Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae. Upper image : antenna.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

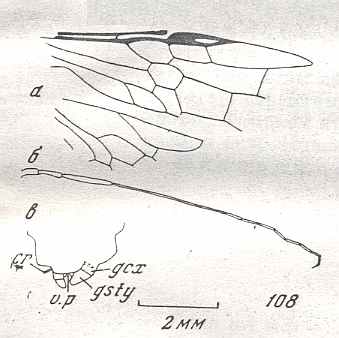
Figure 80 : Syntexyela inversa Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Middle image : antenna. Bottom image : genitalia.
cr - cercus, gcx - gonocoxa, gsty - gonostylus, v. p - valve of penis.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

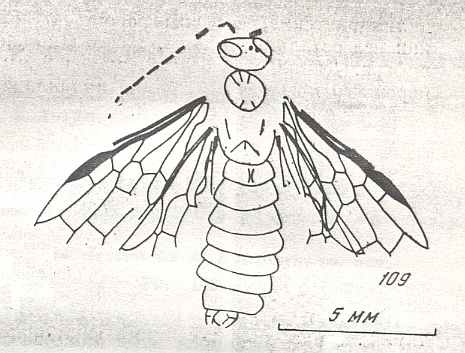
Figure 81 : Syntexyela gracilicornis Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)
Figure 82 : Urosyntexis drepanura Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
. (After RASNITSYN, 1969)

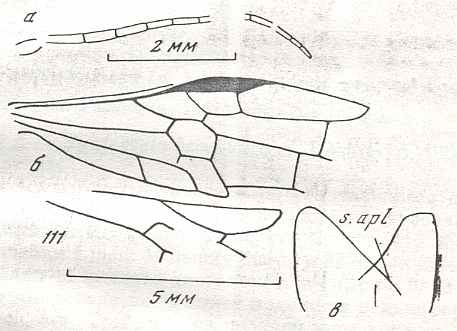
Figure 83 : Urosyntexis magna Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Top : antenna. Center : wings. Bottom right : mesothorax. s. apl - anapleural suture.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 84 : Urosyntexis depressa Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

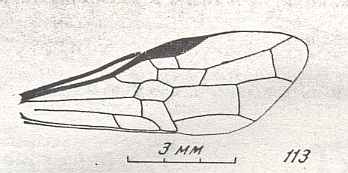
Figure 85 : Karatavites medius Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Karatavitidae. The normally curved A2+A3 is straightened out.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

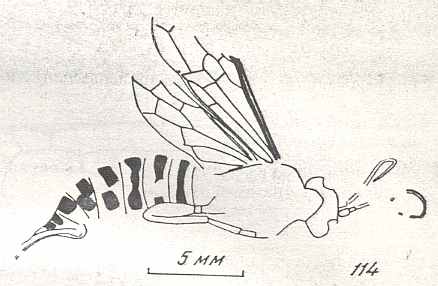
Figure 86 : Karatavites angustus Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Karatavitidae. See also the holotype of this species (that is, the specimen first used to describe the species) Figure 47 .
Compare with Xyela (recent) . (After RASNITSYN, 1969)

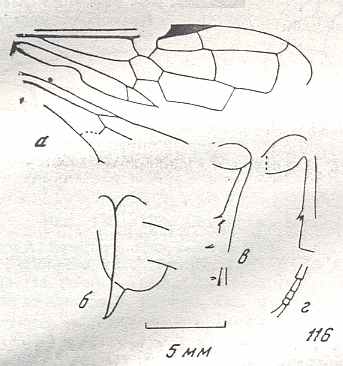
Figure 87 : Megaulisca grossa Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Siricidae.
Top : wings. Left : ovipositor. Bottom right : middle- and hind-leg.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 88 : Megura magnifica Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Siricidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

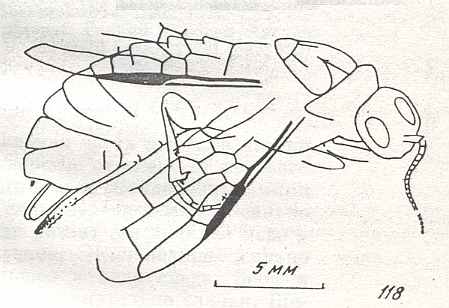
Figure 89 : Aulisca odontura Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Siricidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)


Figure 90 : Aulisca variicornis Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Siricidae.
Top : antenna. Middle : forewing. Bottom : foreleg.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

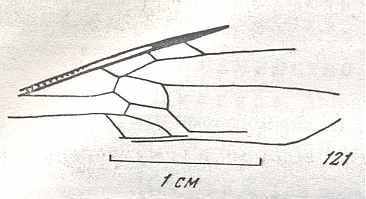
Figure 91 : Myrmicium heeri Westw. Upper Jurassic of England. From a photograph obtained from Dr. Wootton. Family Myrmiciidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

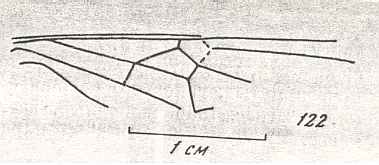
Figure 92 : Formicium brodiei Westw. Upper Jurassic of England. From a photograph obtained from Dr. Wootton. Family Myrmiciidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

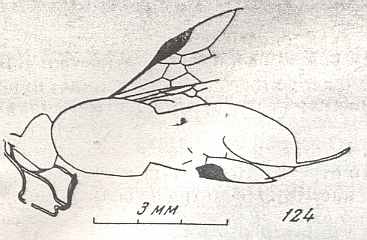
Figure 93 : Praeoryssus venosus Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Paroryssidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

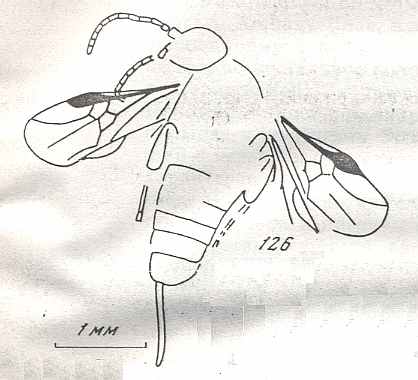
Figure 94 : Microryssus brachyurus Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Paroryssidae.
From R, just before the pterostigma, RS branches off. It merges for a short part of its course with M, then meets the cross-vein 1r-rs and then heads into the direction of the wing-apex (the cross-vein 2r-rs is absent). The Radial Sector is not branched. M, after having left RS, heads to the apex of the wing. At the point of the separation of M from RS we see a short oblique vein which is the cross-vein 1m-cu. It connects M (in fact M+RS) with CuA.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 95 : Microryssus subtilis Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Paroryssidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)
B a i s a. Deposits, apparently formed in a lake, are exposed by the river Vitim (right east of Lake Baikal. The Vitim ultimately flows to the north and joins the river Lena), close to the settlement Baisa (Burjatskaya Republic [USSR, now Russia], Jeravninsk district). The layers with insect impressions belong to the baisinsk suite (Kolesnikov, 1964).
The turginsk (upper subsuite), cholbolzhinsk (lower subsuite), kutinsk, argunsk, ust-karsk (upper subsuite), utansk, narasunsk, ulan-ganginsk, mayaksk, baleisk, nertjinsk, romanovsk, baisinsk, and possibly, also sepchindinsk, are suites of Zabaikalj. The talandsk suite [is of ] Priamur, the dzunbainsk suite of Mongolia, and the formation Layjan of China. The naming of the suites is done by geologists, having collected material, or by the authors listed later.
These suites are in a certain degree of different age. At least among them there exists some immediate alternation of one with the other, for instance the romanovsk, baisinsk, sepchindinsk or kutinsk and argunsk (Kolesnikov, 1964). Variuous authors indicate for the listed suites an interval [gap] from the top of the upper Jurassic up to about the middle of the lower Cretaceous. More rarely they grant them only a late Jurassic age or early Cretaceous age.
As to the baisinsk suite, directly to its Jurassic age only Martinova (1961) and Tjernova (1961) point. In this, Martynova does not give support for her opinion, and Tjernova refers to the fact that the genus Hexagenites Scudd., closely related to Ephemeropsis, is known from the Malm (upper Jurassic) of Europe. However, not only closely related genera, but also species of those same genera, found in Baisa are not seldom not only found in the Malm, but also in the early Jurassic, late Cretaceous, and even persist today. Among Symphyta for instance the genus Xyela Dalm. lives on up to the present time. Ponomarenko lists a series of analogous cases for beetles.
Therefore, an early Cretaceous age of the baisinsk suite, taken by the majority of investigators, and confirmed by the discovery here of an impression of Mesocephus, that is, of an insect already connected with angiosperm plants, appears more likely, albeit not definively proven.
The fauna of the Baisinsk site is not uniform : The complexes of forms collected in various parts of the investigated (geological) section differ strongly from each other. Thus, among the aquatic forms in the upper part of the section larvae of the genus Ephemeropsis Eichw. dominate, having lived, apparently, in densely grown sections of the water-basin with slowly moving water. In precisely such conditions are characteristic also the cases of caddis-fly larvae found here. The lower layers, on the other hand, contain in large numbers impressions of larvae of dragonflies (Odonata), being -- according to Pritikina by word of mouth -- characteristic of more open water. Here are found many larvae of the beetle Coptoclava longipoda Ping, also connected with open water (Ponomarenko, 1961). The structure of cases of caddis-fly larvae from these deposits points to a greater mobility of the water. Mayfly larvae are not abundant here, as well as larvae of dragonflies and Coptoclava [are not abundant] in the upper part of the section. Layers between the ones described above are in-between also with respect to the insect-remains contained in them, which allows to suppose a gradual growth of the water-basin (probably a lake), in which sedimentation of the investigated deposits took place.
According to data of Sinitsyn (1962) in the beginning of the Cretaceous in Zabaikalj a mountainous, even volcanic, relief prevailed, and chiefly pine forests. The climate was moderate with seasonal fluctuations. The site lies at the southern border of the Siberian paleofloristic region, as it is drawn by Wachrameev (1964).
The insects, in their present level of being studied, provide comparatively little supplementary data as to this question. Especially the above referred-to Mesocephus Rasn. belongs to the recent subfamily Cephinae, connected with angiosperm plants, and belongs even directly to the tribe Pachycephini, of which the recent representatives develop in composites (Compositae). This bears witness to the very probable presence in the early Cretaceous of Zabaikalj of angiosperm plants, and, perhaps, even of the very Asterales, with certainty known only from the cenomane [about lower upper Cretaceous].
By word of mouth communication Arnoldi holds that the Curculionidae (beetles) in the baisinsk fauna are represented by the subfamilies Nanophyinae and Notarinae, in recent times also connected with angiosperm plants.
Interesting are some features of the fauna of the Baisinsk site, bearing witness of possible cyclic fluctuations of the climate. Thus, in the intermediate part of the (geological) section the number of thermophilous insects has increased, insects such as Orthoptera [grasshoppers and the like], Phasmatodea [stick-insects] (six times higher than in other layers), Neuroptera [lace-wings and the like], and especially Psychopsidae (4.5 times and more). In certain in-between layers of this part of the deposits are unusually many impressions of cockroaches, also in their majority being thermophilous animals. The high abundance of remains of thermophilous insects in this part of the [geological] section allows us to assume a hotter climate in the period of formation of the corresponding layers as compared to the ones lying beneath or on top of them. In favor of this also bears witness the relative abundance of remains of terricole insects, which here predominate above the aquatic ones (in other parts of the section this relationship is the other way around).
The distribution of the remains of Symphyta, in each case, does not contradict this supposition. Thus, in the upper and lower parts of the section are found 22 impressions of 11 species and 9 genera of the family Xyelidae, and not a single representative of the other families, whereas in the in-between layers out of 5 remains only one belongs to the Xyelidae, and the other belong to four other families (Cephidae, Siricidae, Anaxyelidae, and Tenthredinidea incertae sedis). Among these four forms the above mentioned Mesocephus is the most interesting, which, apparently, belongs to the recent thermophilous (steppe) tribe Pachycephini. As to the Xyelidae, predominating in the other parts of the section, they are, at least today, adapted to chiefly the forest zone and mountains of the Holarctis [the temporate and colder part of the northern hemispere].
K e m p e n d j a i. Remains of insects are found at the tributary Viljuja of the river Kempendjai (Suntarsk district in the Jakutsk Republic) in deposits of the lower Cretaceous sangarsk suite, that is, [these remains being] of the same age the baisinsk insects, or a little younger. Insects were collected in 1953 by Tsjumakov. The climate and vegetation of this district and the district of Baisa were, in early Cretaceous times, also similar (Sinitsyn, 1962), but the relief was flat, not mountainous. The paleofloristic region is, according to Wachrameev (1964), Siberian (outside the middle zone). The only representative of Symphyta found in Kempendjai is Kempendaja jacutensis Rasn. (Anaxyelidae), which is more closely related to the Karatau-representatives of the family than to the baisinsk representatives.
As to the first Symphytum in the list of Cretaceous lower Hymenoptera we may refer to Figure 9, above , that is to Gigantoxyela quadrifurcata Rasn., of the Family Xyelidae.

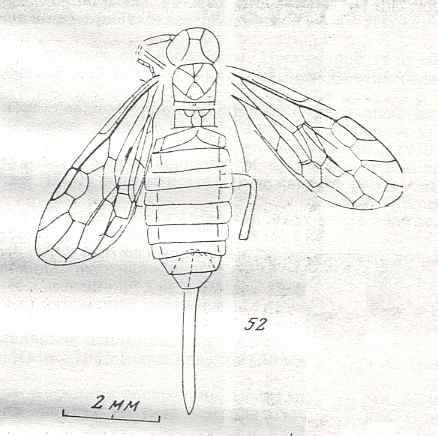
Figure 96 : Spathoxyela fossilis Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae. Combined drawing from two specimens.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

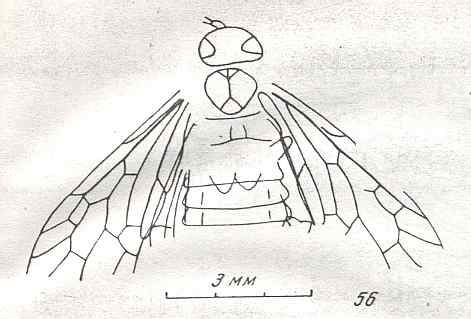
Figure 97 : Eoxyela siberica Rasn. Lower Cretaceous (?) Zabaikalj (Baisa). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

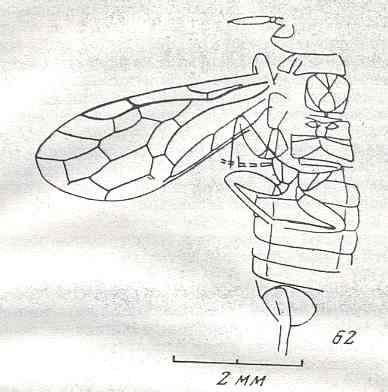
Figure 98 : Xyela (Mesoxyela) mesozoica Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)
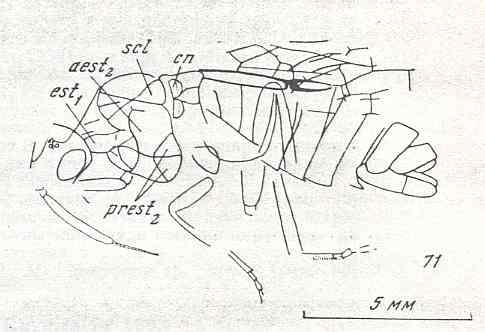
Figure 99 : Baissoxyela tarsalis Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae. cn - cenchrus, scl - scutellum of thorax, aest2 - anepisternite of mesothorax, est1 - episternite of prothorax, prest2 - preepisternite of mesothorax.
(After RASNITSYN, 1969)

Figure 100 : Angaridyela vitimica Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae. Combined drawing from three specimens. (Hindlegs and hindwings of holotype of A. pallipes Rasn.)
Compare with Xyela (recent) . (After RASNITSYN, 1969)

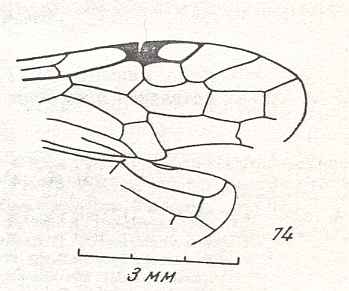
Figure 101 : Angaridyela minor Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

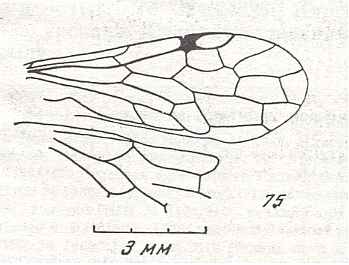
Figure 102 : Angaridyela pallipes Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 103 : Chaetoxyela hirsuta Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 104 : Ceroxyela dolichocera Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae. Combined drawing from two specimens.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

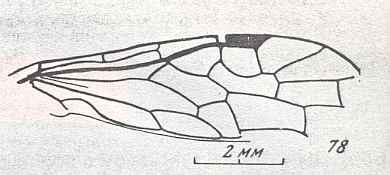
Figure 105 : Xyelites trigeminus Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae.
Compare with Xyela (recent) . Here the basal segment of the Radial Sector is almost completely suppressed by the up-curving Media. (After RASNITSYN, 1969)

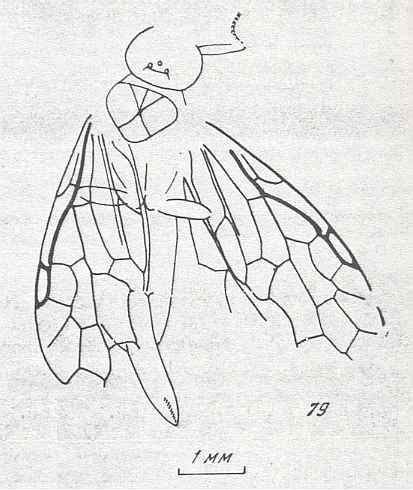
Figure 106 : Uroxyela sicicauda Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

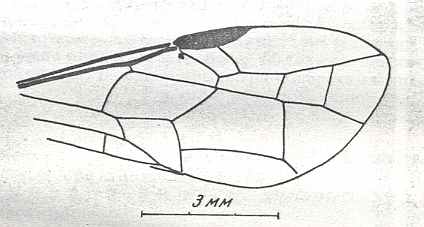
Figure 107 : Mesocephus sibericus Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Cephidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

Figure 108 : Dolichostigma tenuipes Rasn. Lower Cretaceous (?) of Zabaikalj (Baisa). Family Anaxyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

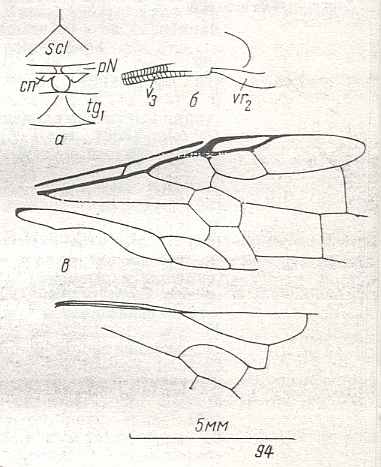
Figure 109 : Kempendaja jacutensis Rasn. Lower Cretaceous of Jakutija (Kempendjai). Family Anaxyelidae.
Top left : part of notum of mesothorax, notum of metathorax, and first abdominal tergite.
Top right : preserved part of ovipositor.
Below : wings.
scl - scutellum of thorax, cn - cenchrum, pN - postnotum of thorax, tg1 - first abdominal tergite, V3 - third valve of ovipositor, vr2 - second valvifer.
Compare with Xyela (recent) . (After RASNITSYN, 1969)
With the beginning of the Tertiary (the geohistoric epoque coming after the Cretaceous, and followed by the last one, the Quaternary) we leave the Mesozoic and enter the Cenozoic.
The first half (i.e. the older) Tertiary is called Paleogene, wheras the second half is called the Neogene.
The Paleogene begins with the Paleocene (the oldest period of the Tertiary), is then followed by the Eocene, and concluded by the Oligocene.
The Neogene begins with the Miocene and concludes with the Pliocene.
Symphyta of the Tertiary

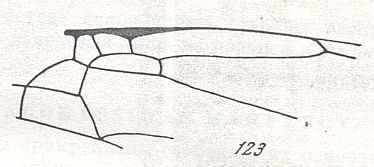
Figure 110 : Megapterites mirabilis Cockerell. Eocene of England. Family Myrmiciidae. (After COCKERELL, 1921, in RASNITSYN, 1969)

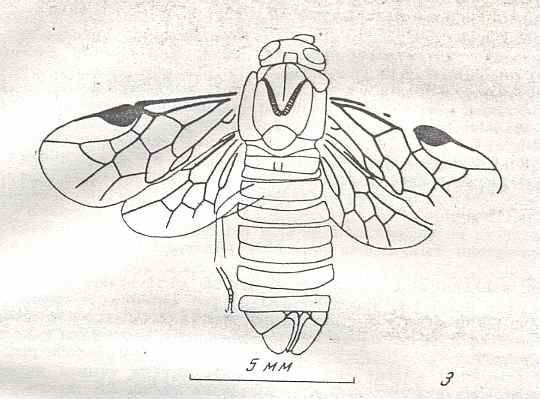
Figure 111 : Schizocerus konowi Rohwer. Oligocene of Colorado [USA] (Florissant). Family Argidae. Drawing after a photograph sent by professor F.M. Carpenter.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

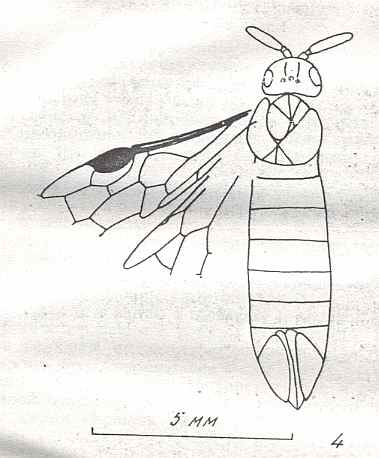
Figure 112 : Paremphytus ostentus Brues. Oligocene of Colorado [USA] (Florissant). Family Blasticotomidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

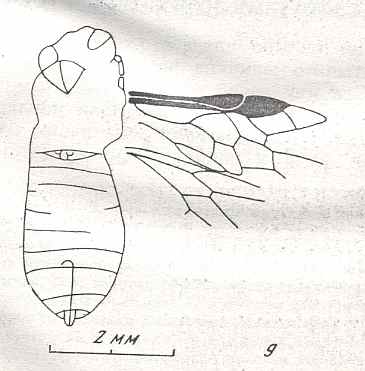
Figure 113 : Lithoryssus parvus Brues. Oligocene of Colorado [USA] (Florissant). Family Tenthredinidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)

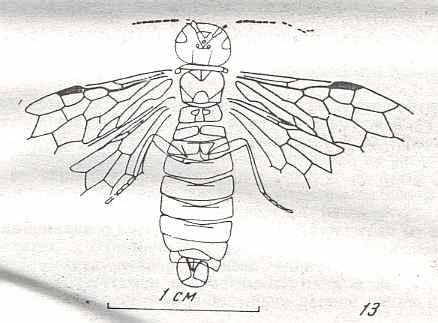
Figure 114 : Cephalea caplani Cockerell. Oligocene of Colorado [USA] (Creed [I hope I've spelled this name correctly] ). Family Pamphiliidae. Combined drawing from several specimen
Compare with Xyela (recent) . (After RASNITSYN, 1969)

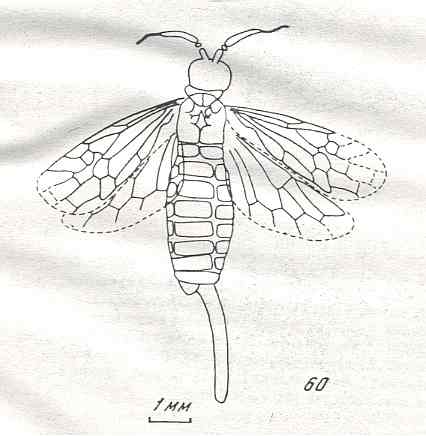
Figure 115 : Pinicolites graciosus Meunier. Oligocene of Germany (Rott). Family Xyelidae.
Compare with Xyela (recent) . (After STATZ, 1937, in RASNITSYN, 1969)

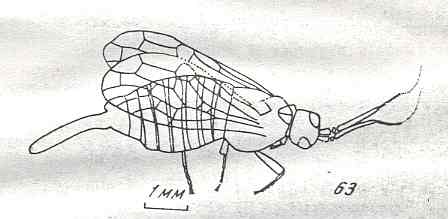
Figure 116 : Xyela magna Statz. Oligocene of Germany (Rott). Family Xyelidae.
Compare with Xyela (recent) . (After STATZ, 1937, in RASNITSYN, 1969)

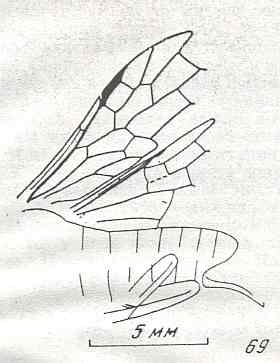
Figure 117 : Megaxyela petrefacta Brues. Oligocene of Colorado [USA] (Florissant). Family Xyelidae.
Compare with Xyela (recent) . (After RASNITSYN, 1969)
Here we will depict wings of recent Symphyta, and have them compared with those of the fossil representatives of the Suborder. Also here, we let accompany each Figure with a schematic (colored) drawing of the, for the Hymenoptera, approximate original condition of the main-vein systems in the forewing.
The first example of recent Symphyta was already given above : species of the generalized family Xyelidae : Figure 8 , and Figure 1 .

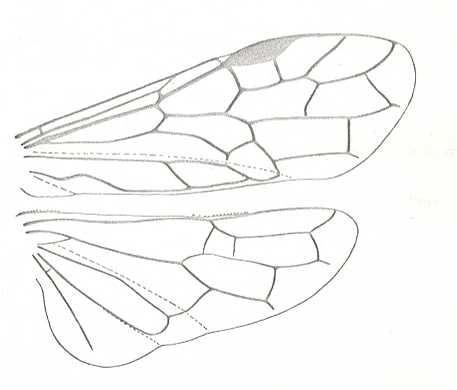
Figure 117A : Wings of Macroxyela. Family Xyelidae. Recent. Forewing : Subcosta 2-branched. Radial Sector 2-branched. Media, after having arched up, coalesces with RS (forming M+RS) for a relatively long distance. Cross-veins 1r-rs and 2r-rs present.
(After COMSTOCK, 1918)

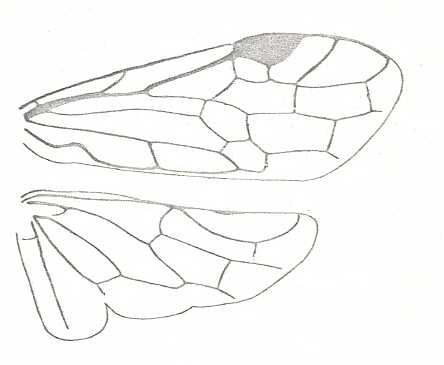
Figure 117B : Wings of Manoxyela. Family Xyelidae. Recent. Forewing : Subcosta unbranched, or, second branch coalesced with R. Radial Sector 2-branched. Media, after having arched up, just touches RS and then leaves it independently again. Cross-veins 1r-rs and 2r-rs present.
(After MacGILLIVRAY, in COMSTOCK, 1918)
Above we already had pictured the wings of a member of the Pamphiliidae, which is also a generalized family : Figure 7 .


Figure 127 : Wings of Cephalcia Panz. Family Pamphiliidae. Recent. (After RASNITSYN, 1969)

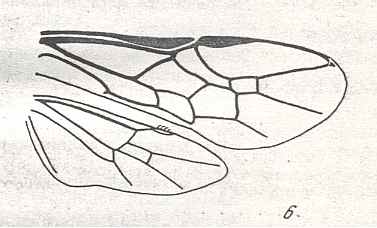
Figure 118 : Wings of Acordulecera Say. Family Pergidae. Recent. If we go around the forewing's apical margin, beginning at the pterostigma, we see successively the tip of RS (meeting with the tip of R), the tip of M, and the tip of CuA.
The segment of RS connecting M with the cross-vein 1r-rs has disappeared (likewise the cross-vein 2r-rs has disappeared). This segment is still well developed in, for example, Xyelinus. From the strong, curved 1r-rs the Radial Sector runs to the apex of the wing.
(After MacGillivray, 1906, in RASNITSYN, 1969)

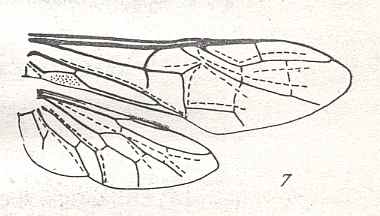
Figure 119 : Wings of Cimbex Oliv., male. Family Cimbicidae. Recent. The Media suppresses the origin of the Radial Sector, and the segment of the latter, between the Media and the place where 1r-rs is supposed to be, has disappeared. (After RASNITSYN, 1969)

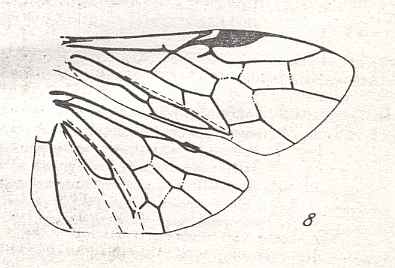
Figure 120 : Wings of Neodiprion Rohw. Family Diprionidae. Recent. (After RASNITSYN, 1969)

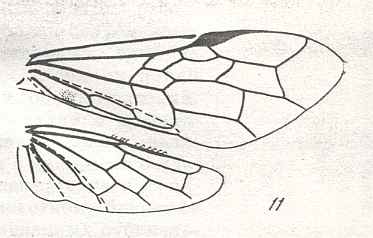
Figure 121 : Wings of Megalodontes Latr. Family Megalodontidae. Recent. The base of RS runs obliquely backwards (See Figure 124). (After RASNITSYN, 1969)

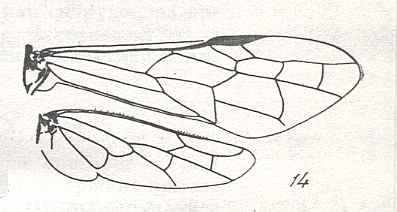
Figure 122 : Wings of Syrista Knw. Family Cephidae. Recent. The vein A2+A3 is straightened out. (After RASNITSYN, 1969)

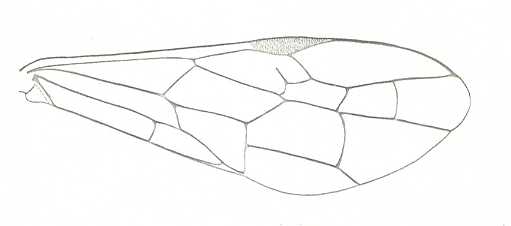
Figure 122A : Forewing of Janus abbreviatus. Family Cephidae. Recent. Subcosta absent. Radial Sector unbranched. Media, after having arched up, coalesces with RS for quite a long distance and then leaves it independently again. Cross-vein 1r-rs only partially present. The cross-vein 2r-rs present. The cross-veins 2r-m and 3r-m present.
(After COMSTOCK, 1918)

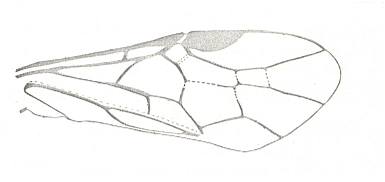
Figure 122B : Forewing of Pteronidea ribesii. Family ? Cephidae. Recent. Subcosta merged with R, but with a free end. Radial Sector unbranched. Media, after having arched up highly, coalesces with R and RS for a short distance and then leaves it independently again. As a result of this merging the base of RS is suppressed by M. Part of RS from the point of leaving M to its meeting point with the cross-vein 2r-rs more or less atrophied. The latter cross-vein looking like the continuation of RS. The cross-vein 1r-rs present as a strong oblique vein. As to its position with respect to the pterostigma, the mentioned cross-vein 2r-rs might well be the cross-vein 1r-rs, but in that case the identity of the short vein proximad to it is unclear. It could be the end of SC or part of R. The latter interpretation seems the more probable one. In that case we must say that the cross-vein 2r-rs is wanting, while 1r-rs is present as a strong short vein. The cross-veins 2r-m and 3r-m present.
(After COMSTOCK, 1918)

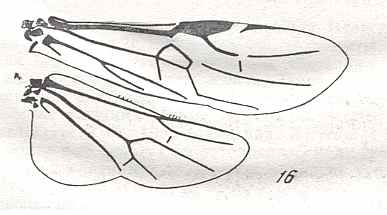
Figure 123 : Wings of Orussus F. Family Orussidae. Recent. (After RASNITSYN, 1969)

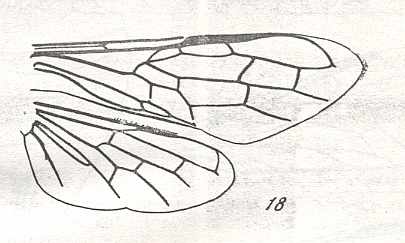
Figure 124 : Wings of Urocerus Geoffr. Family Siricidae. Recent.
As to the forewing : The short basal segment of RS is directed obliquely toward the wing-base. This we see in the wings of many (fossil) representatives of the second suborder (of Hymenoptera), the Apocrita [Terebrantia (= Hymenoptera parasitica) + Aculeata [sting-bearing Hymenoptera (such as ants, bees, and true wasps)]]. So the actual course of RS is rather peculiar : Having originated from the Radius, it first runs obliquely backwards, then it almost makes a U-turn, and finally proceeds to the apical part of the wing where it meets the downward-curved end-segment of the Radius. (See also Figure 121) (After RASNITSYN, 1969)

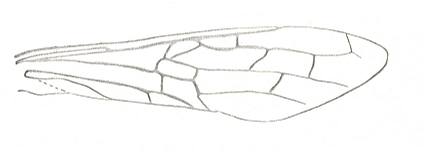
Figure 124A : Forewing of Sirex. Family Siricidae. Recent. Subcosta present, ending up in R. Radial Sector unbranched. Distally, the Radius -- as in Urocerus (previous Figure) -- bends down to meet RS. Indeed, RS has a curious course (like in the previous case) : It branches off from R in a slightly backward (but mainly downward) direction, then meeting the up-arched M, just touching it (i.e. merging with it for a very short distance), and then strongly arching up again until meeting the cross-vein 1r-rs, and then heading off straight to the wing-apex. Cross-vein 2r-rs present. Pterostigma not developed. The cross-veins 2r-m and 3r-m also present.
(After COMSTOCK, 1918)

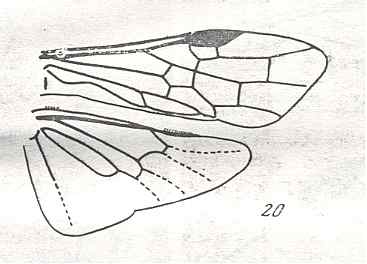
Figure 125 : Wings of Syntexis Rohw. Family Anaxyelidae. Recent.
Forewing : RS obliquely branches off from R, and, after having merged with M for a short part of its course, abruptly turns toward the anterior wing-margin, gives off the cross-vein 1r-rs, then heads into the direction of the wing-apex, and after a very short part of this course gives off the cross-vein 2r-rs, and then proceeds all the way to the wing-apex. The Radial Sector is unbranched. At the end of RS+M we have 1m-cu.
(After ROHWER, 1915, RASNITSYN, 1969)

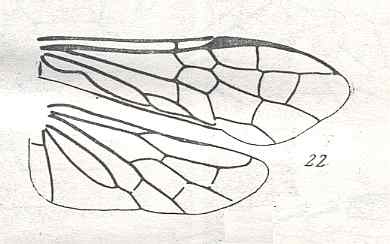
Figure 126 : Wings of Xiphydria Latr. Family Xiphydriidae. Recent. (After RASNITSYN, 1969)

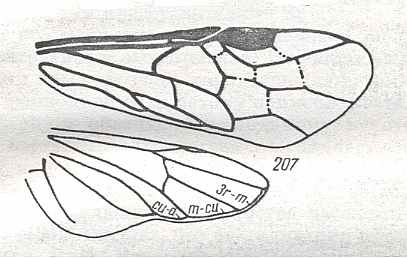
Figure 128 : Wings of Caliroa Costa. male. Family Tenthredinidae. Recent. (After MacGillivray, 1906, in RASNITSYN, 1969)

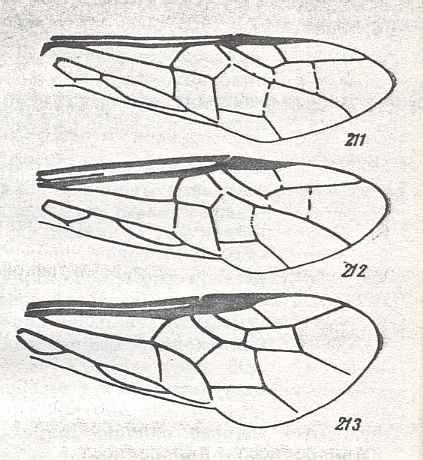
Figure 129 :
Top (211) : Forewing of Cimbex Oliv. female. Family Cimbicidae. Recent.
Middle (212) : Forewing of Zaraea Leach. Family Cimbicidae. Recent.
Bottom (213) : Forewing of Corynis Thunb. Family Cimbicidae. Recent.
In all three (fore)wings the Media has suppressed the origin (basal segment) of the Radial Sector. Further, there is no independent segment of RS that connects its suppressed basal segment with its distal part. The cross-veins 1r-rs and 2r-rs are well developed.
(After RASNITSYN, 1969)

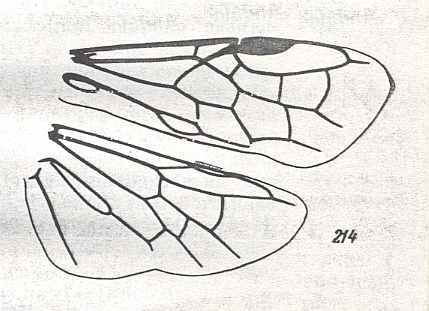
Figure 130 : Wings of Arge Schrnk. Family Argidae. Recent. (After RASNITSYN, 1969)

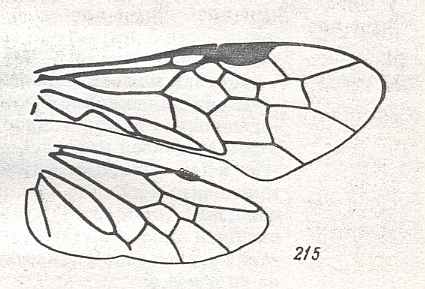
Figure 131 : Wings of Eriocampa Htg. Family Tenthredinidae. Recent. (After RASNITSYN, 1969)

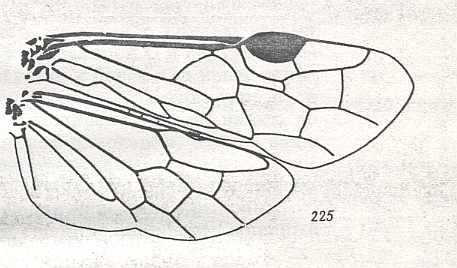
Figure 132 : Wings of Blasticotoma Klug. Family Blasticotomidae. Recent. (After RASNITSYN, 1969)
Transition from Symphyta to Apocrita
In the upper Jurassic of southern Kazachstan three Symphyta individuals of the (fossil) family Karatavitidae, viz., Karatavites angustus ( Figure 47 and Figure 86 ) and Karatavites medius ( Figure 85 ) are found. It is believed by RASNITSYN, 1969 (and 1975) that the upper Jurassic family Karatavitidae phylogenetically stands close to the origin of the suborder Apocrita (higher Hymenoptera).
In 1975 RASNITSYN (Higher Hymenoptera of the Mesozoic, pp. 22) describes yet another fossil apparently also belonging to the family Karatavitidae : Proapocritus praecursor from the Lias (?) of central Asia, that is, allegedly, from the lower Jurassic.
It is instructive to compare the forewing of Karatavites medius (accompanied by a diagram of the original venation of Symphyta) with that of Proapocritus praecursor, and also with the forewing of an ancient representative of the Apocrita Stephanogaster magna (family Ephialtitidae, upper Jurassic) in the drawing of which the names of the cells are indicated.


Figure 85a : Karatavites medius Rasn. Upper Jurassic of southern Kazachstan (Michailovka). Family Karatavitidae.
The normally curved A2+A3 is straightened out, that is, the bend (concavity) of this vein, so typical of Symphyta, has disappeared.
(After RASNITSYN, 1969)
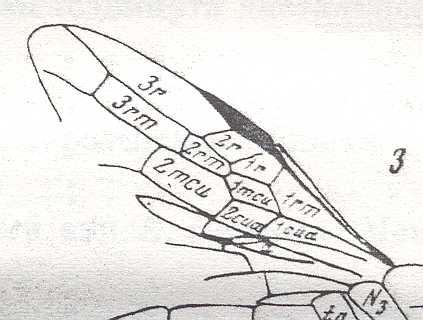
Figure 133 : Wing of Stephanogaster magna Rasn. Upper Jurassic of southern Kazachstan (mountain chain Karatau). Family Ephialtitidae.
Cells in the wing indicated. The two cells under the pterostigma (from about right to left) are 1r and 2r. (After RASNITSYN, 1975)
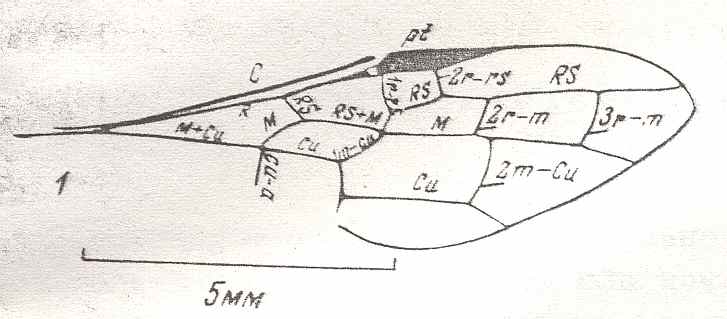
Figure 134 : Proapocritus praecursor Rasn. Lower or middle Jurassic of Sagul, central Asia (Kirgizen Republic, Oshk region, Batensk district, [settlement] Sagul ). Family Karatavitidae.
Anal veins not preserved. Veins and cross-veins indicated. From the pterostigma from left to right two cross-veins go down : 1r-rs and 2r-rs. The course of the Radial Sector, from its origin to its end, is indicated : RS - RS+M - RS - RS. M = Media, Cu = CuA (anterior Cubitus). 1r-rs, 2r-rs, 2r-m, 3r-m, cu-a, 1m-cu, and 2m-cu are cross-veins. The parallellogram-shaped cell in about the middle of the wing (bordered above by RS+M and below by Cu) is the cell 1mcu. Compare with previous Figure and with Karatavites . (After RASNITSYN, 1975) .
We will now give the description of the forewing of a representative of the above mentioned genus Proapocritus. (compare with the Figure above it).
Wing-venation (forewing) principally complete (the structure of the anal region is not known). Costal field moderately narrow, not widened at the base of RS. There is no Subcosta. At the base of the pterostigma is a light spot of weakening. The first segment of RS is straight, short (shorter than the first segment of M and much shorter than RS+M ), obliquely directed to about the apex of the wing.
1r-rs, although complete, but weak, is markedly thinner than 2r-rs. The latter is at the middle of the pterostigma.
RS+M reaches the cross-vein 1m-cu. The cell 2r is strongly widened toward its base, and, consequently, the cell 2rm is strongly narrowed toward its base. The cell 1mcu is long, and the cross-vein cu-a is at its base.
Comparison (of Proapocritus)
The overall look of the wing is extremely similar to wings of primitive Apocrita, especially Ephialtitidae (see below).
Such features, as -- (1) the costal field, totally not broadened at the base RS, (2) the weakened spot at the base of the pterostigma, (3) the short straight first segment of RS, (4) the weakened cross-vein 1r-rs, (5) the cell 2rm being strongly narrowed toward its base, (6) the long cell 1mcu, not [for some distance] lying against the base of the cell 2rm, and, finally, (7), with the cross-vein cu-a at its [1mcu's] base -- are more characteristic of Apocrita than of Symphyta. However, the complete, albeit weakened, cross-vein 1r-rs, and the basal segment of RS (obliquely) pointing to about the wing tip, make the place of Proapocritus inside the Symphyta more probable, and especially in the family Karatavitidae. As to the latter family, a close phylogenetic relationship was assumed also already earlier [by Rasnitsyn, 1969]. The discovery of Proapocritus confirms this assumption : If this form is not a direct ancestor of the higher Hymenoptera, then a form close to it will. The absence of data about the structure of the body is, in the present case, not so significant : In those features that usually are visible in fossils (impressions) of the body of Hymenoptera, the Karatavitidae and Ephialtitidae do not differ. From the genus Karatavites the described genus differs in the above listed features.
The family Ephialtitidae (upper Jurassic of Spain and southern Kazachstan), see Figure 133 , is supposed to be the most primitive family of the Hymenoptera Apocrita. The wing-venation [we only consider the forewing] usually is complete. In the forewing a rudiment of 1r-rs is often present, sometimes almost reaching the pterostigma. The cross-vein 2r-m is always developed. Not seldom the cell 2a is closed. The costal field is broad.
Second abdominal segment is well developed, it is comparatively little, or completely not, changed to become a hinge-segment in contrast to the next absominal segments.
The Ephialtitidae differ from the Stephanidae [a recent apocritan family] in their more complete venation (in Stephanidae 2--3r-m and 2m-cu are absent in the forewing) and in the absence of the of the Stephanidae typical specializations of the head, thorax, and legs.
The Ephialtitidae are morphologically in such a high degree primitive that we can, without special qualifications, see them as an ancestral group of all remaining Apocrita. As to their structure, they are still very close to the lower Hymenoptera ( Symphyta), especially to the Karatavitidae. Apparently, we may indicate only two reliable diagnostic characters by which the Ephialtitidae and Karatavitidae may be distinguished (but not all Apocrita from all Symphyta!) : (1) The position of the first segment of RS of the forewing -- In Karatavitidae it is directed obliquely to the wing-apex ( Figure 85 and Figure 47 ), whereas in Ephialtitidae it is perpendicular to R ( Figure 133 ), or, more often, directed to the base of the wing, see next Figure :
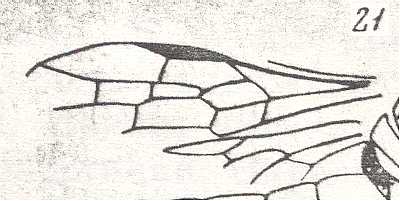
Figure 135 : (Part of) Forewing (and part of hindwing) of Leptephialtites euryarthrus Rasn. Upper Jurassic (Malm) of Karatau (southern Kazachstan) (Michailovka). Family Ephialtitidae. The cross-vein 1r-rs has vanished. (After RASNITSYN, 1975)
and (2) (as a second diagnostic character distinguishing the Ephialtitidae and Karatavitidae) if it were only the partial reduction of 1r-rs. Thus, the Ephialtitidae, judging from the available data, represent the "missing link" between Symphyta (as represented by the Karatavitidae) and the remaining Apocrita. In this, the morphological gap is, in both cases, albeit neat, not deep. Especially, the mentioned differences between the Karatavitidae and the Ephialtitidae are, apparently, not directly connected with the change of the way of life as it is supposed to have taken place in the Ephialtitidae, that is, with the transition from feeding [of the larvae] on wood to zoophagy (a similar position of the first segment of RS is known in a whole series of lower Hymenoptera, including also Siricidae ( Figure 124 ) [of which the larvae still live in wood, i.e. are xylophagous] which are rather closely related to the Karatavitidae, and the reduction of the cross-vein 1r-rs as it is seen [not only in Ephialtitidae but also] in Cephoidea and Orussoidea ( Figure 123 )).
We have now concluded our treatment of the evolution of the suborder Symphyta (as to their wing-venation), following RASNITSYN, 1969, and the evolutionary transition (also as to the wing-venation) from them to the early Apocrita.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for further study of Organic Evolution, Part LXVII.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII
Back to Evolutionary Part LVII
Back to Evolutionary Part LVIII
Back to Evolutionary Part LXII
Back to Evolutionary Part LXIII
Back to Evolutionary Part LXIV