

This document gives some existing additional (secondary) strategies of (aculeate) wasps, taken from EVANS and EBERHARD, 1973, The Wasps, pp. 207.
Cleptoparasitic Wasps
The substances stored in the nests of wasps, bees, and ants, represent rich supplies of food, and it is perhaps not surprising that a variety of organisms take advantage of this. These are the cleptoparasites, sometimes also called brood-parasites or labor-parasites. For the most part they do not feed directly on the cell contents, but deposit an egg so that their larvae may do so. The offspring of the host must, of course, be disposed of, and most cleptoparasites have developed behavioral mechanisms for destroying the egg or larva of the host so that it cannot compete with their own larva for the limited cell contents. Hence cleptoparasites are also predators of a sort, although they attack the host not primarily as food but simply to make its food-store fully available to their own offspring.
It has often been pointed out that many cleptoparasites are closely related to their hosts. For example, the most abundant cleptoparasites of spider wasps are other Pompilidae resembling their hosts very closely. This resemblance extends to even their most subtle structural features and indicates that this is by no means mimicry but a reflection of the fact that parasites and hosts shared a recent common ancestry. In all probability cleptoparasitism began as simple brigandage, that is, as stealing of paralyzed prey as it lay near the nest entrance. This was postulated many years ago by the noted French wasp observer Charles Ferton, who wrote as follows :
" The parasitic habit would . . . appear to have been built up in the following manner : [Episyron] rufipes [a common pompilid], living in colonies [that is, aggregations [JB]], has aquired the habit of stealing the prey of its neighbor and even of fighting for the possession of prey not its own. Some individuals finally learned to steal the spiders that had been buried, either by driving away the rightful owner while she was sealing the burrow, or by ferreting through the soil occupied by the colony in search of sealed burrows. Their decendants, inheriting this habit, gave up constructing a nest and transporting the stolen prey to it and left it in the cell where it was discovered, simply substituting their egg for the one it bore. Thus [the genus Evagetes] was evolved, scarcely distinct from the maternal stock in many of its anatomical characters but become a parasite on the species from which it arose".
This was written in 1905, so perhaps we may forgive Ferton his assumptions that learned behavior may be inherited and that species may evolve in the absence of isolation. Whatever the mechanisms (and the origin of cleptoparasitism and social parasitism is far from fully understood), Ferton may have accurately described some of the intermediate stages. W.M. Wheeler, in an important paper on this subject in 1919, postulated that cleptoparasitism might arise as a result of "urgency of oviposition and temporary or local dearth of the supply of provisions for the offspring".
There are many records of spider wasps taking the paralyzed prey of other individuals, either of the same or different species. In some instances the prey is taken from a plant crotch or other hiding place, in other instances from the burrow entrance or even the burrow itself. Some species seem more inclined to prey-stealing than others, for example the Euopean Episyron rufipes, discussed by Ferton, and the North American Priocnemis cornica. In Japan, certain individuals of Batozonellus annulatus have been seen to dig into nests of other members of the same species, destroy the egg, and substitute their own egg.
Members of the pompilid genus Evagetes, as Ferton mentioned, do not hunt spiders but seek out the provisioned nests of spider wasps of other genera, enter them, and substitute their own egg for that of their host. The genus Evagetes resembles Pompilus so closely that separation of the genera on the basis of museum specimens is sometimes difficult. Yet the behavior patterns of the cleptoparasitic genus have undergone a remarkable reorganization. Evagetes females spend much of their time walking over the ground in areas where spider wasps are nesting. Their somewhat thickened antennae remain in constant motion over the soil surface, and it is believed that they detect nests by odor or by the "feel" of a filled entrance. Often they pause to dig at a certain spot or to explore a depression. If they observe a pompilid nesting, they "freeze" or "hide" behind some object until the pompilid completes its nest and provisions it. Then the Evagetes may rush into the nest, even before the host completes the closure. In most recorded instances, the host makes no attempt to drive the parasite away. Once inside the cell, the Evagetes removes the host's egg from the spider with its mandibles and chews or even devours it. A moment later it lays its own slightly smaller egg on the spider, then leaves the nest, scraping sand into the burrow behind it. It has been shown that if the spider already happens to bear an Evagetes egg, it is nevertheless destroyed and a new one substituted.
The species of Evagetes are sometimes very common, so they evidently thrive by taking advantage of the labors of other pompilids.
There is another genus of cleptoparasitic pompilids which is less commonly encountered in nature and which is structurally rather different from its hosts, so much so that it is usually placed in a different subfamily. This is the genus Ceropales. It is probable that Ceropales split off from the stock of spider-hunting pompilids much earlier than Evagetes. It has had time to develop many structural differences as well as a more advanced type of cleptoparasitism.
The female Ceropales also lurks about where other pompilids are nesting, but her attention is focused not upon the nest, but upon the spider as it is being transported to the nest. See next Figure.

Figure 1 : A female Ceropales maculatus (left) following close behind a prey-laden female Pompilus plumbeus (right). In a moment the Ceropales will quickly attempt to insert her egg into the book-lungs of the spider, perhaps resulting in a fight between host and parasite.
(Günter Olberg, 1959, in EVANS and EBERHARD, 1973)
At a propitious moment, the Ceropales leaps upon the spider and quickly inserts her egg into the book-lungs, where it is invisible or nearly so from the outside. The tip of the abdomen of Ceropales is compressed and somewhat wedge-shaped, evidently an adaptation for inserting the egg into the book-lungs. The host wasp has often been seen attacking the parasite or attempting to pull the spider away or protect it from the oviposition thrusts of the Ceropales, suggesting that in this case there has also been time for the evolution of a response of the host to its rather different-appearing parasite. Whether or not the parasite is successful, the pompilid ordinarily completes its nesting and lays an egg on the spider. If a Ceropales egg is also present, it hatches in a shorter time than the host egg and begins to feed on the spider first. when the host larva hatches, the Ceropales larva devours it, too. Thus the specializations of Ceropales extend to the hatching time of the egg and the behavior of the larva.
It is in the "cuckoo wasps", the family Chrysididae, that this feature reaches its greatest development [See also Part XLVII of the present series of this website]. Cuckoo wasps not only have an unusually thick, strongly sculptured integument, but they are capable of rolling into a ball by applying the concave under surface of their abdomen to the underside of the thorax, in this way offering scarcely any target for the attacks of their host. See next Figure.
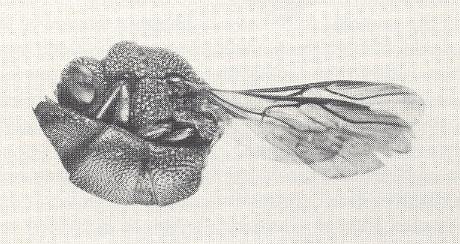
Figure 2 : A cuckoo wasp, Chrysis parvula, in the rolled-up position assumed when attacked. The shieldlike abdomen is concave beneath, and protects the vulnerable parts of the head and thorax.
(U.S. Department of Agriculture, in EVANS and EBERHARD, 1973)
Cuckoo wasps are also unusual in that they have completely lost the ability to sting. Rather, the whole apical part of the abdomen forms an extensible tube which can be extruded like a telescope at the moment of oviposition into the nest-cell of the host. Cuckoo wasps attack a wide variety of wasps and bees and apparently split off from the main line of wasp evolution a long time ago (perhaps from a bethylidlike ancestor). Most species have bright, metallic colors -- green, blue, or coppery red -- but so far as we know no one has satisfactorily explained the significance of these unusual colors.
Social Parasites
The terms cleptoparasitism and social parasitism are sometimes used interchangeably, but we prefer to restrict the latter to parasites of social species which use their hosts as a work force rather than as a direct source of food. The female social parasite waits until the queen of a colony has established her nest and reared a number of worker offspring. Then she enters the nest and usurps the position of the rightful queen, preventing her further reproduction by either killing her, driving her away, or eating her eggs. The host workers tolerate the foreign queen and care for her young as they would the offspring of their own mother. The result is that the colony eventually comes to consist mostly if not entirely of adults of the parasite species -- all males and females, as no workers are produced.
Among the vespid wasps at least seven species are known to be obligatory social parasites. That is, the females are incapble of founding their own colonies independently and produce no workers of their own. So closely do the parasites resemble their hosts that most of them have been recognized only within the last thirty years. All are associated with the best-studied genera of social wasps (Vespula and Polistes), suggesting that investigation of the many tropical social wasps whose biology is almost unknown will reveal others. European workers have tended to put the parasitic Polistes in a separate genus (Sulcopolistes) and the parasitic Vespula in two separate genera (Pseudovespula and Vespula, restricting the names Paravespula and Dolichovespula to the nonparasitic species). This nomenclature is not only unduly complicated but tends to conceal the fact that the relationship of parasite and host is very close indeed, and in each case the parasite may well have evolved from a common ancestor with its host. Parasites differ from their hosts in having a more heavily sclerotized integument, larger head and mandibles, and in some cases a stronger, recurved sting -- all adaptations probably useful in fighting.
In Polistes the interactions of hosts and parasites have been observed directly by Joachim Sheven in Europe. He found that the parasites act as superdominant individuals, overcoming the original inhabitants one by one with an exaggerated form of the dominance behavior common in Polistes colonies (for Polistes colonies see previous document). The female of Polistes ("Sulcopolistes") atrimandibularis touches the host (P. bimaculatus or P. omissus) "slowly and intensively with the antennae, slowly riding upon her, and at last ... bending the abdomen and aiming the sting against the waist and neck of the dominated animal". However, the usurper usually does not kill the host queen, which remains on the nest until she disappears or dies of other causes. P. semenowi (parasite of P. gallicus and P. nimpha) and P. sulcifer (parasite of P. gallicus) use less violent methods, with less stinging and more antennal striking.
The takeover of a Vespula colony by a social parasite has apparently never been observed, but a partial reconstruction of events is possible from inspection of the contents of parasitized nests. Sometimes the body of the host queen is found, indicating that she was killed by the usurper. And in one nest of the European Vespula sylvestris, parasitized by a female of V. omissa, eight host workers were found dead near the corpse of their deposed queen, apparently having come to her aid in vain.
In North America, it is not uncommon to find colonies of the common yellow jacket Vespula arenaria which have been parasitized by a species having white markings instead of yellow, Vespula arctica. In the early summer, parasitized nests are not likely to be recognized, since they contain many arenaria workers plus one female arctica and some of her brood, but by August the arenaria workers dwindle in numbers and a colony of yellow jackets becomes transformed into a colony of "white jackets", all of whom are queens and males.
There are two known "facultative" social parasites (i.e. parasites capable of living and thriving under more than one set of conditions) whose habits shed some light on the mode of evolution of obligatory social parasitism. These species, Vespula squamosa and Vespa dybowskii, are capable of founding colonies independently and always produce some workers of their own. But sometimes they take over colonies established by queens of other species (respectively : Vespula vidua, Vespa crabro or V. xanthoptera). Both species nest somewhat later than those they parasitize. And one observer noted a similarity between the nesting sites of host and parasite. Thus the original social parasites may in some cases have been aggressive, late-nesting females who encountered established nests while searching for nesting sites of their own.
Parasitoid Wasps
In this section we shall consider certain wasps that develop as parasitoids of other wasps, that is, their larvae feed not on the food provided them but directly on the larva of the host, generally after it has completed its feeding. Two major groups fall in this category : (1) certain kinds of true (aculeate) wasps (Mutillidae, some Chrysididae), which have become parasitoids secondarily, and (2) some of the true parasitoids (Terebrantia : a few ichneumon, chalcid, and trigonalid wasps) which have taken to attacking wasp larvae in their nest-cells rather than free-living or boring larvae of other [insect] orders.
Members of the first group attack only solitary wasps, but members of the second group attack both solitary and social species. [Here, i.e. on the present website, we will only deal with the first group, because the present document is on (additional) strategies of true, that is, aculeate, wasps.]
It is probable that the majority of Chrysididae are cleptoparasites, as the name "cuckoo wasps" implies. Their larvae do, of course, destroy the egg or larva of the host, in the manner of many cleptoparasites, and in at least one case it has been shown that the chrysidid larva fails to feed upon the cell contents and to grow if it is deprived of its initial meal, the host larva. From such an antecedent has apparently developed the delayed development of some of the more specialized chrysidids, such as members of the genus Parnopes. The female Parnopes edwardsii enters the nest of ground-nesting fly-predators of genera such as Bembix and Steniolia, often while the host wasps are actively provisioning. Steniolia females have been seen to attack the parasite vigorously, attempting to sting it and even carrying the coiled-up Parnopes several inches and dropping it. When the cuckoo wasp is successful in entering a cell containing a partially grown larva (Steniolia and Bembix are progressive provisioners), she lays her egg on the larva and departs. The egg hatches in a few days, but the Parnopes larva feeds very little at first and remains a very small grub attached to the thorax of the host larva until the latter spins its cocoon. Then the chrysidid larva begins to feed more actively, and within a week or ten days it consumes the host larva and spins its own cocoon inside that of the host. Thus by some physiological mechanism permitting a delay in growth until after the host has completed feeding, these chrysidids have been converted from cleptoparasites to parasitoids of an unusual kind.
One of the chrysidid parasites of mud-daubers (Sceliphron) evidently lays its egg after the cell has already been closed and the host larva has spun its cocoon. In this case the female cuckoo wasp, Chrysis fuscipennis, makes a conical hole through the wall of the mud cell and lays its egg through the breach. Upon withdrawal of the ovipositor the hole is sealed with a brown plug probably formed from a secretion of the wasp. The Chrysis larva then consumes the pupa of the Sceliphron and spins its own cocoon, later emerging by chewing a hole through the mud closing plug. It seems odd that this apparently specialized mode of entry and parasitism occurs in the same genus as other cleptoparasitic species such as Chrysis coerulans, discussed earlier. In the vast majority of chrysidids, we do not know the manner of entry into the host cell or whether development is primarily upon the prey or upon the host larva.
Mutillidae ("velvet ants") [See also Part XLVII of the present series of this website, Figures 11-13a] apparently always attack larvae after they have spun their cocoons. The females enter nests already containing cocoons, either by digging through the soil or breaking through the walls of mud nests or the closing plugs of nests in twigs. They then chew a hole through the wall of the cocoon, turn around and insert an egg through the hole and onto the diapausing host larva, and finally seal up the hole in the cocoon with salivary fluids and particles of soil or mud.

Figure 3 : A female mutillid wasp, also called "velvet ant" or "cow-killer".
(U.S. Department of Agriculture, in EVANS and EBERHARD, 1973)
Mutillids do not appear to be highly host-specific. Some species attack wasps of several genera or even of more than one family, and at least one species appears to attack certain bees as well as wasps. Others attack various ground-nesting or twig-nesting bees, and a few even attack the puparia of flies such as the tsetse fly, sealing up the hole in the puparium in much the way that the hole in the cocoon is sealed. Certain Mutillidae are reported to attack adult bees or wasps, including honeybees and Bembix, biting the host in the neck region and sucking out its body contents.
With all this we conclude our exposition of some additional strategies in wasps. And with it our exposition of the evolutionary phases of true (aculeate) wasps has come to an end.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part LVI.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII