

REMARK : Although the caption of the present, previous, and subsequent documents reads "Organic Evolution in terms of the Implicate and Explicate Orders", the reader finds merely expositions of the phases of evolution of the Hymenoptera in terms of biology, behavior, and instincts, that is, in terms solely of the Explicate Order. However, as has been expounded in the first document of the present series on Hymenoptera, we hold that organic individuals are substances in the metaphysical sense. Such a substance carries a so-called essence determining WHAT such an individual intrinsically is, not as an individual, but as a species or type. The organic substances differ from inorganic substances by the fact that their "essence" presents itself, not merely as some definite structural material pattern that is thermodynamically stable -- as is the case in all inorganic true substances -- but as STRATEGIES to exist and persist. This is because the organic individual is thermodynamically highly unstable. It is as such far from thermodynamic equilibrium, and it will cease to exist as soon as it becomes thermodynamically stable and thus acquiring thermodynamic equilibrium, because this means its very disintegration. The strategy of a given organismic species is thus, to begin with, its internal chemistry and physiology, its morphology, and its complete way of live. And because an organic individual is thermodynamically unstable it must absorb energy, but even then it cannot persist very long, so it must, in order to allow its essence to persist beyond individual life, reproduce itself. And guaranteeing successful reproduction belongs to the way of life of the individual too, in addition to feeding, etc. It is part of the organism's strategy or essence. In higher organisms, among which surely the Hymenoptera, this strategy becomes more and more active and sophisticated. However, the origin and evolution of these strategies cannot be accomplished by the Explicate Order all by itself (In epistemological terms : We cannot explain the origin and evolution of these strategies by the model of random genetic mutations and natural selection of advantageous mutants). We must assume that these strategies already 'exist' implicitly, that is, in the form of noëtic patterns, residing in the Implicate Order. When conditions in the Explicate Order are appropriate these patterns can be "projected" from the Implicate Order into the Explicate Order, where they then appear unfolded along space and time dimensions. They appear as material individuals in the course of time acquiring adaptations to a certain way of life in some particular environment.
When the egg of the prey had lost its significance as being the very place and object of feeding of the terebrant larva, certain adults from those terebrants, in their turn, lost the connection with egg-clutches of the host, and began to lay their egg not on or into the egg of the prey, but at some distance from the prey. As a result there now originated the necessity to display activity in order in this or that way to reach the prey. In connection with this, the look of the larva, that emerges from the terebrant egg, has in such a degree changed, that it has received a special name, planidial larva or planidium. On its integument appeared long spinelets, and it acquired the ability to attach itself to the substrate by suction with its anterior end and to boom off itself by its posterior end, in order to execute stepwise and other motions. With respect to its biological task -- independently reaching the prey -- it became similar to the triungulins of the Meloidae (beetles), but according to structure -- missing legs and a well-separated head -- the planidium is very different from the triungulins, and is not homologous with them. It is a secondary (= derived) specially adapted first-instar larva of the present group of terebrants. Having acquired the ability to independently find the prey, some planidial larvae evolved along unusual lines and began to attack even those preys that are totally unreachable by the adult terebrants themselves. The development and the working-out of details of these original relationships of the terebrants with their preys undoubtedly show a series of gradations. The origin of planidial larvae must also be placed at the time of bloom of the archaic inquilinoid phase. Their appearance has been evoked by, apparently, the fact of the inquilines, as this is not seldom observed also today, laying [at each oviposition site] several eggs at a place (originally -- into the gall), a place such, that only one egg can develop in it. Therefore that particular inquiline-larva that had emerged earlier than the others had to destroy not only the egg of the host, but also the surplus of eggs of its own race. In such conditions the attainment of the ability to actively move gave to the newly-born terebrant larva the possibility to resolve a new task, independently reaching its prey, which sometimes is at a considerable distance from the place where the egg was laid and where the larva emerged.
Already among the primitive group of Chalcidoid terebrants there can be observed development with a planidium-like stage. Thus, Syntomaspis eurytomae Puz.-Mal., see next figure, lays its egg on the almost fully-grown larva of the almond seed-eater Eurytoma amygdali End., which develops in kernels of plums.
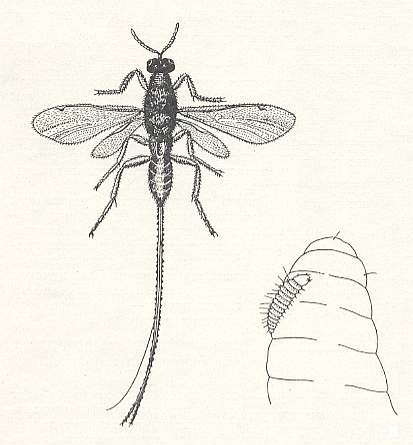
Having emerged from its egg, the planidium-like larva of Syntomaspis, which is covered by rows of spinelets, is able to actively moving around on the body of the prey, then holding itself onto it by suction with the mouth, then bending, and booming off with the posterior end (See Figure 1, right image). On enquiry of larvae of the almond seed-eater 2-3 and even 6 eggs of Syntomaspis were found on one and the same prey. In some cases 2-3 larvae of it were found on one larva of the seed-eater. In this latter case only one single larva of the parasite was in good shape. The others, apparently, died. We, probably, do not need to doubt that also in this case the surplus of eggs and larvae of the parasite were destroyed by the surviving larva of it. In 3-4 days, on having emerged from the egg and got stronger by consuming the juices of the prey, the Syntomaspis-larva moults and transforms into a usual terebrant larva. Further, after having destroyed the larva of the seed-eater, it eats what is left by the seed-eater of the kernel of the plum.
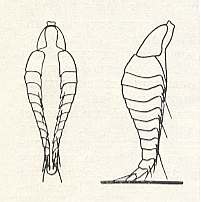
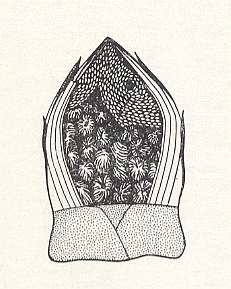
Figure 2 : Planidium (larva of the first instar) of the chalcidid Schizaspidia manipurensis Claus. Ventral and lateral views.
In the latter case in its upright waiting position. [According to the text the chalcidid larva, here depicted, must be that of Perilampus chrysope (Perilampidae) (and thus not Schizapidia manipurensis (Eucharidae)).]
(After CLAUSEN, 1940, in MALYSHEV, 1966)
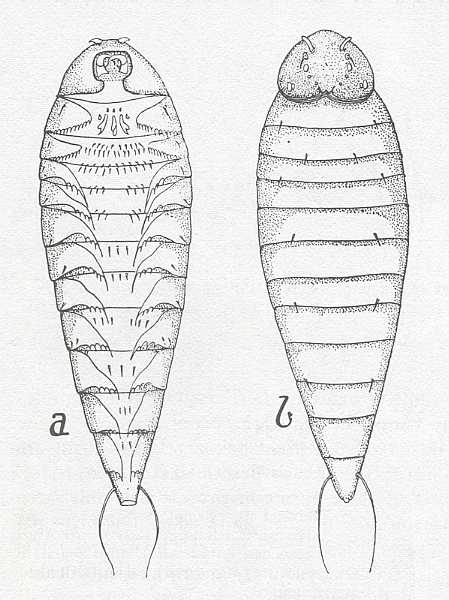
Figure 2a : Planidium of Perilampus. a - ventral view, b - dorsal view.
(After H.S. SMITH, in RICHARDS and DAVIES, Imms' General Textbook of Entomology, 1977)
In this position it remains without moving for days on, until one or another insect appears at its site. Then it suddenly moves furiously stretching and bending forwardly and backwardly trying to attach itself to the possible host. When the Chrysopa-larva happens to come too close, the planidium with hellish rapidity attaches itself to a hair or spinelet of that larva's body. After that it slowly crawls down the hair onto the body of the larva and attaches itself to it with its mouth-hooks. Often one can see a planidium having attached itself to the stalk of the Chrysopa-egg under a right angle with its longitudinal axis. This shows an interesting instinct of the planidium, because it is now in a position to lie in wait for, and indeed does so, as the investigator observed, a young larva of the lace-wing when it emerges from its egg and crawling down along the stalk. In this way for the planidium successfully reaching the appropriate host is guaranteed totally. Having itself attached to the larva of the lace-wing, the planidium does not eat until its prey makes a cocoon and transforms into a pupa.
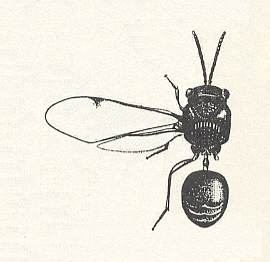
This eucharid drives its very small eggs in clutches into the buds of various trees and shrubs -- white mulberry, horse chestnut, Cladrastris. It was found out that a single female may lay on average 1050 eggs. See next Figure.
Often some females lay their eggs in one and the same bud, and indeed so many buds may be infected that in one single shrub 4320000 eggs could be counted. A large number of these Schizaspidia-eggs, however, perishes, especially in winter time in the case of accidental loss of the cover-scales of the bud and the subsequent influence, on the denuded egg-clutches, by too high a humidity, fungi, and other detrimental factors. The planidia exit from their eggs not until July. They hardly become 0.1 mm long and are able to make fairly limited loop-like movements with the help of the mouth sucker and a group of stiff spines replacing in them the "cerci" (tail-threads) of other planidia at the posterior end of their body. Having left the bud, the planidia crawl in the vicinity of the place of birth over the branches and leaves. Here they, as the case may be, encounter worker-individuals of the timber ant Camponotus japonicus, who are visiting colonies of aphids or fruits of the tree. Having come into contact with an ant, the very small larvae clasp themselves onto the hairs of its feet and in this way are transported by it into the depth of the formicary (ants' nest). There the planidia get to the larvae of the ants. After having crawled over the body of the prey, the planidium nestles on its back, in the deepening between the head and the first segment or between the first and second segments. The planidium consumes a little sap of the prey, moults, and becomes worm-like. As soon as the prey transforms into a pre-pupa or pupa, the development of the parasitic larva speeds up significantly, and as a result of this its feeding is finished in a few days (about a week) after its second moult. Pupation of the eucharid takes place inside the cocoon of the ant, and the adult terebrants independently get there way out of the ants' nest. The males swarm above the formicary, and here mating takes place. The emerged (winged) females live all in all two or three days.
With all this we have concluded our exposition of the vagabondish-parasitic phase of the evolution of the Hymenoptera.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XLI.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX