

REMARK : Although the caption of the present, previous, and subsequent documents reads "Organic Evolution in terms of the Implicate and Explicate Orders", the reader finds merely expositions of the phases of evolution of the Hymenoptera in terms of biology, behavior, and instincts, that is, in terms solely of the Explicate Order. However, as has been expounded in the first document of the present series on Hymenoptera, we hold that organic individuals are substances in the metaphysical sense. Such a substance carries a so-called essence determining WHAT such an individual intrinsically is, not as an individual, but as a species or type. The organic substances differ from inorganic substances by the fact that their "essence" presents itself, not merely as some definite structural material pattern that is thermodynamically stable -- as is the case in all inorganic true substances -- but as STRATEGIES to exist and persist. This is because the organic individual is thermodynamically highly unstable. It is as such far from thermodynamic equilibrium, and it will cease to exist as soon as it becomes thermodynamically stable and thus acquiring thermodynamic equilibrium, because this means its very disintegration. The strategy of a given organismic species is thus, to begin with, its internal chemistry and physiology, its morphology, and its complete way of live. And because an organic individual is thermodynamically unstable it must absorb energy, but even then it cannot persist very long, so it must, in order to allow its essence to persist beyond individual life, reproduce itself. And guaranteeing successful reproduction belongs to the way of life of the individual too, in addition to feeding, etc. It is part of the organism's strategy or essence. In higher organisms, among which surely the Hymenoptera, this strategy becomes more and more active and sophisticated. However, the origin and evolution of these strategies cannot be accomplished by the Explicate Order all by itself (In epistemological terms : We cannot explain the origin and evolution of these strategies by the model of random genetic mutations and natural selection of advantageous mutants). We must assume that these strategies already 'exist' implicitly, that is, in the form of noëtic patterns, residing in the Implicate Order. When conditions in the Explicate Order are appropriate these patterns can be "projected" from the Implicate Order into the Explicate Order, where they then appear unfolded along space and time dimensions. They appear as material individuals in the course of time acquiring adaptations to a certain way of life in some particular environment.
The essential and very important transition in the further development of the way of life of the Hymenoptera took place when the larvae, hatching from the eggs laid into the surface layer of the plant-tissue, began penetrate into the depth of the plants instead of going to their surface to eat. This transition to a life within plants had certain gradations, the expression of which is observed among saw-flies also today.
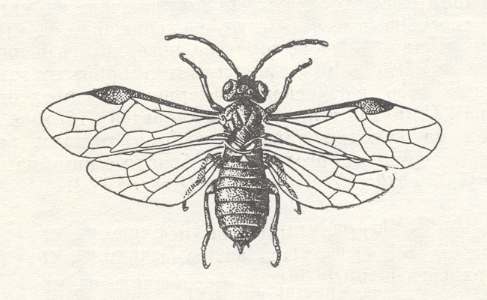
As an indication of the fact that we are not far away from the sources of endophytic feeding of the larvae, may serve a series of phenomena in their life. First of all we note that only in relatively few saw-flies the larvae live within plants, penetrating in shoots, leaves, and fruits, where sometimes around them galls are formed. It is remarkable that among these endophytic saw-flies there is a series of undoubtedly very primitive forms. First of all this is the saw-fly of the above mentioned family Blasticotomidae which forms the transitional link between lower and true saw-flies, namely Blasticotoma filiceti Kl. See next Figure.

The larva of this rare hymenopteron develops in the cavity of the leaf stalk of the fern Athyrium felix femina, secreting small foamy lumps, that surround the leaf stalk. With the primitive morphological features of this saw-fly thus correspond also its archaic habits -- the endophytic connection with the fern.
According to Ross, the saw-flies of the family Tenthredinidae possess a series of interesting structural differences that connect them with the group O r t h a n d r i a [Representatives of the archaic family Xyelidae are characterized by features relating them (also) to the higher group (consisting) of the horntails -- the 'spurious' horntails are the stem-horntails, the Cephidae, while the true horntails (trunk horntails) are the wood-boring Siricidae and allies -- forming with them, as it were, a single whole, the group O r t h a n d r i a, according to Ross.]. Together with this he holds that they are the least changed in the whole superfamily of the true saw-flies Tenthredinoidea.
Apart from horsetails, the food plants of the other Dolerinae are rush (Juncaceae), sedge, and grasses.
It is observed that there exists also a certain instability (variability) in the very way of eating channels within plants by larvae of saw-flies. For example, the larvae of the saw-fly Monophadnus elongatus Kl. that live in shoots of rose plants (family Rosaceae) are "ascending borers", while the larvae of the other rose saw-fly, Ardis brunniventris Htg. are "descending borers". The larvae of Heptamelidae bore channels in ferns [that is, in the "vajach" of the fern, which word, unfortunately, I cannot translate].
In the further development of the saw-flies within the tissues of plants, special adaptations were formed that regulate the outflow of vegetable saps to the place where the larvae feed. So we see that in some saw-flies, for example Pontania proxima Lep., and P. viminalis L. (see next Figures) (subfamily Nematinae, of the same family Tenthredinidae), while the egg is being laid in the tissue of the leaf or shoot of the willow, they produce a special secretion, that causes a rapid uncontrolled growth of plant cells and the formation of a gall. In this way are formed usually one-roomed galls. See next Figures.
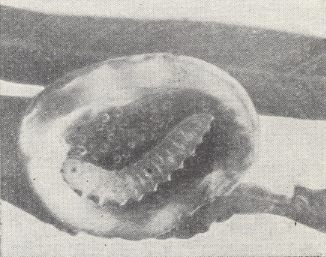
Around the larvae of Euura amerinae, that live together (up until 5 individuals) in shoots of willow, in the beginning individual galls are formed, but later they coalesce resulting in one common room.
In the next phytophagous hymenopterous superfamily of saw-flies, that is, in the so-called stem- or cephoid saw-flies ( Cephoidea ) endophytic feeding has become already a common phenomenon : Their larvae exclusively develop inside green stems or offshoots. Today the whole superfamily is represented by only one family, the cephids -- Cephidae. See next Figure.
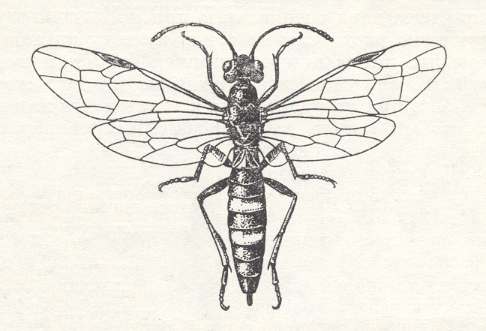
This family contains about 100 species that are encounterd chiefly in the steppes of Eurasia (57 species), and also in the adjoining woodland zone (29 species), while in North America are found all in all 12 species, on Madagaskar 2, and in tropical Asia 4. The biology of the Cephidae is until now (1966) insufficiently studied. For a series of genera and even some larger groups there are no data about their food-plants, implying that "there is still no possibility to guess in what degree future information will clarify the final picture" (BENSON, 1946).
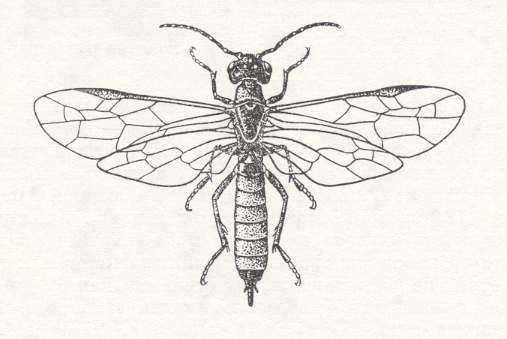
With this also corresponds the fact that this genus has a wider circle of food-plants than other known cephids, within the species, as well as between species. Indeed, the larvae of Janus develop in different trees and shrubs -- pear, apple, rose, currant, guelder rose, willow, poplar, and oak. Thus, the larva of the European "shoot horntail", Janus compressus, bores a spiral channel in the pith of the shoot of pear.

On the other hand, the group Cephiini, consisting of the three genera Cephus, Trachelus, and Calamenta, develops in grasses. With respect to the way of life of the Cephidae the biology of the "cereal saw-fly" (cereal "stem horntail"), Cephus pygmaeus (Figure 3 ), is instructive. The stems of cereal grasses (rye, wheat) are most attractive to the "saw-fly during the time of threshing and bloom. Because the flying period of this saw-fly coincides usually with the threshing of the winter wheat, this plant is infected by it in the highest degree. Having found an appropriate stem, the female with her ovipositor saws a narrow opening through which she lays an egg inside the stem, placing it in the cavity of the stalk at the level of one of the upper nodes. The egg, in line with its development, becomes larger as a result of liquid that is imbibed by it from the tissue of the stem by osmosis. After 10 days from it a larva emerges which feeds on the pith, descending to the base of the stem while gnawing the nodes that it finds in the way. Not seldomly, after having so descended, the larva again crawls up and concludes its feeding with the more delicate tissues of the upper part of the stem. At the time of ripening of the cereals the larva descends to the very base of the plant, and there, directly below the node of the stump, builds for itself a winter refuge. Having prepared in the root-part of the stem a cell which measures 1.5 - 2 times the length of its body, the larva gnaws in the surrounding wall a ring-shaped groove, such that only the outer layer of the wall of the stalk is untouched by it. After this the larva covers the opening of the cell with a plug (consisting) of chips from the stem glued together with saliva, and weaves around itself a thin transparent cocoon which looks like a a little sack of parchment. The gnawed, or we might say, sawed, stem very easily snaps off and the larva remains in the stump that projects above the ground for only 1-5 cm. Walled up in this way, the larva is well protected against the influence of external factors and quietly rests in its refuge until the spring of the next year, changing into a pupa which gives the adult insect halfway May. The "sawed off" stems snap off above the plug at the slightest touch or wind, fall down, and coming to lie in all directions, giving the field a characteristic look.
Similar habits are observed in Trachelus tabidus F. The Cephids (here Cephinae) of the genus Calamenta develop in stems of quitch grass and reed.
So, we can hold that the chief food-plants of the cephoid "horntails" are, on the one hand, woody rose plants with their tendency to grow as shrubs as well as herbs, and grasses on the other. According to Benson, "the chief centers of the origin of the Cephidae must be there, where the moderate woods enter the steppe of Eurasia". Precisely in this zone, as is assumed, also their foodplants appeared. The investigator holds that the very specialized vegetative associations of the Cephidae, like the other saw-flies, developed after the morphological separation of the different families which are known today, and that generally the cephoid archtype developed in the pith of shoots. This feeding habit inside plants also provided the possibility to the Cephidae to survive the direct action of the summer drought in the steppe conditions.
Markedly farther from the starting point did the fourth group of phytophagous Hymenoptera go -- the true horntails, Siricoidea, consisting of three families : Syntexidae (1 genus, 1 species, from California), Xiphydriidae (1-2 cosmopolitic genera), and Siricidae (with two subfamilies -- Siricinae and Tremicinae). See next Figure.
To this complex sometimes is also added the family O r u s s i d a e, the systematic position of which is disputed. Today it is taken as an independent superfamily Orussoidea, containing just the one mentioned family. We shall deal with this family later.
All true horntails lay their eggs already not on the green parts of the plants anymore, but directly in the wood of trees, of pine-trees (Siricidae) or of leaf-bearing trees (Xiphydriidae). For executing such an act, possessing a short ovipositor is not enough. In order, in laying an egg, to bore through the thick bark of trees the development of a special ovipositor was needed, the drill. In some species it was even almost twice as long as the body of the insects.
The young larvae of the true horntails having emerged from the eggs at first feed on the newly deposited wood rings, but soon begin to eat the harder rings. While eating they gnaw in the wood curvy, and circular in transverse section, channels, the diameter of which increases in line with the growth of the larvae. Because of the scarcity of nutritive substances present in wood the larvae of the horntails must eat much of it. As a result the whole channel is totally filled with chewed wood having passed through the intestinal tract of the larva. The feeding of the larva lasts several years. It is interesting that also in such apparently unfavorable conditions new possibilities arose in the life of the larvae. There is an indication that in the mass of wood, surrounding the larva in its gnawed-out channel, threads of fungi (Basidiomycetes) will grow, increasing the quality of the food of the larva of the horntail. As is known, feeding on fungi, seen in different Orders of insects, underwent in some of them a special development : true cultivation of fungi, even such ones that are not encountered in nature elsewhere. But, among Hymenoptera only the ants took this way of development.
In the present phase of evolution of Hymenoptera the most significant moment was another : The morphological changes of the larvae, that went from living in the open to a life within plants. When the sawfly-like ancestors of the Hymenoptera had, in their larval stage, to go from their original way of life on the plants to a life within stems and galls, then, corresponding to the new situation, also the outer appearance of their larvae changed : Their large head with developed sense organs disappeared, so also their functional limbs, colored integument adapted to passively and even active protection. In their place other features appeared : A non-developed head, weak thoracic legs, absence of abdominal limbs, and delicate colorless integument. In this way the origin was set of the powerless, almost immobile, larvae of the higher Hymenoptera, of which the existence turns out to be totally dependent on the presence of correspondingly developed instincts in the adult individuals. In addition we must note that in the present phase of development, in which the origin of the powerless larvae had been already determined, their adult individuals, essentially, still possessed rather simple maternal instincts. As before, they were able, although doing it in other ways, just to lay their eggs precisely on that (or in that) plant-substrate, where their larvae will live completely independently. Not any care whatsoever, by the adult forms, of the young developing offspring was here present, if we exclude the behavior of the above mentioned Australian saw-fly Perga lewisi.
In the above described habits of adult saw-flies we still almost do not see anything that might fill that great gap that separates them from the higher Hymenoptera.
Everything indicates that the next basic moment in the development of maternal instincts of Hymenoptera consists of the transition from the phytophagy of the larvae to their zoophagy (i.e. carnivorous habit). As will be shown, this will take place in the ancestors of the Cephoidea -- the stem saw-flies -- precisely there where the endophytic feeding of the larva takes place in a gall caused by the saw-fly. And, as we know, a gall containing an egg or larva of such a saw-fly can be conquered by other insects including close relatives of its original inhabitant.
In the next document we will describe the origin of the first parasitic Hymenoptera (Terebrantia) : the Archaic Inquilinoid Phase.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXXIV.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII