

Paratrichoptera and Diptera
We now depict some Mecoptera (suborder Paratrichoptera and other Mecoptera) that tend -- as regards their wing-venation -- to be close to the Diptera, and some dipterous wings that are venationally still more or less close to Mecoptera.
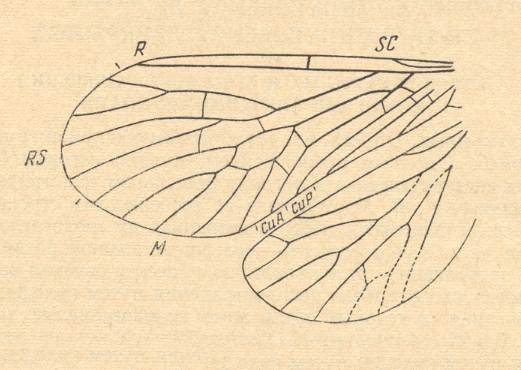
Figure 1 : Pseudopolycentropus latipennis MARTINOV, Order Mecoptera, Suborder Paratrichoptera, Family Polycentropodidae.
Wings. Length of forewing 8 mm. Middle Jurassic of Karatau, southern Kazachstan.
(After MARTINOV, 1927, from ROHDENDORF, 1964)
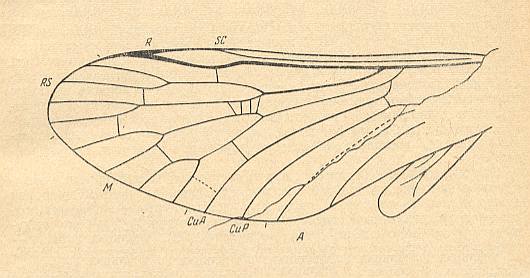
Figure 2 : Wings of Pseudodiptera gallica LAURENTIAUX ( Pseudodipteridae, order Mecoptera, suborder Paratrichoptera). Length of forewing 10 mm. Lower Triassic (Buntsandstein) of France (Vogesen). The drawing is made by ROHDENDORF after a photograph of the holotype. The hindwing, the one on the right side of the body, is transposed [to the left] [MARTYNOVA, O.M., 1959].
(From ROHDENDORF, 1964)
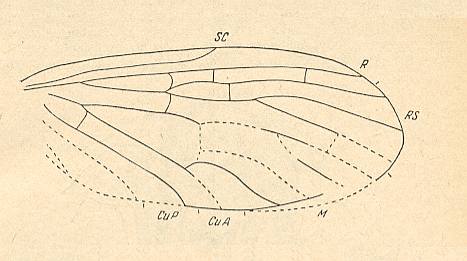
Figure 3: Ptychopteropsis mirabilis MARTINOV, wing. Order Mecoptera, Suborder Paratrichoptera, Family Ptychopteropseidae. Length 11 mm. Lower Jurassic of Central Asia.
(After MARTINOV, 1937, from ROHDENDORF, 1964)
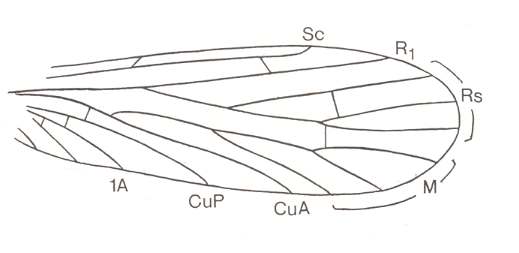
Figure 4 : Wing of Nannochoristella reducta RIEK. Order Mecoptera. Upper Permian of Australia. (After RIEK, 1953, from HENNIG, 1969)
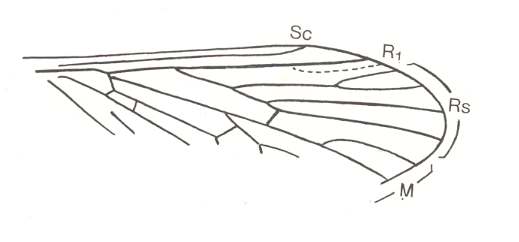
Figure 5 : Wing of Permotanyderus ableptus RIEK. Order Mecoptera, Suborder Protodiptera. Upper Permian of Australia. (After RIEK, from HENNIG, 1969)
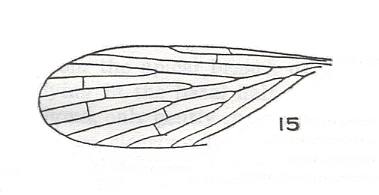
Figure 6 : Forewing of Mesotanyderus jonesi RIEK. Order Mecoptera, Suborder Protodiptera, Family Permotanyderidae. Length of wing 6.5-8.0 mm (there are some other specimens (C2168-9 and C2249) found). C1054 (University of Queensland, Department of Geology collection). Trias of Queensland (Australia) at Mt. Crosby.
(After RIEK, 1955)
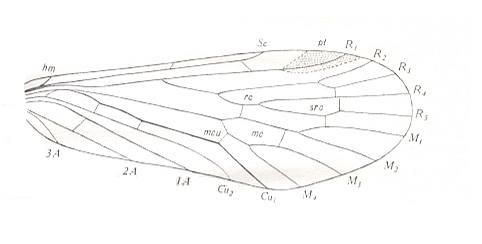
Figure 7: Forewing of an undescribed primitive Paratrichopteron from the Upper Permian of New South Wales, Australia.
hm = humeral veinlet, mc = median cell, mcu = medio-cubital cross-vein, pt = pterostigma, rc = radial cell, src = subradial cell.
(After TILLYARD, 1935)
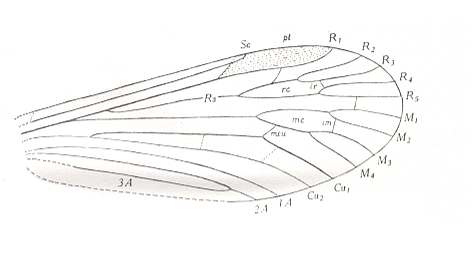
Figure 8 : Forewing of Liassophlebia hydromanicoides TILL. Order Mecoptera, Suborder Paratrichoptera, Family Liassophlebiidae.
Lower Lias of England. im = inter-median cross-vein, ir = inter-radial cross-vein. Pigmentation omitted.
(After TILLYARD, 1935)
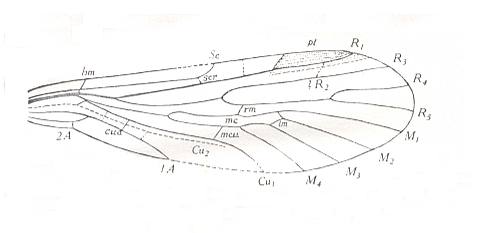
Figure 9 : Forewing of Permotipula patricia TILL. Order Protodiptera, Family Permotipulidae. Upper Permian of New South Wales, Australia. cua = cubito-anal cross-vein, scr = subcosto-radial cross-vein.
To see a qualitatively better and somewhat enlarged figure of the same object, click twice on image.
(After TILLYARD, 1935)
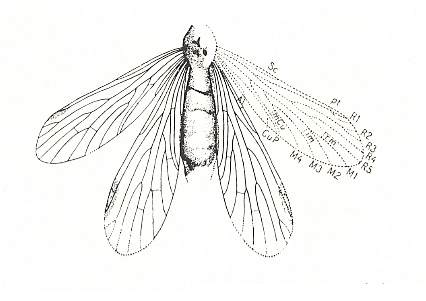
Figure 10 : Permotipula patricia TILL. Ca. 13/1 natural size. Upper Permian of Warners Bay, New South Wales, Australia.
For a better and somewhat enlarged figure, click twice on image.
(After TILLYARD, 1937, from MÜLLER, 1978)
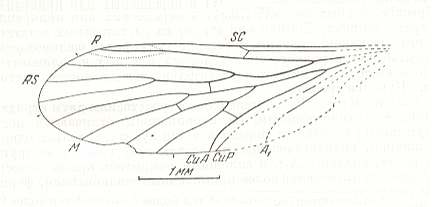
Figure 11 : Forewing of Permotipula borealis O.MART. Coll. PIN No. 676/583. Length of impression 4.15 mm, probable length of whole wing 4.4 mm. Surijokova I. Upper Permian of Basin of Kuznetsk, Siberia.
(After MARTINOVA, in ROHDENDORF et al., 1961)
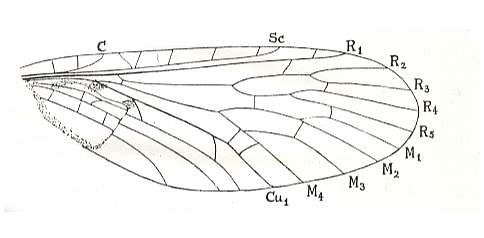
Figure 12 : Forewing of Aristopsyche superba TILL. Upper Trias of Ipswich, Queensland, Australia.
Greatest length of fossil 24 mm (representing a complete wing about 25 mm long.
C = distal end of short costal vein.
(After TILLYARD, 1919)
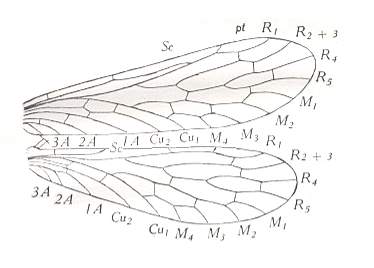
Figure 13 : Wings of Nannochorista dipteroides TILL., Order Mecoptera, Suborder Eumecoptera, Family Nannochoristidae. Recent, Tasmania.
(After TILLYARD, 1935)
The above Figure was already given earlier in the previous document , there provided with labeling all the wing features [Perhaps the reader should have to scroll a little to find this Figure].
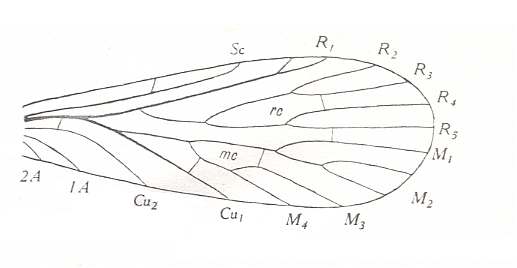
Figure 14 : Forewing of Petrochorista minuta MART., Order Mecoptera, Suborder Eumecoptera, Family Permochoristidae. Upper Permian of Russia. After Martynov, 1930, p. 186, fig. 24, with labels of veins added.
mc = median cell, rc = radial cell.
(From TILLYARD, 1935)
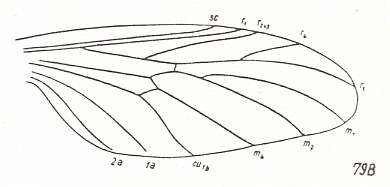
Figure 15 : Wing-venation of Eoplecia primitiva HANDL. Upper Liassic of Mecklenburg, Germany.
[It clearly belongs to the present wing-type.]
(After HANDLIRSCH, 1938, from HENNIG, 1954)

Figure 16 : wing of Cramptonomyia spenceri ALEX. Order Diptera, Family Phryneidae [ = Rhyphidae].
(After ALEXANDER, 1931, from HENNIG, 1954)

Figure 17 : Wing of Protorhyphus stigmaticus HANDL. Order Diptera, Family Protorhyphidae. Upper Lias of Mecklenburg, Germany.
(After HANDLIRSCH, 1938, from HENNIG, 1954)
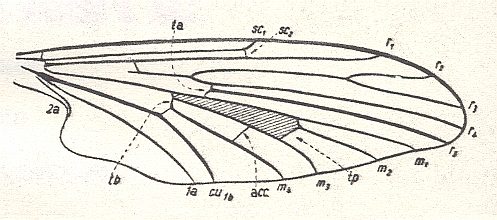
Figure 18 : Wing of Protoplasa fitchii O.S.. Order Diptera, Family Tanyderidae.
(After HENNIG, 1954)
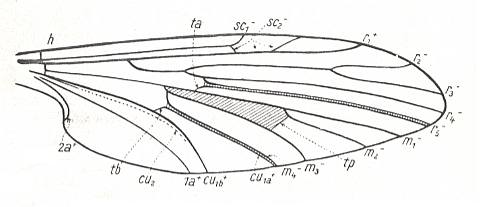
Figure 19 : Scheme of groundplan of dipterous wing-venation, inspired by, and based on, the wing features of Protoplasa fitchii O.S. as depicted in the previous Figure.
(After HENNIG, 1954)
The notation "tb" indicating the connection of the discoidal cell and CuA has been more or less inconsistently used by Hennig (as he himself points out in his 1969 book, p. 379) : tb consists of two parts. The anterior part is the basal piece of M4, the posterior part is the basal piece of CuA1.
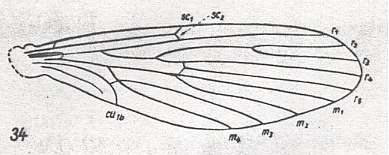
Figure 20 : Wing of Nemopalpus zelandiae ALEX. Order Diptera, Family Nemopalpidae, Superfamily Psychodidea.
(After ALEXANDER, 1927, from HENNIG, 1954)
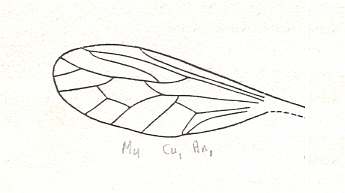
Figure 21 : Wing of Eremochaetus asilicus USSATCHOV. Holotype (almost complete insect), PIN No. 2066/2121. Positive impression of male. Length of wing 5.5 mm. Jurassic of Karatau, southern Kazachstan.
Order Diptera, Family Eremochaetidae.
(After USSATCHOV, 1968)
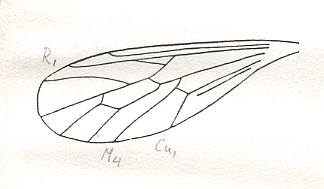
Figure 22 : Wing of Pareremochaetus minor USSATCHOV. Holotype (almost complete insect), PIN No. 2066/1560. Positive impression of male. Length of wing 3 mm. Jurassic of Karatau, southern Kazachstan.
Order Diptera, Family Eremochaetidae.
(After USSATCHOV, 1968)
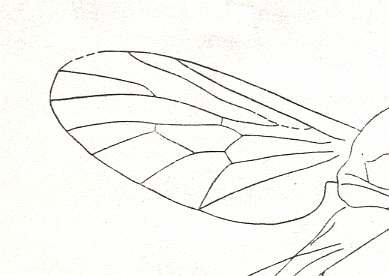
Figure 23 : Wing of Probolbomyia modesta USSATCHOV. Holotype (almost complete insect), PIN No. 2239/2162. Positive impression of female. Length of wing 3 mm. Jurassic of Karatau, southern Kazachstan.
Order Diptera, Family Rhagionidae.
(After USSATCHOV, 1968)
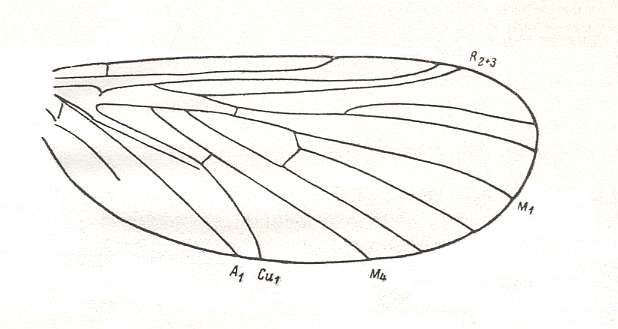
Figure 24 : Scheme of basic plan of the wing-venation of Asilomorpha [ = about Brachycera-orthorrapha].
(After USSATCHOV, 1968)
In connection with the above presented scheme of wing-venation we now consider specifically the position of the vein M4. See next Figure.
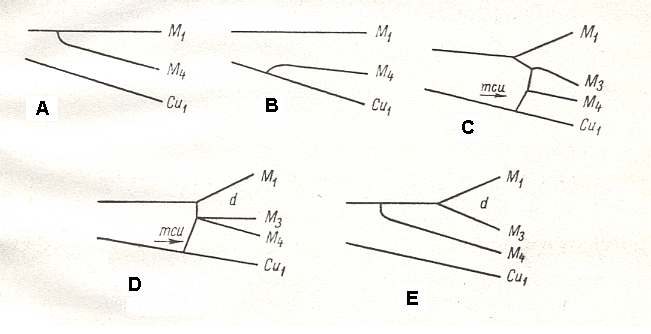
Figure 25 : Different positions of the vein M4 in Diptera.
(After USSATCHOV, 1968)
In the Diptera this vein (M4) usually stands in a two-fold connection with other veins that are its direct neighbors :
In the case of the primary absence of the discoidal cell, M4 either branches off from the chief stem of the media (M), or from Cu1 (Figure 25, A and B). Many examples of which we find in the Nematocera.
In the case of the presence of the discoidal cell the free branch M4 is always connected with this cell, either directly or by a cross-vein (Figure 25, C and D). This we find in a part of Tipulomorpha, Anisopodomorpha, Asilomorpha (USSATCHOV, 1968, p.625). In the new family Eremochaetidae (see the two Figures further above ), in contrast, we encounter the interesting combination of these two mentioned situations : In the presence of the discoidal cell the vein M4 independently branches off from the chief stem of M, having no connection whatsoever with the discoidal cell and not taking part in its structure (Figure 25, E). This fact legitimates us to agree with the point of view of Tillyard (1919) according to whom the vein lying between M3 and Cu1, in Diptera, represents the last branch of the medial system of veins, that is the true M4, and consequently is not the product of coalescence of M4 and Cu1, as is supposed by Hennig (1954) (see the groundplan of dipterous wing-venation as conceived by HENNIG).
(USSATCHOV, 1968, p. 625)
It might be interesting to compare the above wings of three Jurassic diptera, asserted to belong to the Suborder Brachycera-orthorrapha, to those of recent relatively primitive members of this suborder. In the next Figure we show the wing-venation of one of them.
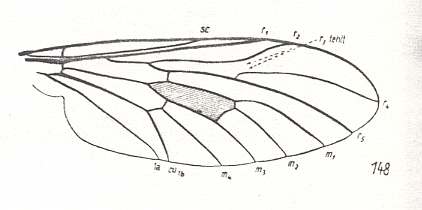
Figure 26 : Wing-venation of Rhagio scolopaceus LW. Order Diptera, Suborder Brachycera-orthorrapha, Family Rhagionidae.
(After HENNIG, 1954)
Although with Rhagio we find ourselves well within the next suborder (the first one being the Nematocera), the wing-venation can nevertheless be very archaic as can be seen in the above Figure. This indicates that at least some groups of Brachycera-orthorrapha must have had their origin deep down in the Nematocera (perhaps somewhere close to the Rhyphidae).
The origin of the order Diptera cannot be read off solely from the wing-venation of certain Mecoptera and archaic Diptera. But insofar as we have to rely on fossils, wing-venation is the only thing we have. And wing-venation is in this case -- the origin of Diptera -- very significant because it comprises structural features of the wings and thus expresses different types of flight-regimes. And it is precisely the diversifying of flight-regimes which seems to be the chief characteristic of the further evolution of the Diptera. Probably a prerequisite for being able to evolutionarily evolve a vast array of different flight-regimes is the two-winged condition (together with the formation of halteres at the sites of the former hindwings). So once having evolutionarily achieved this two-winged condition, a radiation of flight-regimes (among other things expressed by the wing-venation) will, we can expect, necessarily follow. What is not so clear, however, is how and why a two-winged condition (of certain mecopterons) was evolved in the first place. It cannot have taken place because this condition is, already from the start, a universal improvement of arial locomotion, because there are many successful insect groups whose members still have four wings, such as the Hymenoptera (wasps, bees and ants) and the Lepidoptera (moths and butterflies), and, not to forget, the dragonflies. And among these there are that fly extremely well. In order to see two-wingedness as an improvement, we could think of the possible fact that a concentration of all the muscle power on just one pair of wings might be the necessary condition to increase wing-beat frequency, and so allowing the wings to become smaller (because the higher wing-beat frequency compensates for the decrease of surface area of the wings with respect to their generating lifting force). But high wing-beat frequency is not unique to Diptera (and in the Family Tipulidae [Diptera, daddy-long-legs] it is even relatively low) : We also find it in many Hymenoptera ( It is true that the hindwing in such Hymenoptera has become relatively small and that, moreover, it is attached (by a row of hooks) to the forewing, forming with it in fact a single structure that is morphologically -- and probably also functionally -- similar to a single dipterous wing). So while two-wingedness can lead to the development of powerful flight it is not the only way to achieve that, and thus it will not necessarily follow upon the need to fly better. And indeed, one can legitimately assume that not only the order Diptera originated from mecopterous ancestors, but also the orders Trichoptera (caddis-flies), Lepidoptera, and Hymenoptera. And, as has been said, among the latter there are skilled flyers, moving their four wings up and down with high frequency. And while it is thus perfectly understandable that the four-winged flight-apparatus of Trichoptera, Lepidoptera, and Hymenoptera, originated from the four-winged flight-appparatus of Mecopteroid (=scorpionfly-like) ancestors, it is much harder to understand the origination of the all-round two-winged flight-apparatus of the Diptera from such ancestors. But all entomologists agree (and rightly so) that the order Diptera originated (somewhere in Permian or Triassic times) from mecopterous ancestors (probably from the mecopterous suborder Paratrichoptera).
In the next document we will investigate the possible basic plans of wing-venation (and their subsequent modifications) in the different groups of Nematocerous Diptera, and their bearing on the question of the poly/monophyletic origin of the order Diptera.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XVI.