

After having dealt with the wing-venation of the Order Hymenoptera from a structural point of view, it is perhaps instructive to do the same with the wing-venation of the Order Diptera. We, in the Parts preceding those on Hymenoptera, have, it is true, already dealt with the wing-venation in Diptera, but mainly so from a phylogenetic viewpoint, meaning that we have investigated whether and where in Diptera venational patterns possessed by given species may be derived from those of other species. What we will do now, on the other hand, is to see, as we did in Hymenoptera, how all dipterous wing-venations can be derived directly from the venational prototype of the Order. This must be seen in the following theoretical context (as explained in the previous document) : The different types of wing-venation that exist or had existed have not materially evolved from each other (in a linear or branched sequence). They are, more or less commonly-possessed (= common in smaller or larger groups), elements of strategy. And because these different types of wing-venation are elements of strategy (that is elements making existence in the Explicate Order possible) they come, not from the Explicate, but from the Implicate Order, where they are added to the corresponding strategy to be projected. And a given wing-venation can only be assigned to some given strategy (upon projection becoming an organismic species) if it (1) complies with the primary venational scheme discovered by Comstock and Needham, (2) is formally derivable from the order-prototype, and (3) does not interfere with any other element of that strategy. Every type of wing-venation (at least in Orders such as Diptera and Hymenoptera) determines a special and subtle variant of flight-regime and is common to a larger or smaller number of species. The particular flight-regime co-determines in what way the insect finds itself in its environment. This is why we (here and in the previous document) consider the several wing-venations from a structural and functional viewpoint (but unfortunately not knowing in what way the venation precisely determines flight-regime other than assuming that it will influence the air-flow [such as causing small deflections] over the wing-surface during flight).
Although it was assumed that, in the Implicate Order, an appropriate (type of) 'ready-made' complete wing-venation is assigned to each pterygote insect strategy, i.e. an assignment that is independent of the noëtic or material existence of other venations, empirical evidence on existing venations demonstrates that in the process of the mentioned assignment acts of derivation must nevertheless play a role. That is to say that certain elements of a given assigned venation have come from other venations (of related forms). That's why at least short derivational sequences between existing venations can be found. And it is also to these sequences that we will pay attention in discussions to come. In the mentioned earlier documents on the venation of Nematocera these derivational sequences have been discussed, it is true, but the emphasis there was on the existence of derivational gaps. Now we will deal with the possible derivational sequences themselves, including derivation from the order-prototype, in order to find out how the mentioned assignment actually goes its way. But because in the mentioned assignment the nature of wing and venation to be assigned (i.e. which one of the many natures is appropriate) is first of all determined by their functional (in contrast to formal) properties, we will let precede the exposition of venational derivation in a given collection of Diptera by the description of the functional wing-type and subtypes prevailing in this collection. The theoretical considerations concerning the status of wing-venation given here is still incomplete and preliminary. Therefore :
in the next document we have worked out still further, and even more or less completed, the theory about intrinsic and extrinsic venational elements, their mutual relationsship, how they involve derivational sequences, and in what relation they stand to the functional main types distinguished by Rohdendorf.
But let us begin with the origin and order-prototype of Diptera.
With the Origin of the Order Diptera we have dealt in Part XIV and Part XV of the present Series on Evolution.
While for the hymenopterous venational prototype we cannot find any existing (fossil or recent) venation that more or less mediates between this prototype and the venation of some existing (fossil or recent) representative of some insect Order other than Hymenoptera,- we do find such mediating venations for the Order Diptera. Among the many existing examples we show the following two Figures (5 and 8 there) from Part XIV :
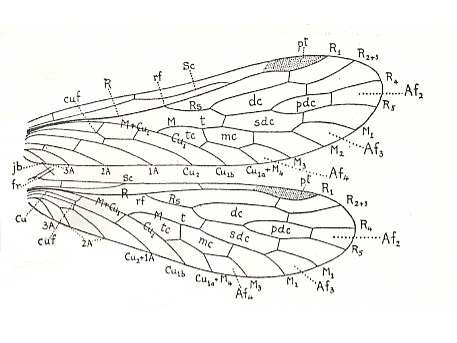
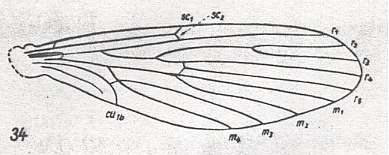
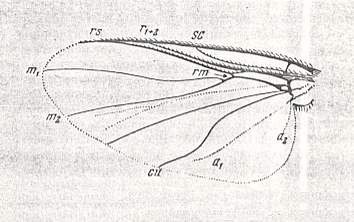
Figure 1 : Wing-venation of Nannochorista dipteroides TILL. Recent. Order Mecoptera, Family Nannochoristidae. Comstock-Needham nomenclature and notation [which is still a generally accepted way of signifying venational details].
Af2, Af3, Af4, second, third, and fourth apical forks. cuf = cubital fork, dc = discoidal cell [not homologous with "discoidal cell" in Diptera], mc = median cell, pdc = post-discoidal cell, pt = pterostigma, rf = radial fork (origin of Rs), sdc = subdiscoidal cell, t = thyridium (medial fork), tc = thyridial cell.
(After TILLYARD, 1917)
If we look to the wing-venation (forewings) in Mecoptera and to that in Diptera, we see that the latter has wings in which the venation is strongly reduced as compared to that in the former. In the Nannochoristidae this originally very rich mecopterous wing-venation has been reduced already so far that we must depart from their venation (concentrating on forewings only) -- in addition to that of some other Mecoptera -- in order to understand and interpret the venation in the Order Diptera. And to understand in turn the venation in Nannochoristidae, we must go back to the more primitive wing-venation as we see it in the large mass of fossil representatives of the Mecoptera.
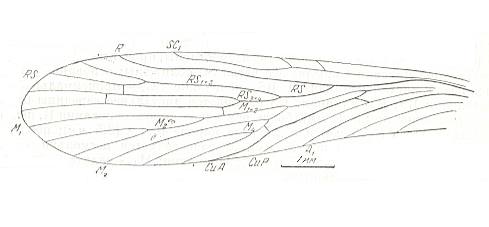
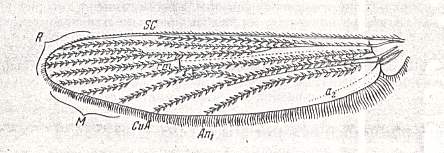
Figure 2 : Fore wing of Mesochorista novojilovi O.MART. (Mecoptera). Coll. PIN No. 673/306. Length of impression 8.5 mm, probable length of whole wing 8.8 mm. Sokolova II. Permian of Basin of Kuznetsk, Siberia.
Description of venation in : Paleozoiskije Nasjekomije Kuznjetskovo Bassjeina [Paleozoic Insects of the Basin of Kuznetsk (Siberia)], ROHDENDORF, et al., 1961.
The wing was found in the Jerynakovskaja suite of the Basin of Kuznetsk (p. 583). The geological age is given (p. 11) as P2, which should then be upper Permian.
(Figure after Martinova, in ROHDENDORF et al., 1961)
From Part XV we give another example (Figure 6 there) from the possible venational precursors of Diptera :
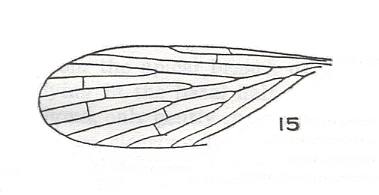
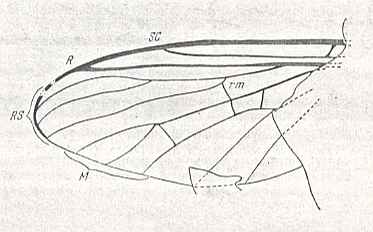
Figure 3 : Forewing of Mesotanyderus jonesi RIEK. Order Mecoptera, Suborder Protodiptera, Family Permotanyderidae. Length of wing 6.5-8.0 mm (there are some other specimens (C2168-9 and C2249) found). C1054 (University of Queensland, Department of Geology collection). Trias of Queensland (Australia) at Mt. Crosby.
(After RIEK, 1955)
The venational prototype of the Order Diptera
The venational prototype or groundplan of the Order Diptera is given by HENNIG, 1954, 1969. It is supposed that two longitudinal veins in it are in fact results of the coalescence of two original veins. See next Figure.
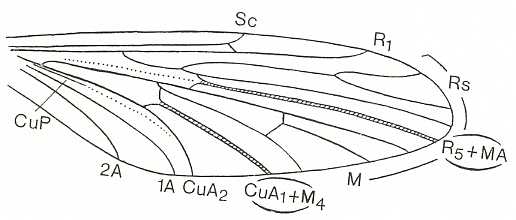
Figure 4 : Prototype of the wing-venation of the Diptera. Those longitudinal veins that are, as to their identity and naming, disputed (R5 + MA and CuA1 + M4), are emphasized. (After HENNIG, 1969)
In this dipterous prototype we see :
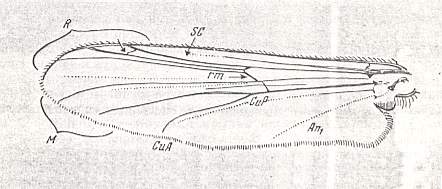
This prototype is based on the venation of Protoplasa fitchii O.S. :
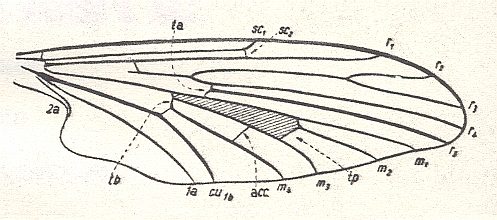
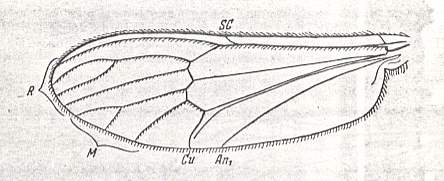
Figure 5 : Wing-venation of Protoplasa fitchii O.S., Tanyderidae, Diptera.
(After HENNIG, 1954)
The name "tb" indicating the connection of the discoidal cell (shaded in the figure) and CuA (or if one wishes CuA2) is used by Hennig in his 1954 essay on dipterous wing-venation inconsequently (as he himself indicates at p. 379 of his 1969 book on fossil insects) :
The (cross)vein tb consists of two parts. The anterior part of it is the basal section of M4, while its posterior part is the basal section of CuA1.
The cross-vein tp closes the discoidal cell posteriorly.
In reproducing also (Figure below) the hymenopterous venational prototype we can see how much indeed they (the dipterous and hymenopterous venational prototypes) differ (while still complying with the comstock-needham scheme of principal veins) :
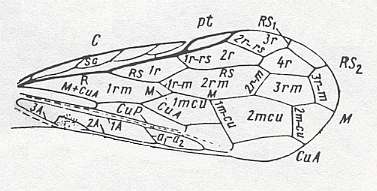
Figure 6 : Prototype of the venation of the forewing of the Hymenoptera.
(After RASNITSYN, 1980)
For a description of it, see Figure 1 in previous document .
Functional Types of Wings in the Order Diptera
Below we will inspect the many venations as they occur in the wings of representatives of the Order Diptera, and in what features they differ from the Order-Prototype. We will do this from a structural point of view -- that is, not exclusively concentrating on the fate of individual homologous veins (such as their number of branches) insofar as the knowledge of this fate would contribute to the reconstruction of the Order's phylogeny, but also (concentrating) on qualitative differences of formally equal venational patterns -- and at the same time [we will do this] from a functional point of view. The latter means that we provisionally as it were accept the idea that, in addition to the features of the wing-as-a-whole, and to those of the wing-base (basial), also many details of the venation have some functional meaning. They may determine, for example, the deformation of the wing during flight, and then, as a result, (may determine) the direction of (micro-)air-flows over the wing surface. In this way, the details of the venation may constitute "the last determinations of the flight-regime", that is to say, they are added 'on top' of the basic and necessary features that determine the flight-regime. Also the covering of the wings (all over or markedly on certain places) with hairs or spines undoubtedly has aerodynamic significance.
Rohdendorf (1949) already considered the functional characteristics of the wings of all Orders of the Pterygota (winged insects), and in a second book, 1951, he wholly devoted the attention to determining functional types of legs and wings of the Order Diptera : "Organi dwizjenija dwukrilich nasjekomich i ich proischozdjenje". ( The organs of locomotion of two-winged insects and their origin). In this book a number of main types and their subtypes are established. Roughly we can say that the main type is characterized there by (1) the size and shape of the wing, (2) by the global characteristics of the venation (that is, whether it is more or less complete, whether the veins are evenly or unevenly distributed over the wing-blade, the state of differentiation in strong and weak veins, etc.), (3) the nature of the wing-covering (hairs, scales, and the like), and some other general features, while the subtypes are characterized by changes in the details of the venation. It is in fact these latter features that came 'on top' of those of the main type.
In earlier documents on the Order Diptera we have already considered the functional types and subtypes of the wings of the dipterous Suborder Nematocera. There it was done just to add characteristics of the families and superfamilies. We will repeat it here, because we now are thematically considering the functional wing-types. The reader is thereby referred to the introduction to functional wing-types in Part II of the present Series on insect evolution, that is, the (long) Section (close to the beginning of the document) named "Functional Interpretation and Evolutionary Evaluation of the Features of the Wings of the Order Diptera".
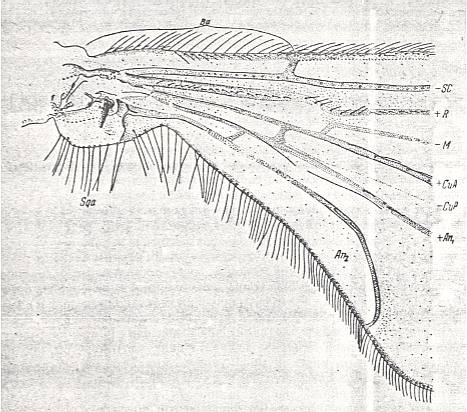
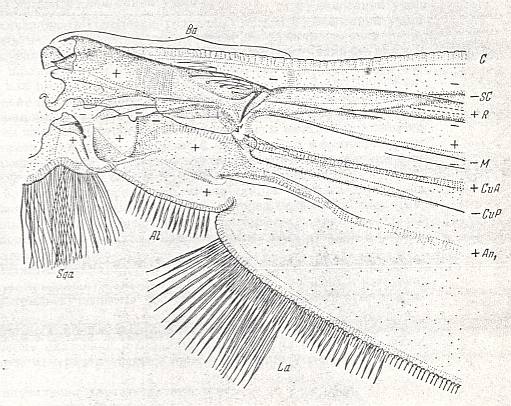
Special attention must be given to the study of the basal part of the wing-proper, i.e. its individualized part following upon the hinge by which [latter] the wing is articulated with the thoracic wall. That is to say, we must give special attention to the wing's basiala. See next Figure.
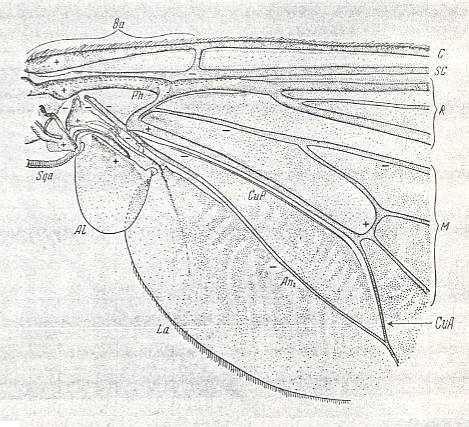
Figure 7 : Eremomydas bek SEM. ( Asilomorpha-Mydaidae). Proximal half of the right wing of a male. Top view.
Specimen Nr. 2002. Length of complete wing 14.5 mm. Of basiala 2.5 mm. Punctuation expresses stronger or weaker coloration and undulation of the wing-membrane.
Abbreviations : The veins are symbolized as usual. From top to bottom : C = Costa, SC = Subcosta, R = Radius (radial veins), M = media (medial veins), CuA = anterior Cubitus, CuP = posterior Cubitus, An1 = first Anal vein.
La = anal lobe, Al = alula (winglet), Sqa = squama alaris (wing-scale), Ba = basiala, Ph = phragma.
The signs + and - signify respectively the convexity or concavity of the given part of the wing.
The distal border separating the true wing-blade from the basiala runs about from the humeral cross-vein [the small cross-vein between Costa and Subcosta] to the boundary between the anal lobe (La) and the alula (Al).
(After ROHDENDORF, 1951)
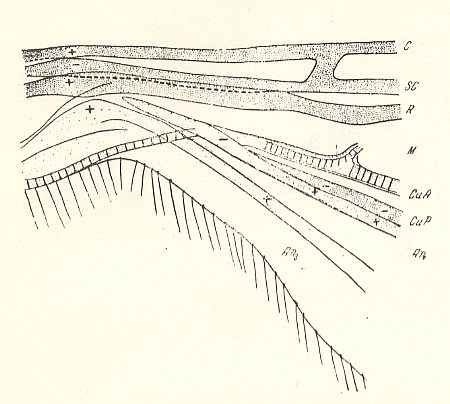
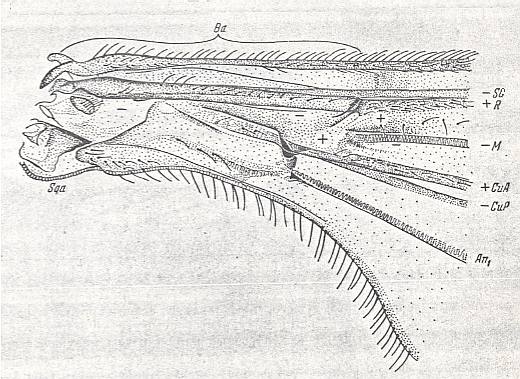
Figure 8 : Pachyneura fasciata ZETT. ( Pachyneuridae). Wing-base, basiala. Schematic.
Abbreviations as usual.
Here we have an example of a weakly developed basiala, that is, lots of elements are missing.
(After ROHDENDORF, 1946)
We will now describe, one by one, the many functional types and subtypes of the wings in the Order Diptera.
Primitive traction (tipuloid) wing type.
Description of the type (and its subtypes) as such :
To begin with, ROHDENDORF adds as a note :
In naming the types I wanted to express the most important and the very characteristic functional features of the wings of the given type. Of course, the presented names do absolutely not indicate the exclusiveness of the trait, as expressed in the name, for the type [that is, other types could also possess this trait], but only indicate the type's most characteristic feature.
Representatives of the type.
To the primitive traction (tipuloid) wing type -- one of the most primitive types, [the representatives of] which are closely related to the ultimately first forms, namely to some Mecopteroidea (namely the fossil Paratrichoptera) which evolutionarily have led to the order Diptera -- today belong wings of the representatives of the families Tipulidae, Limoniidae, Liriopeidae [ = Ptychopteridae], Trichoceridae [ = Petauristidae], all belonging to the superfamily Tipulidea of the infraorder Tipulomorpha, further, of Pachyneuridae, as superfamily Pachyneuridea also belonging to the infraorder Tipulomorpha, and, finally, of the relict family Nemopalpidae belonging to the superfamily Psychodidea, also a superfamily of the Tipulomorpha). So we see that the primitive traction (tipuloid) wing type is not just confined to the superfamily Tipulidea. It is also present in some other superfamilies of the infraorder Tipulomorpha.
[ The systematic position of the family Pachyneuridae is uncertain. It could be that this family does not belong to the infraorder Tipulomorpha (and then, consequently, not to the superfamily Tipulidea) but to the infraorder Bibionomorpha. Functionally, of course, their wings do belong to the present type].
Here we present some Figures depicting wings of this type.
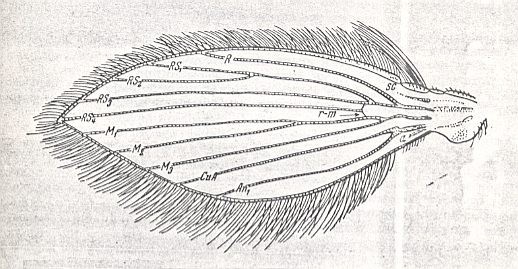
Wing-venation of a member of the Tipulidae.
8 -- Idiotipula confluens ALEX.
Interpretation of wing-venation (i.e. the identity of the individual veins and cross-veins) according to HENNIG, 1954.
(From HENNIG, 1954, after ALEXANDER, 1921)
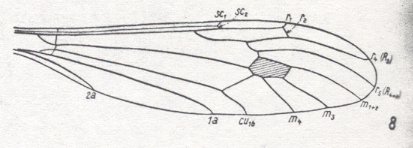
80 -- Wing of Pachyneura fasciata ZETT.
Interpretation of wing-venation according to HENNIG, 1954.
(From HENNIG, 1954, after DUDA, 1930.)

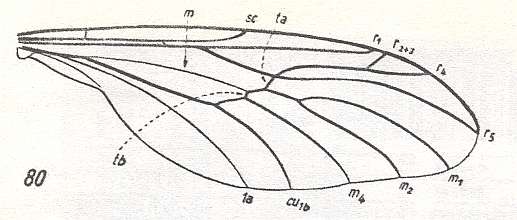
Wing of Protoplasa fitchii O.S. ( Tanyderidae).
Interpretation of wing-venation according to HENNIG, 1954. The venation of Protoplasa could be close to the original venation of the wings of the first Diptera.
(After HENNIG, 1954.)
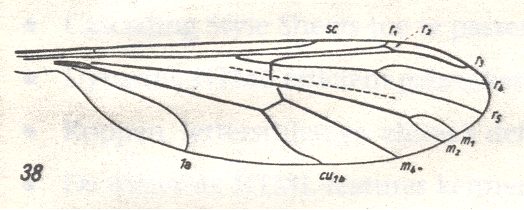
Wing of Liriope scutellaris MEIG. ( Liriopeidae [ = Ptychopteridae] ).
Interpretation of wing-venation according to HENNIG, 1954.
(After HENNIG, 1954.)
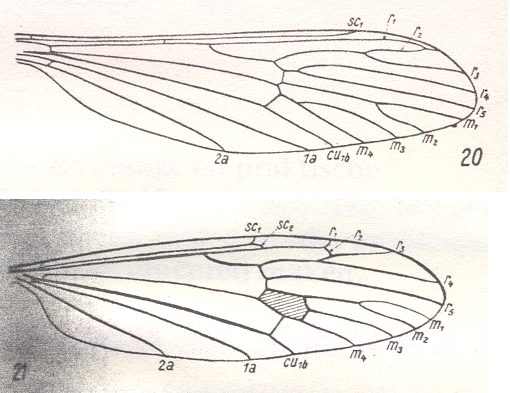
20 -- Wing of Tricyphona protea ALEX. ( Limoniidae). (From HENNIG, 1954, after ALEXANDER, 1927).
21 -- Wing of Limnophila ferruginea MEIG. ( Limoniidae). (After HENNIG, 1954.)
Interpretation of wing-venation according to HENNIG, 1954.
Wing of Nemopalpus zelandiae ALEX. ( Liriopeidae [ = Ptychopteridae] ). [according to ROHDENDORF Nemopalpus belongs to the family Nemopalpidae].
Interpretation of wing-venation according to HENNIG, 1954.
(From HENNIG, 1954, after ALEXANDER, 1927.)
Dimensions of the wings.
Shape of the wings.
The wings are always elongated -- their width three times, sometimes even four times smaller than their length. The anterior wing-edge is straight almost over two thirds or even three fourths of the [anterior] edge, rarely only over its half (Pachyneuridae). After this the edge starts to bend and to form the wing tip, its apex. The basiala is not clearly individualized [that is, not clearly distinguished from the wing-blade], and consists of a more or less expressed constriction. Transverse anastomoses and phragma as a rule not expressed. Sometimes there are only transverse veins [within the confines of the supposedly basiala] ( Petauristidae [ = Trichoceridae], see next Figure).
Petaurista maculipennis MEIG. ( Petauristidae [ = Trichoceridae] ).
Base of right wing of male. Topview.
Specimen Nr.2534. Length of wing 7.35 mm. Length of part starting from the level of the base of the costal vein to that of the humeral cross-vein [the cross-vein between Costa and Subcosta], supposedly delineating the basiala, 0.94 mm.
For the abbreviations, see Figure 7 above . The brace (in upper part of figure) with the sign Ba indicates the extent of the supposed basiala.
(After ROHDENDORF, 1951)
Sometimes, in the most specialized forms ( Tipulidae), the basiala is transformed into a complex tube-like construction. Rarely there is a germ [or rudiment] of a phragma ( Liriopeidae [ = Ptychopteridae], see next Figure).
Liriope contaminata MEIG. ( Liriopeidae [ = Ptychopteridae] ).
Base of right wing of male. Topview.
Specimen Nr.3684. Length of wing 7.50 mm. Length of part starting from the level of the base of the costal vein to that of the humeral cross-vein [the cross-vein between Costa and Subcosta], supposedly delineating the basiala, 1.35 mm.
For the abbreviations, see Figure 7 above . The brace (in upper part of figure) with the sign Ba indicates the extent of the supposed basiala.
(After ROHDENDORF, 1951)
The anal lobe is weakly expressed. The alula and thoracic scale are always absent. There is a germ of the wing-scale in the form of a projection of the hind margin of the basiala.
Skeleton of the wing.
See Figures above [ It should be clear that not all features mentioned in what follows can be expected to be found in these Figures. This is because there is a lot of variation, even within a single type of wing].
The wing-venation is rich, very little reduced, and [as such] comparable with other Mecopteroidea [containing the order Diptera, Trichoptera (caddis flies), Lepidoptera (butterflies and moths), Mecoptera (scorpionflies), and one or more fossil orders], especially with certain Paratrichoptera [a fossil Mecopteroidean order]. Costal, Subcostal, and Radial veins always run parallel, having become more or less near to each other, and extending up to the place of curvature of the anterior edge, that is, up to where the apex of the wing starts. It is only this feature that guarantees [a degree of] costalization of the primitive traction (tipuloid) wings. It [that is, costalization] is almost not expressed by the rest of the wing's structural features (apart from the presence [where indeed it is] of the anal lobe ! ). [Costalization of the wings can be realized in different ways. Generally we can say that costalization means that the wing-skeleton concentrates on the anterior half of the wing. It has either shifted towards that half (and from there, often towards the wing-base), or the posterior half of the venation is partly reduced or weakened and/or the veins of the anterior half have thickened. So the wing-blade has, in the case of costalization, qualitatively differentiated into an anterior and posterior half. It is clear then that the development of a large anal lobe, totally or almost devoid of (strong) veins, contributes to this differentiation and thus to the wing's costalization. The same goes for the development of a fringe of long hairs along the hind margin of the wing.]
The structure of the venation of the wing-blade shows almost no sign of whatever [form of] displacement of veins [meant is here shifting of veins toward the wing's fore margin], which generally run parallel to each other and end up at the apical part of the hind margin. Only the cubital and anal veins [will sometimes] fan-like move away from each other (See Figure above ). The general number of branches of the radial system (up to 5) and of the medial system (up to 4) is the greatest of all wing types. There are cross-veins such as the basic ones, namely the radio-medial cross-vein (rm) and the medio-cubital cross-vein (mcu), as well as extra intermedial cross-veins, which determine the presence of closed cells. The costal vein runs along the whole wing margin, it goes around the whole wing including the anal lobe. Only sometimes the wing's hind margin is devoid of the costal vein, so at the Pachyneuridae.
The venation of the basiala consists of not-individualized 'handles' of the main veins (See Figure 8 above ), sometimes coming very close together and moved over, one above the other, forming in this way a characteristically tube-like basiala ( Tipulidae). Wing membrane firm. Sometimes, in the most specialized forms ( Tipulidae), in which the wings are strongly elongated, a characteristic transformation of the venation took place, consisting in the shifting of the forks of the medial veins to the distal part of the wing. This process is peculiar and in essence widens [in the direction of the wing's longitudinal axis] the basal [in text : basialar] part of the wing, being provided with the parallel trunks of the veins, which usually occupy only a third of the wing [See Figure above ]. In Tipulidae, on the other hand, more than half of the wing [length] is occupied by those veins [See Figure above ]. The mechanical significance of this process is still not completely clear. Probably a sharp individualization of the apex of the wing is taking place here, which apex is able to swing relative to the rest of the wing membrane. The swing axis lies along the line of forks of the medial and radial veins.
Coverings of the wing.
The wings are completely covered by [a] dense [layer of] very short hairs (microtrichia). Spinelets are sometimes developed on the main longitudinal veins and on certain cross-veins (the medio-cubital), while being absent on the wing membrane. Scales on the wings [like we see them in Culicidae] are absent.
The nerve system of the wing is studied only in one species of Tipulidae and consists of two main nerve branchings, many times splitting up and sending nerves along the main veins of the basiala and of the front part of the wing (costal, subcostal, radial, and, partly, medial, veins). These nerves consist of neurons, nerve cells, being placed below the base of hairs and spinelets. In addition, in certain parts of the wing (at the end of the anterior edge before the apex, and at the boundary of the basiala and the wing-blade, and especially at the base of the basiala on its [part of the] subcostal and radial veins) there are accumulations of sense organs, sensoria, which are densely innervated [that is, in which many nerve endings can be observed]. The hind part of the wing, the greater part of the cubital and anal veins, including the anal region of the wing, are devoid of nerves.
Functional characteristic.
The representatives of the primitive traction (tipuloid) type have a rather slow flight, which has no relation with getting food (hunting for prey, the sucking of nectar in flight [that is, sucking nectar from a flower while hovering above it], etc.). The flight usually is rectilinear, rather ponderous. Sometimes these insects fly while alternately going up and down, that is, performing so-called "dances" connected with sexual behavior (for example the winter-gnats of the genus Petaurista [ Petauristidae [ = Trichoceridae] ] and others). The wing-beat frequency is known only of one species of Tipulidae and equal to 44-73 beats per second. It seems to me that these numbers are even a bit too high, and that in reality insects with primitive traction (tipuloid) wings perform flight in which the wing-beat frequency is lower. The little biological significance of the flight function for the representatives of this type, among other things, is made evident by existing cases of reduction of the flight-ability, the existence of short-winged or even wingless species, which are clear derivatives of forms that had wings of the primitive traction (tipuloid) type.
History of the type and its transformations.
Primitive traction wings were possessed by representatives of the most ancient diptera which were discovered in already lower- and middlejurassic sediments. Rohdendorf adds as a note :
The by Tillyard described at the time as being a dipterous insect Permotipula Till. from permian sediments in Australia belongs to a peculiar family of the closely related [to the order Diptera] mecopteriod order Paratrichoptera, in whose representatives, just like in Diptera, occurred processes of reduction of the wing-venation.
Such were the peculiar Eoptychopteridae, Eolimnobiidae, Tanyderophryneidae and the closest relatives of the recent Tipulidae, the Architipulidae. In [geologically] recent times this type contains comparatively different forms from many families, having in common a rich venation and a weakly individualized basiala. The [historical] differentiation of this type consisted in the separation of different forms of wings which are characterized by the development of a peculiar strengthening of the base of the wing, without, however, having worked out an individualized basiala. The determining processes leading to the formation of the individual subtypes of the tipuloid wings consisted in the increase of the size of the body and in a moderate refinement of flight in the direction of increase of tractional force which expressed itself in the elongation of the wings [as we se it in the most specialized members of the type, the Tipulidae]. We are still not in a position to present a complete overview of all the forms of tipuloid wings and can only distinguish the most conspicuous subtypes :
1. Conditionally, we must set, as the first subtype of (primitive traction) tipuloid wings the original [ancestral] form of the dipterous wing, which undoubtedly was most closely related to precisely the primitive traction (tipuloid) wings. This subtype is purely hypothetical, not illustrated by whatever concrete examples.
2. A special subtype of the primitive traction wings, which branched off from the original wing-forms of Diptera is represented by architipuloid wings. This subtype is characterized by a moderate increase of size, by an elongation of the wings, and with it in the wing-venation a shift of cross-veins and of forks of the medial and radial veins in the direction of the wing apex was developed. The greater part of the basal half of the wing is occupied only by the straight [rectilinear] parts of the longitudinal veins. The basiala does not undergo mechanical refinement. In it there have only taken place some reductions and mutual approaches of the basal parts of veins.
To this subtype belong many species of the jurassic family Architipulidae (see HERE for a figure), Tanyderophryneidae (see HERE for a figure), tertiary and recent Limoniidae (See Figure ABOVE ), Cylindrotomidae, Tanyderidae (See Figure ABOVE ), and Nemopalpidae (See Figure ABOVE ).
3. The tubular subtype of the primitive traction type of wings, which is a direct derivative of the previous subtype, is represented by wings of tertiary and recent Tipulidae (See Figure ABOVE ), which are characterized by an increase in size, a shift of forks and cross-veins in the direction of the apical part of the wing, and especially, a strong narrowing of the wing-base, in which the basal parts of the veins closely approach each other and partly one covering the other, and in this way forming a characteristic tubular structure.
This tubular subtype corresponds to the form of flight apparatus of insects earlier (1949) called by me paddle-shaped two-wingedness (copedipterygia) and undoubtedly is a very peculiar specialization of wings of large dipterous insects, which show certain analogies as regards the wing-beat mechanics with some other insects (the scorpionflies of the family Bittacidae and dragonflies of the family Coenagrionidae).
4. Another derivative of the architipuloid subtype were wings of the jurassic Eolimnobiidae and tertiary and recent Liriopeidae [ = Ptychopteridae] in which the process of shift of forks of medial veins and the shift of cross-veins towards the apex of the wing went slowly. At the same time in the basal [i.e. proximal] part of the wing several refinements of the venation took place and the presence of a phragma could be observed (that is, the unification by means of a transverse vein or fold, of the bases of the radial and cubital veins, see Figure above ).
This subtype, which can be called liriopeoid, in addition is characterized by the increase of size, not, however, becoming especially large.
5. From the original dipterous wings (the conditional first subtype of tipuloid wings), in addition to the wings of the architipuloid subtype, undoubtedly forms of wings branched off which were characterized by a refinement of the wing-beat, that is, the increase of its beat frequency, whereby the general size did not increase and the wings were not lengthened. This subtype, which can be called eoptychopteroid, is in fact only little known, but it is very interesting, because it appeared as [evolutionary] source for all other forms of dipterous wings.
The only known representatives of this subtype are [the] members of the family Eoptychopteridae, whose rare species are found in jurassic sediments. Most characteristic for this subtype is the absence of the shift of radial and medial forks towards the apex of the wing. The eoptychpteroid subtype formed the basis not only of the other types, but also the source of a special form of the primitive traction (tipuloid) wings, which we will now discuss.
6. The special diptera of the family Pachyneuridae, which in the recent fauna exists as a relict group, are characterized by clearly expressed processes of reduction of the wing-venation, which processes have the character of costalization [processes], namely in this particular case of the lightening of the posterior half of the wing [with "lightening" is here meant : making (the posterior half) free of (strong) veins]. See Figure above [In a figure of the wing, like this one, we must realize that in drawing it the emphasis was layed on the pattern of the wing-venation, not on possible differences of strength and thickness of the veins]. However, the structure of the wing-base (see Figure 8, above ) shows not only the absence of an individualized basiala, but also the absence of any partial changes whatsoever of the proximal parts of the veins, which remain very similar to those of other representatives of the tipuloid type, to which these wings almost undoubtedly belong. They must be distinguished as a separate specialized, pachyneuroid, subtype. This subtype may functionally be characterized as a first "attempt" to work out costalized wings on the original still little refined basis of the tipuloid type.
Traction costalized (tendipedoid) wing type.
Description of the type (and its subtypes) as such :
Representatives of the type.
To the traction costalized (tendipedoid) type belong the many forms of the large superfamily Chironomidea [ = Tendipedidea], which includes three separate families Chironomidae [ = Tendipedidae] (non-biting midges), Ceratopogonidae [ = Heleidae] (biting midges, gnats), and Simuliidae (black-flies or buffalo-gnats).
Wings of some representatives of the type are depicted here :
Wings of the traction costalized (tendipedoid) type.
Top : Pelopia sp. (Chironomidae).
Bottom : Simulium sericatum MEIG. (Simuliidae).
(From ROHDENDORF, 1951, after HENDEL)
Sizes of wings
Shape of the wings
As to their shape, the wings of this type are divided among thee subtypes, partially corresponding to the three families.
The wings of the first subtype, to which belong the [wings of the] representatives of the family Chironomidae (see Figure above, top image ) and many Ceratopogonidae, are very elongated, about four times as long as their width, and having their margins almost parallel. The anterior margin of the wing is entirely straight up to the wing-tip. The apex [ = wing-tip] of the wing is very small, about one fifth of the length of the wing. The hind margin is moderately convex in its apical half and almost straight in its proximal half. Anal lobe in the form of a marked projection, qua shape approaching that of a quadrant of a circle.
The wings of some Ceratopogonidae and a few Chironomidae (for example Corynoneurinae), forming the second subtype within the tendipedoid type, are short, only 2-3 times longer than their width. The anterior margin is straight only along three fifths to one half of the wing-length, and with it the apex is very large. The hind margin of the wing is markedly convex. Anal lobe in the form of a moderate convex projection, far from having the shape of a quadrant of a circle.
Still broader are the wings of the representatives of the third subtype, that is, in the Simuliidae (see Figure above, bottom image ). Their wings have a length which is only 13/4 - 2 times larger than their width. The fore margin of the wing is straight up to 1/4 - 1/5 of the length of the wing. The apex is not large. The hind margin is strongly convex, and with it the anal lobe is in the form of a marked projection.
The basiala is well-individualized in the whole type and is characterized by the development of a very strong and firm phragma which actually separates the basiala from the wing-blade. The alula is clearly marked off in the first and last subtype and only in the Ceratopogonidae not clear. The alula has the shape of a convex lobe, usually provided with hairs. In the Chironomidae there is a small wing-scale. When the insect is not flying its wings are held one over the other along its body.
The skeleton of the wing
The venation of the wings of the tendipedoid type are characterized by very far progressed processes of costalization, which [processes] appear in the form of reductions, shifts, and coalescence of veins. The costal vein always runs only along the anterior wing margin, rarely reaching the wing-tip. Sometimes it even does not reach the middle of the anterior margin. The subcostal vein usually is weakly expressed and delicate, lying between two strong and thick veins, the costal and radial vein. Sometims the subcostal vein disappears completely. Rarely it is developed as a distinct vein (Simuliidae). The radial veins have markedly come close to each other, and include no more than three separate longitudinal branches, rarely supplemented by a fourth [branch, forming a] short fork on the middle radial branch. Surprisingly more often we will see a reduction of the number of radial veins down to two (the majority of Simuliidae and Ceratopogonidae) or even down to one (for example the genus Brachypogon from the Ceratopogonidae). The medial veins are always significantly thinner and more delicate, counting three (Ceratopogonidae and Simuliidae) or two (Chironomidae) branches. Cubital and anal veins also very delicate, often badly marked off. Of cross-veins there are only the usual main ones ( rm [between radius and media], mcu [between media and cubitus], and the humeral cross-vein [between costa and subcosta] ), where the medio-cubital is often not clear and shifted in the direction of the wing-base. The last medial branch usually sharply separated from the two anterior branches, where its base has somtimes completely disappeared (Chironomidae).
The processes of costalization reach their extreme expression in Ceratopogonidae (for example the genus Leptoconops) or in Chironomidae (the group Corynoneurinae), in which the wings in fact are transformed into free lobes of the membrane at its base strengthened by a firm thick vein which is the product of coalescence of the radial and costal veins. They lively let us remember the wings of those totally diffent insects which are parasites, namely the minute Chalcididae of the insect order Hymenoptera [sawflies, wasps, bees, and ants] !
Coverings of the wing
The wings of this type are always covered with delicate short hairs (microtrichia). Sometimes on the membrane has developed a larger (Ceratopogonidae) or smaller (some Chironomidae) number of spinelets, which are, however, not transformed into scales. Sometimes the spinelets appear only on the veins, they are then short and strong (Simuliidae). In the basiala, namely on the proximal part of the radial trunk in it, there exist two groups of sensory organs looking like short, and thick, small spinelets or hairs. More in detail these sense organs and the nerve system of the wing are not studied.
Functional features
Up to now the flight [features] of insects having a traction costalized type of wings is not studied accurately, but all of their structure allows us to assume an especially high wing-beat frequency. Biologically, flight is of important significance to the representatives of almost all three subtypes. The first subtype (majority of Chironomidae and some Ceratopogonidae) includes wings of insects which are able to perform prolonged air 'dances', swarming in masses, sometimes high in the air (tens of meters). The females of these midges fly toilsomely, having a relatively heavy abdomen filled with eggs. The flight of insects possessing wings of the second subtype (Ceratopogonidae and some Chironomidae) is little known. We should mention the presence among these insects (Ceratopogonidae) of blood-suckers, and with this not only from vertebrates but also from other insects being themselves able flyers like dragonflies. The high quality of flight in Ceratopogonidae therefore turns out to be biologically a truly essential acquisition in their evolution. Completely clear is the significance of refined flight in the third subtype, the wings of black-flies, Simuliidae, active blood-suckers in mammals and birds. The amount of blood sucked by a black-fly sometimes doubles the weight of its body. Therefore the necessity of possessing a strong flight apparatus is evident. For the black-flies a good flight is also important for hunting their victim which has the ability to move quickly. In describing examples of the great biological significance of the flight function in Diptera having wings of the traction costalized (tendipedoid) type, it is necesary to note cases of the reduction of wings, the development of apterygia [ = wingless condition], especially in females, which have originated from the first subtype-as-a-base. Such are certain Chironomidae, for example Corynoneurinae, Clunioninae. Especially remarkable in this respect are certain marine insects, namely species of the genus Pontomyia, in which the reduction of flight ability also affects the males, where the females are transformed into peculiar worm-like organisms devoid of appendages, which never go ashore.
History of the type and its transformations.
The first representatives of the tendipedoid type are known in the form of remains in jurassic sediments of Karatau [southern Kazachstan], and belong, presumably, to the first subtype whose representatives are closest to the original forms. The dixoid type [see below below ] is undoubtedly the source of origin of the tendipedoid type, more precisely the more primitive forms of the Dixoid type, not possessing such broad wings with rich venation. The refinement of the dixoid type consists in the increase of costalization -- that is, the forwardly-directed shift of the whole system of radial veins, the weakening of the posterior half of the wing by way of either complete reduction of veins, or their weakening -- and the improvement of mechanical structures in the basiala, that is the developing of an especially firm phragma and of various lobes at the hind margin of the wing-base. The partition of the tendipedoid type was already described above in the exposition of the functional features. Here we must only add that while the wings of the type's first subtype (we could call it the "narrow-winged tendipedoid subtype") stand most closely to the original forms of the type, the two other subtypes were already significantly [evolutionarily] moved away from the original forms.
Traction broad (dixoid) wing type.
Description (taken from ROHDENDORF, 1951, with changes) of the type as such :
Representatives of the type
The single recent family of the peculiar relict insects, the midges Dixidae, have wings whose structure is such that justifies placing them into a separate functional type, the traction broad (dixoid) type. To this type also belong some fossil jurassic forms. See next Figures.
Wing of Dixa sp. (Dixidae). (from recent fauna).
Specimen nr. 2647. Length of wing 4.5 mm.
This wing belongs to the traction broad (dixoid) type.
(After ROHDENDORF, 1951)
Wing of Dixamima villosa ROHD. (Dixamimidae). Jurassic of Karatau [southern Kazachstan], PIN No 334/167.
This wing also belongs to the traction broad (dixoid) type.
(After ROHDENDORF, 1951)
Size of wings
The shape of the wings
The wings of this type are elongated, about three times longer than wide. Fore-margin straight up to the apical fourth [the latter] consisting of a rounded apex. The hind-margin is strongly convex. The anal lobe is well expressed. The basiala is sharply individuated, with a clearly developed phragma. The alula is in a germinal state, in the form of a lightly projecting broad lobe. Wing-scale unclear, also in a germinal state. When the insect does not fly it holds its wings backwardly spread out.
Skeleton of the wing
The wing-venation is characterized by an expressed costalization, determined by a shift of the subcostal vein ( running up only to the middle of the wing margin ! ) and the first radial vein towards the wing's fore-margin. Very characteristic is the position of the end pieces of the radial branches which run parallel to the apical fore-margin, curving a little backwards, and end up on the margin nearly at the very wing-tip. The number of radial (four) and medial (three) veins is the same as in the wings of the culicoid type (See next superfamily). Of cross-veins there exist only the main ones (rm and mcu). Costal vein firm an thick only along the wing's fore-margin, along the hind-margin it is very thin, almost vanishing. The venation of the basiala is characterized by the development of a sharp projection from the basal part of the rather wide radial vein, the phragma. The general character of the venation of the wing-blade brings this type close to the specialized tipuloid wings, namely to those where a reduction of veins had taken place (some Limoniidae and, partially, Ptychopteridae [ = Liriopeidae] ). See for the tipuloid wings, that is, the primitive traction (tipuloid) type, above . Characteristic is the absence of the medial cross-veins and of the closed cell in the center of the wing-blade.
Coverings of the wing
The wing carries, in addition to hairs-microtrichia, on the majority of the longitudinal veins series of thin and short spinelets which have not been transformed into scales (as in Culicidae). The hind-margin of the wing and the alula with longer spinelets. Scales are completely absent on the wings, which [feature] sharply distinguishes this type from the culicoid type. Sensoria and nerve system of the wing are not investigated.
Functional features
The flight of Dixidean midges is not accurately investigated. Probably it is weak and is biologically not connected with obtaining food. These midges live not far from water-basins, among bushes and trees, performing the known airy 'dances', alternately ascending and descending in the air, like the many other and most different groups of Nematocera.
The history of the type and its transformations.
Undoubtedly, the first representatives of this type appeared already in jurassic times. This is indicated by the discovery of peculiar jurassic insects from Karatau [southern Kazachstan], the Dixamimidae, see Figure above , possessing characteristic wings, coming closest of all to the dixoid type. The differences of the wings of Dixamima from the true, recent, Dixa boil down to the large absolute size of the insect and at the same time the smaller size of the wings. In the evolution of the dixoid type undoubtedly a decrease of size of the insect took place, which also was one of the factors determining the formation of the type. The source of the dixoid type were special forms of tipuloid wings (namely from the architipuloid subtype) in which the basiala started to refine as a result of the intensification of the wing-beat, the increase of its frequency, together with the development of costalization. [It is perhaps more probable that the evolutionary increase of wing-beat frequency in the development of the dixoid type became possible only after the basiala had been refined (and the degree of costalization had reached a certain level).] [See for tipuloid wings, that is, wings of the primitive traction (tipuloid) type, and subtypes above ]
Traction scaly (culicoid) wing type.
Description (taken from ROHDENDORF, 1951, with changes) of the type as such :
Representatives of the type
Wings of the traction scaly (culicoid) type are possessed by the representatives of the two closely related families of the superfamily Culicidea, viz., the Culicidae (mosquitoes) and the Chaoboridae. See next Figure.
Wing of Culex pipiens L. (Culicidae).
(From ROHDENDORF, 1951, after HENDEL)
Size of the wings
The shape of the wings
The wings of this type are always strongly elongated, 4 - 41/2 times longer than their width. Fore-margin straight almost up to the very wing-tip. The curve of the margin begins very close to the end of the wing. The apex of the wing is [therefore] short. The hind-margin of the wing is uniformly convex, and with it the widest part of the wing-blade lies markedly distally from its middle. The basiala is clearly distinguished, see next Figure.
Theobaldia alaskaensis LUND. ( Culicidae).
Basiala and base of wing-blade of right wing of male. Top view.
Specimen nr. 2570. Length of whole wing 6.5 mm., of basiala 0.75 mm. Macrotrichia and scales not drawn.
For abbreviations see Figure 7 .
(After ROHDENDORF, 1951)
The anal lobe is clearly expressed, sometimes in the form of an almost rectangular rounded projection. The alula is well developed, in the form of a broad rounded projection. The [two, on each wing one,] wing-scales are developed, having the shape of rounded projections, along the margin provided with long spinelets. Thoracic scales are absent. When the insect is not flying it holds its wings one upon the other over the abdomen. Sometimes the wings are held obliquely backwards.
Skeleton of the wing
The wing-venation of the representatives of this type is rich, showing a moderately expressed costalization : The long subcostal vein and the first radial branch run parallel to each other and to the wing-margin, but not coming very close to the latter. At the same time the costalization consists in a clear suppling of the [area at the wing's] hind-margin where the veins are more or less broadly separated from one another leaving the anal lobe almost free of veins. All radial veins (numbering four) and the anterior medial vein run parallel, where the radial veins end up at the fore-margin and at the wing-tip, while the medial veins end up at the hind-margin. There are three medial branches. Of the cross-veins in the wing-blade there are only the two main ones, the radio-medial [rm] and the medio-cubital [mcu], where the medio-cubital has been transformed into the basal trunk of the last medial vein, which is usually observed in recent Diptera. The costal vein runs all around the wing. The basiala carries a sharply expressed phragma, which is the transverse outgrowth delimiting the basiala from the wing-blade and which unifies the 'handles' [ = swollen proximal ends of longitudinal veins] of the veins : the proximal end of the radial vein is very broad, completely covering the [corresponding part of the] subcostal vein.
Coverings of the wing
In addition to hairs, microtrichia, which are distributed over the whole wing-surface, all longitudinal veins carry series of scales. The margin of the wing carries differently-built scales. The fore-margin carries smaller adjoining spinelets-scales that gradually become smaller when going along the margin from base to tip. Beyond the wing-tip the marginal spinelets suddenly increase in length and keep on increasing up to the projection of the anal lobe along which the spinelets reach their maximum length. Along the margin of the wing, especially along the anal lobe, there are three sorts of spinelets (see Figure above ) : The longest are the lanceolate spinelets-scales, then shorter spinelets placed between the adjacent long ones, and, finally, the shortest hairs, densely bordering the wing-edge [and lying] among the bases of the spinelets. The spinelets that lie along the margin of the alula differ totally from those fringing the anal lobe. They are thinner and shorter. On the wing one can easily distinguish special sense organs lying in transverse rows of minute bumps with spinelets, in the form of seemingly transverse stripes in the basiala, namely on the anterior surface of the broadened part of the radial vein.
Functional characteristic
The blood-sucking mosquitoes (Culicidae) have a fairly fast and well-steerable flight. They are capable of flying fairly great distances (some species of the genus Anopheles up to 3 km). The biological significance of flight is very great for the females, which are actively and prolongedly seeking out and 'hunting down' their victims which are vertebrates. The males perform characteristic mass flights, swarming in large aggregations. Characteristic to the flight of mosquitoes (Culicidae) probably is a large store of energy : the mosquito must perform long flights, and, in addition, after having taken a blood-meal significantly increasing its weight (more than two times), must be able to fly to a sheltered place. Undoubtedly the flight of the mosquito is fully refined. This is being testified by its wing-beat frequency, that varies between 248 to 307 beats per second, which until now appears to be the highest value known in all insects. Indeed, it is probably characteristic for mosquitoes to have a high wing-beat frequency which makes possible to develop a strong lifting-force and a refined steerability. The, as it seems, insufficient mechanical specialization of the wing-venation which has little changed and only insignificantly costalized is amply compensated for by the most remarkable covering [of the wings], the abundance of spinelets and hairs, which apparently create useful aerodynamic effects during the fast wing-beat. The presence of spinelets at the wing's hind margin undoubtedly take over the effect of the absent, better, little developed, posterior membrane of the wing. Another important refinement is the structure of the basiala, undoubtedly possessing a high degree of firmness and at the same time flexibility.
[How to deal functionally and ecologically with the other family (Chaoboridae) having this type of wing, will not be easy. They are non-biting. The larvae of them are -- in contrast to those of the true mosquitoes (Culicidae) -- aquatic predators. They presumably have gathered enough protein-containing food for letting to ripe the eggs in the female, making a blood-meal superfluous.]
History of the type and its transformations
Fossils can very little illuminate the history of this type. They are only known from tertiary deposits. All these fossil remains belong to genera also living today. Recent Culicidea are very uniform in their wing structure. It is, however, certain that such uniformity of the wings is only a consequence of their being still insufficiently studied. Actually there are differences of the evolutionary pathways of the wings of the different midges or mosquitoes of this type, which is evident by the fact of their existing in a large number of forms and by the presence of essential differences in their way of life. The first forms that had led to culicoid wings belonged to the tipuloid type, namely to little costalized forms of them, and moreover those forms in which a firm basiala with a strong phragma started to develop. Me (Rohdendorf) thinks that among recent insects such wings are possessed by the representatives of the family Nemopalpidae, not known to me concretely ( They, as relict forms (family), belong to the superfamily Psychodidea, and are known from countries of the southern hemisphere). Such an ancestral 'proculicoid' type did not only form the basis of [the formation of] the true culicoid wings but also of the costalized wings of the dixoid type.
Primitive lifting (psychodoid) wing type.
Description (taken from ROHDENDORF, 1951, with changes) of the type as such :
Representatives of the type
Wings of the primitive lifting (psychodoid) type are characteristic of species of two families of the superfamily Psychodidea, namely the moth-flies, Psychodidae, and the sand-flies, Phlebotomidae. The Nemopalpidae, also belonging to the superfamily Psychodidea, is a relict group, and the wings of its representatives probably also belong to this type.
Here we give some Figures (a number of them already given earlier) depicting wings of this type.

Wings of recent Psychodidea, belonging to the primitive lifting type.
34 -- Nemopalpus zelandiae ALEX.
35 -- Ulomyia fuliginosa MEIG.
36 -- Phlebotomus papatasii SCOP.
37 -- Trichomyia urbica CURT.
(From HENNIG 1954, after ALEXANDER, 1927)
Wing of the primitive lifting type.
Psychoda sp. (Psychodidae).
(From ROHDENDORF, 1951, after HENDEL, with changes)
Size of the wings
Shape of the wings
The wings of this type are of 'middle' length or are elongated. Their width is 2.5 - 4 times shorter than their length. Sometimes the wing is even more than 5 times narrower than long. A sharp narrowing of the wings is observed in the most minute forms (some species of the genus Phlebotomus, for example Ph. dentatus SIN., Ph. minutus ROND.). The relative large Psychodidae possess very broad wings (the genus Pericoma, [ROHDENDORF refers to Figure 24 in his work (see Figure above ), but the depicted wing is, according to his subscript, belonging to the genus Psychoda] ).
Fore- and hind-margin of the wing are convex, more or less similar to each other. The apex of the wing is sharply pointed (the angle is a little smaller than a right angle). The apex is not clearly distinguished [from the rest of the wing]. The basiala is fairly sharply distinguished from the wing-blade and has a peculiar structure. The anal lobe is expressed by the general convexity of the wing's hind margin. The alula and the thoracic scale are absent. The wing-scale is present in the form of a germ ('a beginning') on the basiala.
Skeleton of the wing
The wing-venation consists of many straight longitudinal veins, fan-like distributed over the wing-blade. The branchings of the radial and medial systems do not lie at a single transverse level in the wing, but positioned in the wing-blade at different places. The venational pattern does not show any sign of costalization. In this respect this wing type sharply differs from all other wings. The costal vein runs all along the wing. The subcostal vein is almost totally reduced. The radial (5 branches) and medial (3-4 branches) are of equal thickness, lying at equal distances from each other, uniformly and insignificantly diverging. Almost all radial veins end up at the wing's fore-margin, while the last radial (fifth) branch ends up at the sharp wing-tip (i.e. at its apical corner). Cross-veins are present only in the form of the short radio-medial (rm) and medio-cubital cross-vein (mcu) being placed near the wing-base [The mcu (and also the rm) is well visible in 37 ( Trichomyia ) of the Figure above ]. The basiala is well developed mainly in Psychodidae. In the Phlebotomidae it is reduced. The venation of the basiala is peculiar. This part of the wing itself consists of two parts : a broad proximal one, and a narrowed distal one. The proximal part, which is the part of the basiala closest to the thorax, carries a germ of the wing-scale, and includes the distinguished basal parts of the costal, radial, and anal veins. The narrow distal part of the basiala includes a large number of individual elements, which consist of (1) the lower part [Russian : nyz] of the cut-off piece of the costal vein (see Figure above ), (2) the vestigial subcostal vein, (3) the basal part of the radial vein, and (4) the two basal parts of, respectively, the cubital and anal vein [ in the Figure just referred to, we can see that, in addition to these four mentioned elements, the distal part of the basiala contains the common trunk of the medial system]. The general structure of the basiala of the psychodoid wing can be characterized as being a peculiar elastic joint which makes it possible for the wing to swing, letting, during the wing-beat, the basiala stand still. The wing-membrane is not especially investigated.
Covering of the wing
In addition to being covered by short delicate hairs (microtrichia), the wings of this type are covered by long and flattened spinelets, or better, scales, distributed along almost all veins of the wing. Especially long spinelets-scales sit on the margin of the wing, where the spinelets of the hind-margin are markedly longer than those of the fore-margin (Here the germ of costalization of the wings only expresses itself by this one feature ! ). A dense covering of spinelets-scales is present on almost the whole wing, and is especially dense in the representatives of the family Psychodidae. Sensoria are not especially investigated and are visible in the basiala only.
Functional characteristic
The representatives of the primitive lifting (psychodoid) type have a very weak flight, in which the basic effect is the overcoming of the forces of gravity, that is, generating lifting power. A more active flight is worked out in the sand-flies (Phlebotomidae), undoubtedly connected with the development of blood-sucking by the females. Minute sizes determined the character of flight in the sand-flies which flight comes close to that of those insects whose wings belong to the type named "feather-wingedness" (ROHDENDORF, 1949), and [which flight] is characterized by its slowness, by extremely low load, and by the ease of the flying insects to be carried by even weak air currents. Accurate data about the character of flight of moth-flies and sandflies are absent. There are indications that sandflies do not fly about farther than 150 m from the place of emergence of the adult, but it seems to me that these observations do not exactly anwer our question. It is very likely that we here have to do with the flying insect passively being carried away over different distances, which is not dependent on the quality of its flight. In all, the flight of insects with psychodoid wings does not have equal significance. In some cases (Psychodidae) flight is connected primarily to the sexual function and has little significance for collecting food. In other cases, in sand-flies (Phlebotomidae), flight is a necessary aspect of their life-activity, guaranteeing blood-sucking.
History of the type and its transformations
Reliable fossil material only exists in Baltic amber (Tertiary, upper Eocene) and belongs to genera of the family Psychodidae. Apart from this there exists an indication from the literature about the discovery of an alleged species of Psychodidae from lower jurassic deposits of the Angara region [the Angara is a river] (namely from the Ust-balei finding-place [ Irkutsk ] : Mesopsychoda dasyptera B., R. etc). However, the incompleteness of the description and the totally imperfect drawing of the fossil do not allow judgement as to the correctness of the identification which can be assessed as being doubtful. Investigation of recent wings of the primitive lifting (psychodoid) type outlines the evolutionary pathways of this type. The source of its formation undoubtedly were forms of the tipuloid type [ See in above ], namely its first forms which had not yet developed costalized wings. The formation of the type of psychodoid wings went its way on the basis of the general direction of the evolution of the superfamily Psychodidea, which had distinguished itself first of all by the decrease of body size. The recent state of the psychodoid type indicates a clear subdivision of it into two separate subtypes. In one group [subtype], the sandflies (Phlebotomidae), the wings did not undergo a significant increase in size. Apparently, their wing-size did not increase as compared with those of their ancestral, ancient, tipuloids. The wings merely became pointed [at the apex], acquired a dense covering of spinelets, and lost the ability to fold the wings back over the abdomen [when the insect is not flying], that is, paleopteria was developed. The absolute size of the insects decreased strongly, and the ability to suck blood appeared. These features turned out to be the chief determining factor in the formation of the present subtype. Another evolutionary pathway was realized in the moth-flies (Psychodidae), which had their [relative] wing-size significantly increased. Their wings became peculiar broad, pointed blades densely covered by spinelets-scales. The moth-fly began to hold [when resting] its huge wings like the roof of a house, that is, fold them back along the abdomen, having it covered by them, as do many moths, cicadas, and other insects. The wings of moth-flies certainly possess a very large relative surface area and a low load per unit surface area, that is, features characteristic of broad-wingedness, a type of flight-apparatus of insects which is most abundantly repesented in the insect order Lepidoptera [butterflies and moths]. The evolution leading to such wings went in the direction of an increase of lifting power of them by decreasing their load, realized by the increase of wing-size, without a corresponding strengthening of the muscular apparatus.
Apart from connections with the tipuloid type, the psychodoid wings are more or less similar to those of the culicoid type [See above ]. This similarity consists in the fact that the wings of both types are densely covered by spinelets or scales. However, this similarity is limited by the following feature : the character of the wing-beat. The very structure of the wings points to a totally different pathway of specialization in the blood-sucking mosquitos (Culicidae).
Ancient lifting venationally-attenuated (fungivoroid) wing type.
Description (and the Figures 6a -- 21a)
Representatives of the type
Wings of the ancient lifting venationally-attenuated (fungivoroid) type are possessed by almost all representatives of some superfamilies of the Nematocera -- Fungivoridea, Bolitophilidea, Bibionidea.
We give some Figures, starting with recent relict representatives.
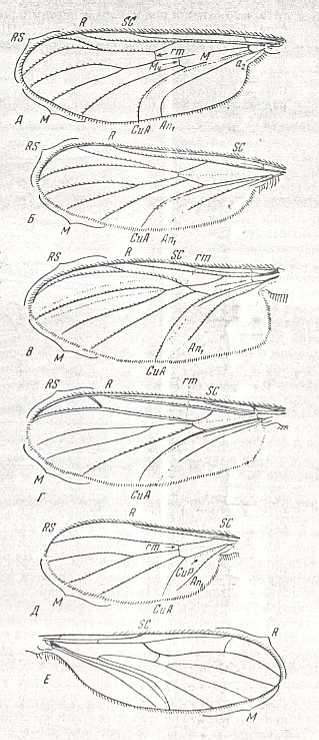
Figure 6a ( A, B, C, D, E, and F ): Wings of recent relict representatives of the ancient lifting venationally-attenuated (fungivoroid) type. Top-Down :
A -- Bibionidea. Hesperinus imbecillus LW. ( Hesperinidae). Length 8.5 mm.
B -- Fungivoridea. Ditomyia fasciata MEIG. ( Ditomyiidae). Length 5-6 mm.
C -- Fungivoridea. Macrocera sp. ( Macroceratidae [ = Macroceridae). Length 4-8 mm.
D -- Fungivoridea. Ceroplatus sp. ( Ceroplatidae). Length 10-12 mm.
E -- Fungivoridea. Diadocidia sp. ( Diadocidiidae). Length about 4 mm.
F -- Bolitophilidea. Bolitophila sp. ( Bolitophilidae). Length 4.5 mm. Specimen No. 2885.
Abbreviations as usual.
(From ROHDENDORF, 1951. A-E after HENDEL, F - after ROHDENDORF, 1951)
The next figure depicts some recent non-relict representatives of the type.
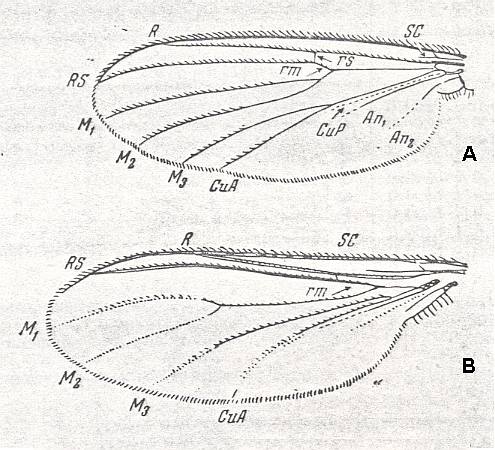
Figure 7a ( A and B ): Fungivoridea. Wings of recent widely distributed representatives of the ancient lifting venationally-attenuated (fungivoroid) type.
A -- Fungivora sp. ( Fungivoridae [ = Mycetophilidae] ). Length 5 mm.
B -- Lycoria sp. ( Lycoriidae [ = Sciaridae] ). Length 2 mm.
Abbreviations as usual.
(From ROHDENDORF, 1951, after HENDEL)
The next Figure depicts some mesozoic representatives of the type.
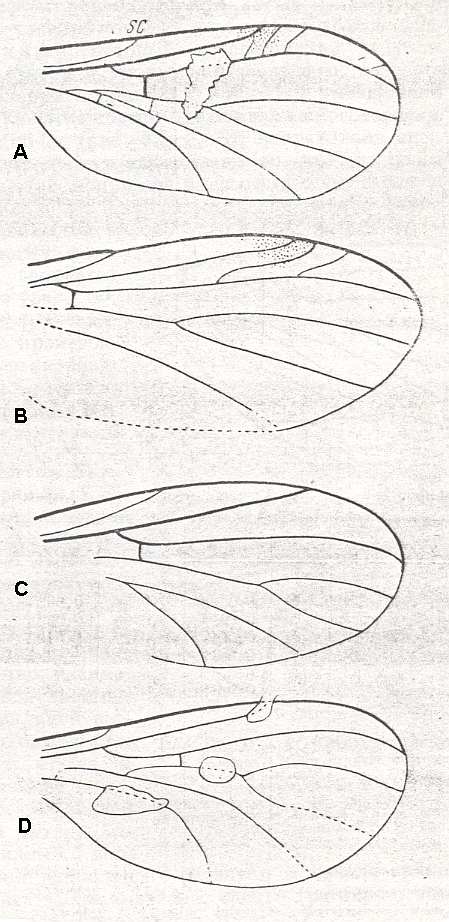
Figure 8a ( A, B, C and D ): Fungivoridea. Wings of fossil representatives of the ancient lifting venationally-attenuated (fungivoroid) type.
A -- Polyneurisca atavina ROHD. ( Pleciofungivoridae), Jurassic of Karatau [southern Kazachstan]. PIN, No. 340/167. length 1.75 mm.
B -- Eopachyneura trisectoralis ROHD. ( Pleciofungivoridae), Jurassic of Karatau [southern Kazachstan]. PIN, No. 2452/673. length 3 mm.
C -- Pleciomima secunda ROHD. ( Pleciomimidae), Jurassic of Karatau [southern Kazachstan]. PIN, No. 287/2465. length 2.25 mm.
D -- Antefungivora prima ROHD. ( Pleciomimidae), Jurassic of Karatau [southern Kazachstan]. PIN, No. 2452/316. length 1.5 mm.
(After ROHDENDORF, 1951)
The next Figures depict a number of schematically drawn basialae [ = proximal parts] of the wings of a number of Fungivoridea (Fungivoridae, Ceroplatidae, Ditomyiidae).
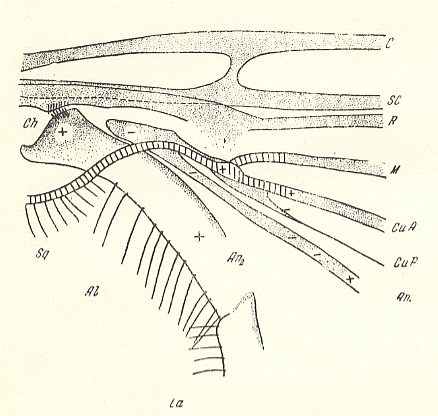
Figure 9a : Fungivoridea. Basiala of wing of Boletina trilineata MEIG. ( Fungivoridae). Schematic.
The veins indicated from top to bottom are : C = Costa, SC = Subcosta, R = Radius, M = Media, CuA = Anterior Cubitus, CuP = posterior Cubitus, An1 = first Anal vein.
La = anal lobe (lobus analis), Al = winglet (alula), Sq = wing-scale (squama alaris). Ch = chaetarium ( = patches of spinelets [forming two brushes] and having [probably] a mechanical function).
+ means : lying on a convex ( = arched upwards) spot or ridge.
- means : lying on a concave ( = scooped out) spot or groove.
(After ROHDENDORF, 1946 )
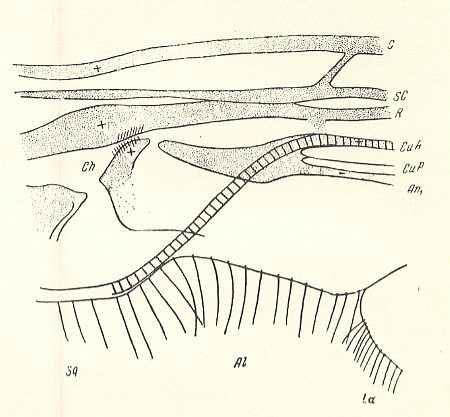
Figure 10a : Fungivoridea. Basiala of wing of Zelmira semirufa MEIG. ( Ceroplatidae). Schematic.
La = anal lobe (lobus analis), Al = winglet (alula), Sq = wing-scale (squama alaris). Ch = chaetarium.
(After ROHDENDORF, 1946 )
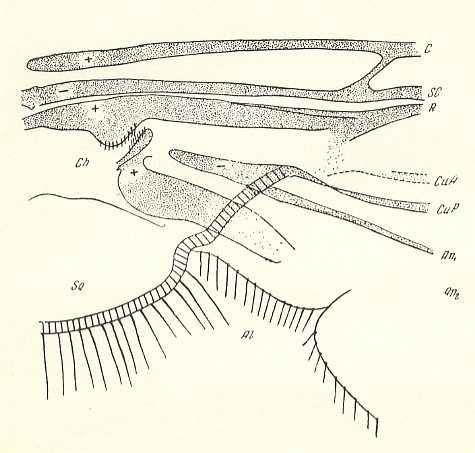
Figure 11a : Fungivoridea. Basiala of wing of Asindulum flavum MEIG. ( Ceroplatidae). Schematic.
Abbreviations as in the previous Figures.
(After ROHDENDORF, 1946 )
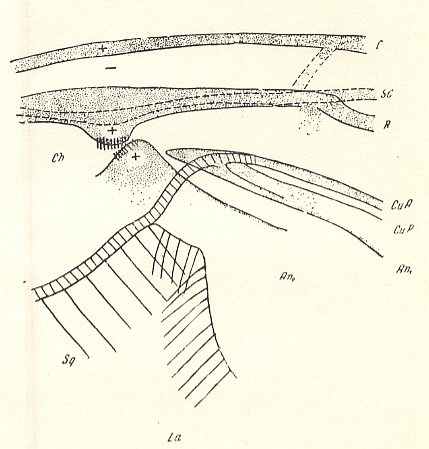
Figure 12a : Fungivoridea. Basiala of wing of Symmerus annulatus MEIG. ( Ditomyiidae). Schematic.
Abbreviations as in the previous Figures.
(After ROHDENDORF, 1946 )
Size of the wings
Shape of the wings
The wings are moderately elongated, usually 2.5 times longer than their width, sometimes shortened (only two times longer than wide) or elongated (up to 3.25 - 3.5 times longer than wide -- Bolitophilidae).
Fore-margin straight in the proximal half or in two thirds of it. The apex is, consequently, relatively large, but not especially sharply distinguished [from the rest of the wing]. Hind-margin uniformly and fairly strongly convex. Anal lobe well developed in the form of an obtusely angled projection. The basiala is, as a rule, sharply individuated by the proximal boundary of the anal lobe and by a fracture or broadening of the end of the basal trunk-piece of the radial vein (See for example Figure 11a, above ). More rarely the basiala is weakly expressed (as in Bolitophilidae, see Figure 3 of Part VII ). The alula usually present in the form of a weakly convex insert. Wing-scale present as a germ.
Skeleton of the wing
When the insect is not flying the wings are held obliquely backward. Sometimes the ability is developed to fold the wings along the abdomen. The venation is characterized by processes of reduction, affecting almost all vein-systems, especially the radial and medial systems (See Figure 6a, above and Figure 7a, above ). Shifting or thickening of veins is relatively uncommon. Costalization basically is realized as a result of processes of reduction, and only partly as a result of strengthened -- by some degree of thickening -- elements of the venation, or as a result of a shift of them.
The costal vein is present at the fore-margin only. It does not run beyond the wing-apex.
The subcostal vein is moderately developed, never longer than half of the wing, often reduced, short, and weak (Fungivorinae [ = Mycetophilinae], Lycoriidae [ = Sciaridae] and some others).
The radial system of veins is the most developed and strong system, consisting of a firm and usually long (often reaching almost 3/4 of the length of the wing ! ) anterior branch, and also of one or two (more rarely more ! ) firm posterior branches.
Of the medial veins there are, as a rule, three branches, rarely reduced to two, or even to one (genus Azana ). The two anterior medial branches form a fork that is very characteristic of this wing-type, that is, a fork formed by two weak veins and lying in the middle of the wing-blade [ = at the level of its median line]. Sometimes there has taken place a reduction of the common trunk of the medial system, as a result of which the [remaining] veins [of this system] look as if they are branches of the radial system, such as in Ceroplatidae, see next Figure,
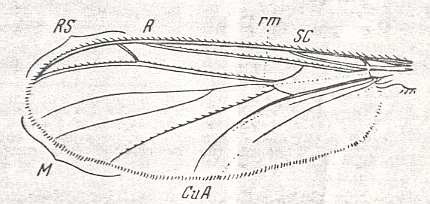
Figure 13a : Wing of Ceroplatus sp. (Ceroplatidae [ Fungivoridea] ). Length 10-12 mm. Reduction of medial trunk.
(Also dipicted in Figure 6 above).
Abbreviations as usual.
(From ROHDENDORF, 1951, after HENDEL)
in Mycetobiidae, in Macroceratidae (see Figure 6a, above ), in Ditomyiidae (see Figure 6a, above ), and others. The third medial branch coalesces with the medio-cubital cross-vein and assumes the position of [as it were] the anterior branch of the cubital vein (see Figure 6a, above, 2nd, 3rd, 4th (top-down) ). Also transformations of the radio-medial cross-vein are common, which rarely preserves its original position between the posterior radial and anterior medial veins as in Bolitophilidae, see next Figure,
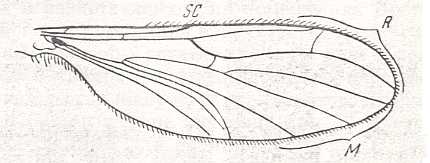
Figure 14a : Wing of Bolitophila sp. (Bolitophilidae [ Bolitophilidea] ). Length 4.5 mm. Original position of the radio-medial cross-vein (rm).
(Also dipicted in Figure 6 above).
Abbreviations as usual.
(After ROHDENDORF, 1951)
in Ditomyiidae, Allactoneuridae, and some others. Usually rm [radio-medial cross-vein] either disappears completely, as a result of the coalescence of radial and medial veins (Ceroplatidae and Macroceratidae, see Figure 6a, above ), or it transforms into a longitudinal vein and then looks like being the immediate base of the posterior radial branch ( Fungivoridae, Lycoriidae, and others, see Figure 7a, above ).
Functional characteristic
The general traits of the way of life of the insects having wings of the present type [ancient lifting venationally-attenuated (fungivoroid) wing type] are known. These minute diptera are closely connected with the soil principally of woodland, with accumulations of vegetable remains and of lower plants -- fungi, which are fed on by the larvae of the majority of forms of these midges. The winged forms have the ability to run fast and sometimes even to dig themselves in and hiding themselves amongst fragments of rotting plants. The fungivoroid flight as such is not investigated. It is probably not distinguished by high speed. However, many representatives of the type have an easy and steerable flight : We can see on indoor plants the behavior of lycoriid (sciarid) midges lively running on the surface of earth (soil) in pots with plants, often also freely flying up and about in the room. The difficulty of catching the midges in their natural habitat among rotting vegetable remains in the forest and among fragments of wood of tree-stumps, dry leaves and pine needles, is also well known. In such environments the insects are very quickly moving around, not only running on the substrate but also often flying off. Flight is of great biological significance to these insects, making it possible to find the sites necessary for reproduction which often are very locally distributed (fruiting bodies of higher fungi, rotting wood) and, in addition, to find hiding-places in which these insects hibernate. Indirect indications of the high quality of flight are the many cases of finding fungivoroid midges on window-glasses of rooms which are many hundreds of meters away from the sites of development of these insects. Together with these data, bearing witness to the great significance of flight to the life activities of the fungivoroids, we must also mention the existence of reduction of the flight ability among this group of diptera. Some forms of these insects are known to be without wings (a series of genera of Lycoriidae [ = Sciaridae], for example Caenosciara, Dasysciara, Peyrimhoffia and others, and of Fungivoridae [ = Mycetophilidae] the genera Pnyxia, Dahlica ).
Also, among the representatives of the family Fungivoridae species are known which possess very large muscular legs and relatively weakly developed wings (the genera Sceptonia, Zygomyia, Delopsis ). Probably these insects fly badly and not often. Until now, precise data on flight features of the fungivoroid midges are absent.
History and transformations of the type.
There exists many fossil documents revealing the history of fungivoroid wings. The diverse jurassic and tertiary species of a whole series of families of the Oligoneura [roughly : Bibionomorpha] illustrate the history and the differentiation of the type, which was present in the form of a series of subtypes already in jurassic times.
We shall now list and describe these subtypes.
1. First of all, the primitive fungivoroid subtype is characterized by the existence of many branches in the radial system of veins ending up at the edge of the wing (for example the genus Polyneurisca of the family Pleciofungivoridae, see Figure 8a, - A, B, above ). This subtype is still insufficiently known. In addition to the peculiar structure of the radial veins, we must note that the wings undoubtedly possessed a whole series of other features, as for instance, a little refined and insufficiently strengthened basiala [which usually is not discernible in fossils]. The primitive fungivoroid wings are a direct derivative of the eoptychopteroid subtype of tipuloid wings, and in their turn were the source of the bibionoid type and of the ancient pleciofungivoroid subtype of the fungivoroid wings (see next subtype). The mentioned eoptychopteroid wings belong to the fifth subtype of the primitive traction (tipuloid) wing type, see above .
2. The pleciofungivoroid subtype is characterized by the existence of a strong well-developed anterior branch of the posterior radial vein [radial sector], by the presence of the common trunk of the medial veins, by the original position of the radio-medial cross-vein (rm), which often is shifted toward the wing-base. Wings of this subtype were possessed by many species of the jurassic family Pleciofungivoridae, see next Figures,
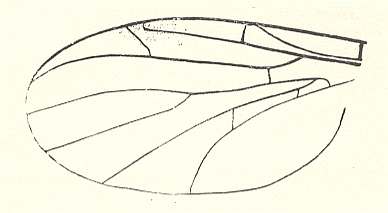
Figure 15a : Wing of Transversiplecia transversinervis ROHD. (Pleciofungivoridae). Length 3.5 mm. Well-developed fork of radial-sector. Original position of the radio-medial cross-vein (rm).
Jurassic of Karatau [southern Kazachstan]. Specimen PIN, No. 2452/358.
(After ROHDENDORF, 1946)
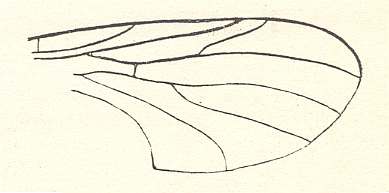
Figure 16a : Wing of Prohesperinus abdominalis ROHD. (Pleciofungivoridae). Length 3.25 mm. Well-developed fork of radial-sector. Original position of the radio-medial cross-vein (rm).
Jurassic of Karatau [southern Kazachstan]. Specimen PIN, No. 2452/580.
(After ROHDENDORF, 1946)
The subtype is barely represented in the recent fauna and only a representative of the single family Hesperinidae [Bibionidea] (See Figure 6a above ) is a residue of this once abundant subtype. The specialization in this relict form is interesting : the females lack wings.
3. The pleciofungivoroid wings were the starting-point of many other subtypes of the present type, of which, first of all we must mention the pleciomimoid subtype, which originated as a result of reduction of branches of the posterior radial vein [radial-sector], that is, the radial-sector lost its fork(s) and became one single vein, and [as a result] of the shortening of the anterior branch [radius], of the increase of the degree of costalization (by means of the weakening of the medial veins), and, finally, as a result of the decrease of the general size of the insects. In the jurassic fauna this subtype was represented by the many species of the Pleciomimidae, see Figure 8a, - D,C, above . The direction of specialization of the wing-venation in its generality reminds us of what we saw in the family Pleciofungivoridae, for example a shift towards the wing-base of the cross-vein rm and the weakening of the medial fork.
In the recent fauna pleciomimoid wings are only observed in some forms of the relict family Diadocidiidae,
see Figure 6a, - 5th (top-down), above .
4. Another derivative of pleciofungivoroid wings, the allactoneuroid subtype, was represented in jurassic times by the family Allactoneuridae. See next Figure.
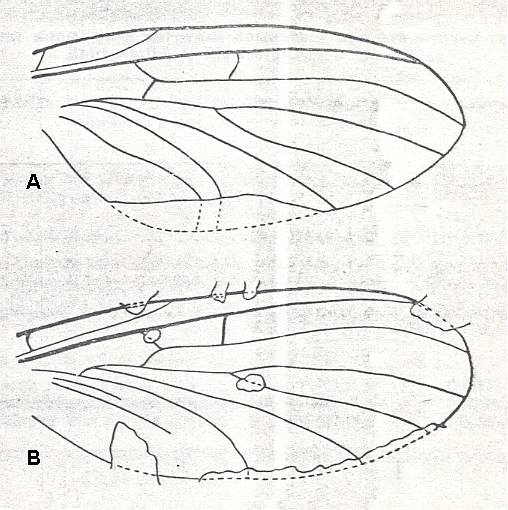
Figure 17a : Wings of the allactoneuroid subtype of the ancient lifting venationally-attenuated (fungivoroid) type.
A -- Mesosciophila venosa ROHD. (Allactoneuridae). Jurassic of Karatau [southern Kazachstan], Geol. Inst. SAGU, No. 26. Length 4.5 mm.
B - Mesosciophilodes angustipennis ROHD. (Allactoneuridae). Jurassic of Karatau [southern Kazachstan], PIN, No. 2452/172. Length 2.75 mm.
(From ROHDENDORF, 1951, after ROHDENDORF, 1946)
In the recent fauna this subtype is represented by the large family Fungivoridae, see Figure 7a, above, - A , and also next Figure.
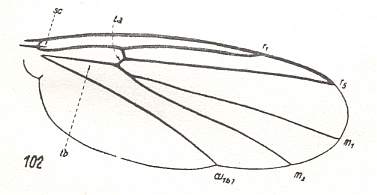
Figure 17aa : Wing of Zygomyia notata STANN. (Fungivoridae), belonging to the allactoneuroid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(After HENNIG, 1954)
In HENNIG, 1954, we found a drawing of a wing of a recent representative of the family Allactoneuridae :
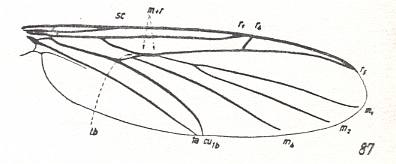
Figure 18a : Wing of Allactoneura nigrifemur ENDERL. (Allactoneuridae), belonging to the allactoneuroid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(From HENNIG, 1954, after EDWARDS, 1925)
This subtype is characterized by an increase of the degree of costalization of the wing, realized in a totally different way, namely by the elongation and strengthening of the anterior radial vein (the radius proper) together with a shift of the cross-vein rm to the wing-base and its transformation into [as it were] the basal [ = proximal] part of the posterior radial vein (the radial sector) (See Figure 7a, above ).
5. The last derivative of the pleciofungivoroid subtype we see in the wings of the tertiary and recent families Mycetobiidae (See next Figure), Ceroplatidae, Macroceratidae, and Ditomyiidae (See Figure 6a - 2nd, 3rd, 4th (top-down), above ).
For the structure of the basiala of members of the Ceroplatidae see Figure 10a, above and Figure 11a above .
For the structure of the basiala of a member of the Ditomyiidae see Figure 12a above .

Figure 19a : Wing of Mycetobia pallipes MEIG. (Mycetobiidae), belonging to the ceroplatoid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(From HENNIG, 1954, after FREEMAN, 1951)
The subtype they represent can be called the ceroplatoid subtype. The direction of changes of the wing-venation consisted in the realization of a peculiar form of costalization : The radial veins did not decrease in number. The middle branch became strong and simple [i.e. not branched] (See Figure 19a and Figure 6a, - 2nd (top-down) ). The medial veins shifted towards the posterior radial vein, and, in the majority of cases the cross-vein rm disappeared as a result of the coalescence of medial and radial veins. The proximal part of the medial vein was reduced and in its place a large basal cell was formed.
6. The allactoneuroid wings (fourth subtype) formed the basis of the formation of the youngest, lycorioid specialized subtype, see Figure 7a, - B, above , which is expressed in the representatives of the soil-dwelling midges, Lycoriidae [ = Sciaridae] (see also next Figure) and some of the gall-midges, namely the Lestremiidae.
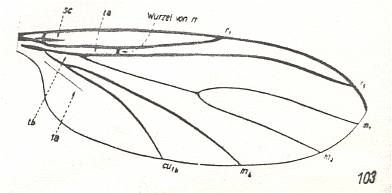
Figure 20a : Wing of Lycoria bicolor MEIG. ( Lycoriidae [ = Sciaridae] ), belonging to the lycorioid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(After HENNIG, 1954)
The subtype is characterized by far-progressed processes of costalization. In this subtype only two radial branches are preserved, and the cross-vein rm changes its original position, becoming the basal trunk of the posterior radial vein, and the direct continuation of the basal part of the media. This complex vein, as also the anterior radial branch [radius proper] and the costal vein, are strongly elongated and become the chief skeletal element of these peculiar costalized wings. All remaining veins of the lycorioid wings are weak, and the subcostal vein is almost completely reduced. The lycorioid subtype is only known from the Caenozoic upwards : The time of its formation must of course be set in cretaceous times.
7. The last subtype of the ancient lifting venationally-attenuated (fungivoroid) wing type, the bolitophiloid subtype, is found in the representatives of the relict family Bolitophilidae, see Figure 6a, - 6th (top-down), above and next Figure,
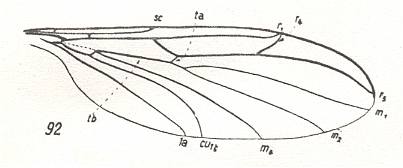
Figure 21a : Wing of Bolitophila hybrida MEIG. (Bolitophilidae) belonging to the bolitophiloid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(After HENNIG, 1954)
and is characterized by a series of peculiar features, bearing witness to a totally different way of origin. First of all the wings of this subtype characterize themselves by the indistinct basiala, in which the proximal parts of the cubital and anal veins are fairly well-distinguished and form an insufficiently compact concave element of the basiala. Together with this feature we can observe (1) a far-progressed reduction of the 'handle' (proximal end-piece) of the second anal vein [The anal chaetarium is, however (judging from ROHDENDORF's drawing of it -- see next link), strongly developed, and this could be the 'handle' of the second anal vein, the rest of the vein being disappeared], (2) the absence of the boundary between the alula and anal lobe, and (3), which is especially important, a strong lengthening of the wings. For the basiala of a representative of this subtype see Figure 3 of Part VII. In addition, this bolitophiloid subtype is characterized by a weakly developed costalization and by other values of surface-area and load of the wings. The latter is very small and is virtually certainly the lowest of all representatives of the order (Diptera) (going down to 0.006 gr/cm2 ).
The connections of the ancient lifting venationaly-attenuated (fungivoroid) wing type with other types are more or less clear. Its point of departure or source of origin were forms of the primitive traction (tipuloid) wings, namely the eoptychopteroid subtype thereof [5th subtype], which led, in a parallel way, to two subtypes of the fungivoroid wings, the primitive fungivoroid subtype and the bolitophiloid subtype. In its turn the primitive fungivoroid subtype was the source of origin of the ancient lifting venationally-dense (bibionoid) type [see for this type there where the superfamilies Bibionidea and Scatopsidea are dealt with] and of the remaining forms of the fungivoroid type. In addition, from the pleciomimoid subtype undoubtedly came forth the characteristic wings of the pseudo-feather-winged ( itonidoid ) type [The latter may also have come from scatopsidae-like wings] [For the pseudo-feather-winged type see there where the superfamily Cecidomyiidea is dealt with].
In evaluating the direction of development of the fungivoroid wing type we can note various changes of features of the wing-venation having the character of particular improvements of the quality of the wing. These include the improvement of costalization of the wing which took place in the history of the type on several occasions and in different ways in the different subtypes.
In the pleciofungivoroid and pleciomimoid subtypes costalization was realized as a result of a shift of the radio-medial cross-vein (rm) toward the base of the wing, but in the latter subtype in addition to it a reduction of the middle radial branches took place. One can notice an extreme shift of the radio-medial cross-vein, a change of its position and its transformation into [becoming] the proximal part of the posterior radial branch, together with the lengthening of those veins, in the allactoneuroid subtype, and, finally, a significant strengthening of this system of veins with at the same time a weakening of the posterior [systems of] veins in the lycorioid subtype. This subtype is certainly the most progressive one. The development of peculiar broad wings of the ceroplatoid subtype, and the elongated wings of the bolitophiloid subtype with a little refined basiala, can naturally be evaluated as examples of a moderately progressive or a narrowly-specialized direction in the evolution of the wings of this type [that is, the ancient lifting venationally-attenuated (fungivoroid) type].
The phenomena of a regressive evolutionary pathway in the fungivoroid wings are very interesting : Regression in this group of diptera expresses itself in the decrease of wing-size, their shortening, a deterioration of mechanical qualities (weakening of costalization) all the way down to the complete reduction of the whole organ, the complete loss of the wings, that is, to the development of winglessness. These processes take place in subtypes in which the general direction of [evolutionary] development is characterized in many cases by progressive features : so in the allactoneuroid and especially in the lycorioid subtype, being relatively very progressive, we see cases of the development of winglessness. This is especially remarkable in the example of the pleciomimoid subtype (almost completely extinct) which was the starting point for the characteristic regressive itonidoid wing type [ See for this type where the superfamily Cecidomyiidea [ = Itonididea] is discussed]. Undoubtedly such a kind of pathway of development of regressive forms on the basis of progressive ones took place in the history of the type. The paradoxical nature of such a position is only seemingly so. In considering cases of development of winglessness in some representatives of the fungivoroid wing type (for example the genus Pnyxia of the Fungivoridae [ = Mycetophilidae], or many Lycoriidae [ = Sciaridae] ), we easily notice the connection of this process with a change in living-conditions : These insects become more closely connected with the soil [such as the forest-floor], more exactly expressed, with every kind of concealed soil-sites -- hollows or clefts in the soil or even simply in caves (various species of Lycoriidae). In their turn, such kinds of changes of the living-conditions directly express changes in feeding [habits] of these insects. For example a species of the genus Pnyxia (a representative of the family Fungivoridae with reduced wings) has larvae living in bulbs of potatoes, but could also be found in the burrow of a mole, that is, in conditions very far from those of the usual larval sites of this family. Especially remarkable are the differences in the living-conditions of gall-midges ( Itonididae [ = Cecidomyiidae] ), which are undoubtedly derivatives of fungivoroid diptera, and of which the wings can rightly be evaluated as a regressive direction in the evolution of the pleciomimoid and lycorioid subtypes.
On the whole, this process of development of regressive wing-forms on the basis of clearly expressed progressive subtypes can be clarified only under the condition of the evaluation of the w h o l e evolutionary development of the given group of organisms. This can serve as a wonderful example of the limitedness of a purely comparative anatomical method of investigation of only a single system of organs and neatly shows how dangerous it is to draw general conclusions on the basis of such one-sided data. The over-all development of a certain group of organisms, which led to the progressive development of one or another system of organs and functions (in our cases of wings and flight), went in some cases further along a different way, along a way, which led to the development of other organs and functions (the digestive system and feeding), and where organs (wings) that earlier had their importance had become of mere secondary importance and their refinement harmful.
We have now reproduced ROHDENDORF's 1951 text dealing with the fungivoroid wing type (and have added explanations and some more figures). This means that we not only have extracted important data from his text, data about the structure of wings and the life-habits of their possessors, but have also doctrinairily largely followed ROHDENDORF, that is, we have here reproduced and followed his general thesis that holds that evolution is driven by ecology. And this implies that many evolutionary changes -- the so-called progressive ones -- must be seen as functional improvements and refinements with respect to the performance of a given organ system. In addition ROHDENDORF also recognizes regressive evolutionary processes, but rightly insists that in evaluating such processes as regressive we must take into account the whole evolutionary development of the given group of organisms. Indeed, when we do so we see that often the group as a whole, or certain subtaxa of this group (that is, precisely there where regressive processes have been observed), are not simply regressive, not regressive groups, not regressive organisms : In such cases, it is true, certain organs have degenerated, but they did so for the benefit of other organs, organs that changed progressively. All these processes are part of 'answers' or 'solutions' to problems which necessarily appear when the habitat of an animal population of a given species changes more or less permanently. And in many cases some regressive processes are necessarily part of the overall process of adaptation. As a third type of evolutionary process ROHDENDORF recognizes narrow specialization. Such a process is neither progressive, nor regressive. The result of such a process is a feature -- morphological or physiological -- (evolutionarily) developed in the organism, [a feature] that lets this organism to be precisely fit into a very special and subtle aspect or 'corner' of the overall habitat. In contrast to a progressive development, the narrow specialization makes the organism evolutionarily vulnerable. Small changes in the environment can cause extinction of this narrowly specialized species.
Because, as we have explained in Part II, the flight-regime has been evolutionarily developed such as to become compatible with how the insect experiences the environment on the basis of the specific morphological and physiological structure of its eyes and antennae, an evolutionary change in the flight-regime (made possible by corresponding changes of the wings) does not necessarily mean an improvement of flight-capabilities (higher speed, better steerability, etc.). True, it can, and often is, such an improvement (which for example was clearly the case in the origin of robber-flies), but in most cases -- especially where the original and derived insects have a more or less similar morphology, size, and way of life -- such a change in flight-regime (from original to derived) results in merely a different flight-regime, different that is, from the original one, not necessarily technically improved. Such a new flight-regime, whether merely different or improved, is an adaptation to the precise new habitat of the adult fly, and allows the latter to move about precisely in it. We must stress that with "precise new habitat" we do not mean a geographically local habitat, that is, not a spatially different location, but only a micro-physiognomically different location. This is thus a type of location or micro-landscape that can be present at many geographically different locations. Only by having acquired such an adaptation -- along with adaptations in other organ-systems -- the adult fly (the winged insect) can perform its overall function which consists in finding food (nectar, blood), in finding a mate, in geographically expanding the (new) species' range, and for the female in finding the appropriate substrate for the development of her larvae.
A given species of fly represents a definite qualitative content. This content is, as a result of the process of injection ( from the Explicate [ = physical] into the Implicate [ = noëtical, i.e. thought-like] Order), present in the Implicate Order as a noëtic entity, that is, as an immaterial thought-like enfolded entity. And as qualitative content of the given species of fly it defines, and thus determines and delimits, the respective qualitative contents of several potential ecological niches, where one of them has become actual. And because these potential and actual ecological niches are qualitatively defined and delimited by the qualitative content of the given species of fly they too can be injected into the Implicate Order and exist there as noëtic entities. And, further, according to our metaphysical theory (expounded in Part I-IV of the Dynamic Metaphysics [nomological] Series), with the transition of an original species of fly into a new species we have to do with a noëtic reaction taking place in the Implicate Order between the qualitative content of the original species -- as such a noëtic reactant -- and the qualitative content of one of its potential ecological niches -- this content also being a noëtic reactant -- resulting in a noëtic reaction-product. This noëtic reaction-product is the qualitative content of the new species of fly still existing as a noëtic entity in the Implicate Order (that is, not yet as a material entity existing in the Explicate Order). When conditions in the Explicate Order are right, this noëtic content will (as a copy) be projected into the Explicate Order ( It might also be assumed that the whole course (i.e. all stages) of the noëtic reaction as such is, together with its product, projected into the Explicate Order ). This means that the projected noëtic contents will be unfolded along space and time dimensions (they become physical), which we (in the Explicate Order) observe as the evolutionary development of certain morphological or physiological adaptations of the new species of fly to its new ecological niche, a development which generally takes much time. And one of these adaptations can be seen in transformations of the wing-structure, especially of its venation. The new wing-venation then is a co-product of the mentioned noëtic reaction. It is fully integrated with the rest of the fly's organization. It is part of the adaptation to the new ecological niche (in the narrowest sense), because it is the morphological expression of a new flight-regime enabling the fly to move about precisely in this new niche, that is, in conformity with this niche. And for the flight-regime to be in conformity with precisely the new niche means the existence of a qualitative compatibility between the way of flying and the nature of the environment precisely as perceived by the fly. And so evolution of flies, as driven by ecology, results -- among other things -- in sequences of transformations of wing-venation (often consisting in reductions and/or shifts of veins) as such [these sequences] not necessarily leading to an aerodynamically improved and refined flight, but merely to a different flight, that is a flight better adapted to certain subtle aspects of the new ecological niche. In such a way, I think, we must interpret the changes in wing-venation as discussed above in the context of the distribution of functional wing-types over taxa of Diptera.
We here have thus described the fact that the qualitative content of a given organismic species (such as a dipterous species) may create a new habitat, namely by the fact that this qualitative content of the species, that is adapted to some particular type of existing habitat, may determine (that is, may qualitatively imply) the qualitative content of a number of potential and new habitats to which, or to one of which, that species might adapt. In the Implicate Order, then, the qualitative content of the species reacts with the qualitative content of one of the species' potential habitats resulting in the qualitative content of a new species provided with the necessary adaptations to the new habitat. And when the proper ecological conditions are actually present in the Explicate Order the qualitative content of the new species will project from the Implicate Order into the Explicate Order.
But, not many documents ago, we have theorized that there may be other cases where all this proceeds more or less the other way around : Possible ecological settings (types of habitats or niches) are, from the outset, present in the Implicate Order in the form of noëtic patterns representing, in the Implicate Order, possible existential conditions in the Explicate Order, existential conditions that is for other potential (complex) noëtic patterns. Noëtic elements come together in such noëtic existential conditions and form 'crystals' which means that in these existential conditions strategies crystallize, strategies that is, to exist in the Explicate Order when these conditions actually exist there. So also here we hold, with Rohdendorf, that evolution is primarily ecologically-driven. But here the ecological conditions (present in the Implicate Order as the qualitative content of the mentioned existential conditions) create the strategy, create, that is, the qualitative content of the new species, and not the other way around. And also here a functional interpretation of even many details of the wing-venation, in at least insects such as Diptera, is the right way to approach the different venational patterns as they are encountered in such insects. Nevertheless, there will also exist, here and there, some non-functional venational details, or differences between such details, being the result of existing factors active in the Explicate Order.
So in both principal cases, just expounded, any given wing-venation (when the organism is a species of the Pterygota [winged insects] ) is, as are many other characters as well, ultimately the result of one or several noëtic reactions having taken place in the Implicate Order.
Before continuing the survey of functional wing-types of representatives of the Suborder Nematocera (reproduced from earlier documents, but expanded where necessary), and of representatives of the Suborder Brachycera-orthorrapha and, finally, of the Suborder Brachycera-cyclorrapha, we, in the next two documents, will concentrate first (next document) on the general biological and formal status of insect wing-venation, and then (next next document) on derivational sequences as they can be found among the wings of the above described functional types and subtypes.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for further study of Organic Evolution, Part LXXa.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII
Back to Evolutionary Part LVII
Back to Evolutionary Part LVIII
Back to Evolutionary Part LXII
Back to Evolutionary Part LXIII
Back to Evolutionary Part LXIV
Back to Evolutionary Part LXVI
Back to Evolutionary Part LXVII
Back to Evolutionary Part LXVIII