
Organic Evolution in terms of the Implicate and Explicate Orders.
Part XLVII
Hymenoptera (wasps, bees, ants) (Sequel)
The evolutionary diversification in the Order Hymenoptera in terms of Strategies (Sequel).
Before entering the various evolutionary phases of the true wasps (that is, including the so-called semi-wasps and all the others [solitary and social], but excluding all terebrants and all 'wasps' (saw-flies and relatives) in the suborder Symphyta, and all bees and ants), we here present an outline of the classification of the major groups of true wasps, and some more or less representative genera, taken from EVANS and EBERHARD, 1973 :
Superfamily Bethyloidea
Bethylidae, Pristocera, Epyris.
Dryinidae, Chelogynus, Gonatopus.
Chrysididae (Cuckoo wasps), Chrysis, Parnopes.
Superfamily Scolioidea
Tiphiidae, Tiphia, Methocha.
Scoliidae, Scolia, Campsomeris.
Mutillidae (Velvet ants), Dasymutilla, Ephuta.
Sapygidae, Sapyga, Eusapyga.
Superfamily Vespoidea
Eumenidae (Mason wasps), Eumenes, Ancistrocerus, Synagris, Zethus.
Masaridae, Pseudomasaris, Euparagia.
Vespidae (Paper wasps, Yellow Jackets, Hornets), Polistes, Polybia, Ropalidia,
Vespula.
Superfamily Pompiloidea
Rhopalosomatidae, Rhopalosoma, Olixon.
Pompilidae (Spider wasps), Pepsis, Pompilus, Ceropales, Evagetes.
Superfamily Sphecoidea
Sphecidae (Digger wasps), Sphex, Ammophila, Bembix, Philanthus.
[In all this, we must realize that there exist differences between classifications presented by different authors. For an overview of the whole Order Hymenoptera and the lines of its evolution, as was given in the previous document, click HERE]
Semi-vespine (bethyloid) phase
Here we must collect those diverse forms which in accordance with their morphological features are considered to be true wasps, but which behavior lets them to be similar to these or those terebrants, and therefore they may be called semi-wasps. The common biological character of them is the absence of the ability to construct cells -- isolated chambers for the individual raising of the young which is so characteristic of typical wasps. There is absolutely no doubt that the absence of the construction ability in the present evolutionary phase of the wasps is primary [that is, it is an original, not a derived, condition], or, in other woerds, we cannot hold that the ancestors of the semi-wasps did once possess this ability. The supposition of this character being primary is also supported by the fact that in all of their life activity, despite their diversity and versatility, only features of generally lower wasps are found, and not those [features] that are reckoned to belong to the higher wasps, as will be explained later.
Among the diverse forms, by their structural features belonging to the wasps, but within them having a disputed and enigmatic position, we must first of all focus on the sapygid wasps, the Sapygidae. This small group, containing in all about 50 species, distributed among 8 genera, is found in all zoogeographic regions, except the Australian one.
See next Figures.

Figure 1 : Sapyga quinquepunctata Fabr. ca. 9 mm.
(After CHINERY, in Elseviers Insektengids voor West-Europa, 1983)

Figure 2 : Sapyga clavicornis Fabr. 8-10 mm.
(After SEVERA, in ZAHRADNIK, Thieme's Insektengids voor West- en Midden-Europa, 1977)
Many opinions were expressed concerning the systematic position of the Sapygidae. In his time LATREILLE, having introduced the generic name Sapyga, placed them into the plicate-winged (or plaited-winged) wasps of the family Masaridae. CURTIS held them to be close relatives of the bees -- Nomadidae. WESTWOOD placed them close to the ant-wasps, Mutillidae, and dagger-wasps, Scoliidae, and LEPELETIER to digger-wasps, Sphecidae. Acknowledging this unclear position, NIELSEN assured that the key to resolve the question of the systematic position of the Sapygidae must be their way of life.
As is now already known for sure, all of them deposit their eggs in the cells of solitary bees, especially of the genera Eriades and Osmia, and partly Megachile, Dianthidium, Xylocopa, and, as an exception, Prosopis communis Nyl. and Anthophora crinipes Sm.
-Indications as to having obtained sapygids from nests of solitary w a s p s -- Sceliphron and Odynerus laevipes Shuck. -- are correctly explained by PATE (1947) by the fact that also in these cases the hosts of the sapygids were nevertheless solitary bees that visit [use] abandoned nests of the mentioned wasps. As was discovered by FABRE (1879-1907), Sapyga punctata Klg. lays its spindle-shaped egg on the egg of Osmia tridentata Dur. The young larva of the Sapyga, having hatched from the egg, is white, transparent, and leggless. Its head is very distinct from the rest of the body, and carries very short and thin antennae. This larva is not similar to the usual form of hymenopterous larvae. It is fairly active, constantly moving up and down the first half of its body. It gnaws the egg of the Osmia, which begins to shrivel, turn pale, and, finally, changes into a flabby shell and [then] completely disappears. The Sapyga-larva molts and, becoming immobile, consumes the honey dough of the osmia.
Based on earlier observations of the author [Malyshev], Sapyga similis F. deposits one or two eggs onto the egg of the bee Eriades florisomnis L. or [lays them] nearby not determining them as to a special position. Sometimes there are more [eggs] -- up to four in one cell. Then some of them lie on the honey provisions or even on the innerside of the cap of the nest. For nest and eggs see next Figure.
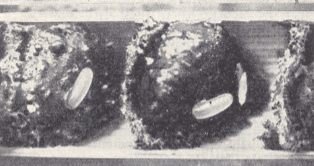
Figure 3 : Egg of Sapyga similis F. in the middle cell of [the nest of] the bee Eriades florisomnis L. (After MALYSHEV, 1966)
[Interpreting this subscript, it must mean that every cell of the nest was provided by the Eriades-bee with one egg (two of them visible in the photograph). In the middle cell we see a second egg, which is added by the inquiline Sapyga similis]
In the same way behaves the sapygid that infects cells of [the nest of] Osmia coerulescens L. The Sapyga-larva having hatched first [i.e. earlier than the Osmia-larva] begins with sucking out the other eggs of its kin that happen to be nearby, and then also destroys the egg of the bee. Having done this, it settles itself at the place where before there was the egg of the host bee, and quickly starts to consume the honey dough. Because of this it might easily be confused with the young larva of the bee. Anyway, in the larva of Sapyga the posterior end of the body is wide with a black content shining through, whereas in the larva of the bee it is narrow with yellow content. Soon after the beginning of feeding, the Sapyga-larva starts to give off excrements in the form of a string of round black small bits connected by short crosspieces. Having finished feeding, it constructs a firm egg-like cocoon and hibernates in it in the adult stage.
According to SOIKA, 1932, in [the case of] Sapyga quinquepunctata F. (Figure 1) the egg is usually attached with a black fluid onto the wall of the cell at the place separating the honey dough from the cap of the nest, or [is deposited] onto the egg of the host bee (Osmia coerulescens L., Eriades fulviventris Pz., Megachile centuncularis L.). The hatching larva is "campodeiform", similar to the young larva of the cuckoo wasp Chrysis dichroa Dahlb., described by FERTON. After a week the larva molts and takes on the form of a usual aculeate larva.
Another sapygid, Polochrum repandum Spin., a giant among its relatives, also, evidently, deposits its egg onto the egg of the solitary bee Xylocopa. Thus, PARKER (1926) found a young Polochrum-larva next to a dead egg of Xylocopa violacea L. He assumes that the Polochrum-larva destroys the egg of the bee, in order to use the honey dough prepared in the cell by the Xylocopa. See next Figure.

Figure 4 : Three cocoons of Polochrum repandum Spin. in abandoned nest channels of Xylocopa valga Gerst. (After MALYSHEV, 1931, in MALYSHEV, 1966)
So, the Sapygidae, enigmatic as to their systematic position, are typical inquilines of bees : They deposit their eggs in strange nests [that is, they do not make a nest of their own] onto the eggs of their hosts or onto the stock of food prepared for the larvae of these hosts. The emerging mobile inquiline larva is not similar to the larvae of the other aculeates, except only those of cuckoo wasps (Chrysididae), about which we will speak a little later. The sapygid larva after having destroyed the egg of the host lives at the expense of vegetable food, not prepared for it -- a mixture of flower pollen and nectar, which it, moreover, assimilates [digest] not in an aculeate fashion, but in a fashion typical of archaic inquilines that defecate during feeding. All this supports the assumption that the Sapygidae are a relict group of aculeates, still standing in the archaic inquilinoid phase of evolution, from which this group, in all probability, directly started off.
This is a conclusion that is very important to us. In it we see the first and very definite indication that the wasp-like Hymenoptera (the true wasps) have very deep [historical] roots, leading, at least in some of them, [all the way] to precisely those archaic forms from which also the Terebrantia arose.
Meanwhile, PATE, 1947, unites the Sapygidae with the enigmatic Fedtschenkiidae. The latter are represented by the one holarctic genus Fedtschenkia with three species which, as PATE assumes, should, in virtue of their structural features, be placed in the fossorial wasps that provide their nests with other insects, which is, however, totally foreign to the Sapygidae.
POPOV, 1948, places the Fedtschenkiidae in the neighborhood of the Thynnidae, and
BRADLEY, 1955, points to the very peculiar, not to be found in other wasps, structure of the 6th sternite forming a kind of cone at the end of the abdomen from where the sting takes its beginning. On this he bases his proposed union of the fedtschenkiids with the sapygids into one single family, the Sapygidae. Assuming the phyletic connection between the two, BRADLEY derives them from a common ancestor -- the fossil wasps of the family Anthoboscidae.
On the other hand, the natural affinity obtaining between the Sapygidae and the Fedtschenkiidae provided the basis to unite them into a single superfamily of sapygoid wasps -- Sapygoidea ( TOBIAS, 1965).
In the light of the just presented ideas of these authors, all these contradictory views lead to a different conclusion. First of all, if indeed the Fedtschenkiidae [the text reads "Fedtschenkiinae"] belong to the family of sapygoid wasps, then we can rightly expect that they are inquilines too, and especially of nests of fossorial solitairy bees such as Andrena and others. The absence of nests of their own, in places where these wasps usually live (Turkestan, North America) and the rarity [low abundancy] of Fedtschenkiidae support this view, because otherwise, taking into account the interest they enjoy among naturalists, their nesting activities would hardly remain unnoticed when they would have nests of their own. On the other hand, if the sapygids, as is held by BRADLEY, are the descendants of the fossil wasps Anthoboscidae, then we must assume that also the Anthoboscidae were inquilines, if not in galls as did their archaic ancestors, then in cells of early bees of the type Colletus and Hylaeus, and possibly also [in cells of] Masaridae.
Let us now turn our attention to the family Chrysididae or Cuckoo Wasps. With already 1500 described species, they are mainly distributed in hot regions. Let us present some Figures illustrating this large family of wasps.
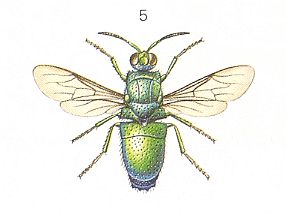
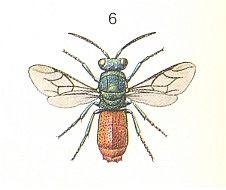
Figure 5 : Chrysididae (Cuckoo Wasps).
Left - Stilbum cyanarum FORSTER. ca. 12 mm.
Right - Chrysis ignita L. ca. 9 mm.
(After CHINERY, in Elseviers Insektengids voor West-Europa, 1983)


Figure 6 : Chrysididae (Cuckoo Wasps).
5 - Chrysis cyanea. 3.5-8 mm.
6 - Chrysis nitidula. 7-13 mm.
(After SEVERA, in ZAHRADNIK, Thieme's Insektengids voor West- en Midden-Europa, 1977)
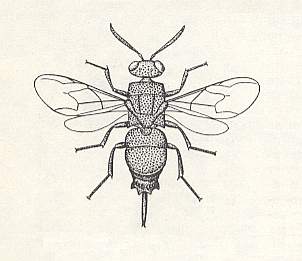
Figure 7 : The cuckoo wasp (Chrysidid) Chrysis coerulans F.
(After CLAUSEN, 1940, in MALYSHEV, 1966)
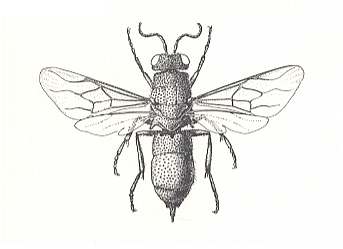
Figure 8 : A cuckoo wasp (Chrysididae). These wasps have lost the power to sting, the last few abdominal segments forming an extensible tube that is used to insert the egg into the nest of the host. (After EVANS and EBERHARD, 1973)
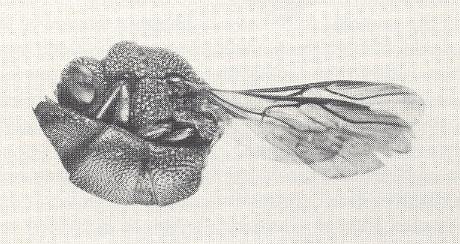
Figure 9 : A cuckoo wasp (Chrysis parvula) in the rolled-up position assumed when attacked. The shieldlike abdomen is concave beneath, and protects the vulnerable parts of the head and thorax. This is believed to be a device for escaping the stings of their hosts, for the majority of cuckoo wasps lay their eggs in the nests of wasps and bees, and their larvae develop as parasites of those insects.
(U.S. Department of Agriculture. In EVANS and EBERHARD, 1973)
Based on the peculiar structure of the cuckoo wasps (Chrysididae) they are placed nearest to the bethyloid wasps [One may say that they are a family of the Bethyloidea]. Earlier they even were distinguished as an independent Suborder of the Hymenoptera -- Tubulifera [= tube bearers]. It is remarkable that the abdomen of the females in all consists of only two or three externally visible segments, whereas the remaining segments are transformed into a narrow telescoping tube, serving as an ovipositor ( Figure 7 ). Apart from one species, the sting is not developed in Chrysididae, and there are no glands that give off venom. So these wasps cannot sting. The chief feature of their development is the moblile planidial [-like] first-instar larva. Of all the [true] wasps such a larva is known only in them and in the Sapygidae. See next Figure.
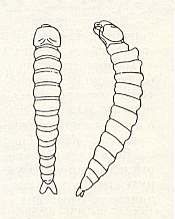
Figure 10 : First-instar larva of Chrysis ignita L.
(After MALYSHEV, 1911, in MALYSHEV, 1966)
Regarding the way of life of the cuckoo wasps, different views exist. Already in the beginning of the 19th century LEPELETIER, basing himself on his own observations, stated that the larva of the Chrysidae, having hatched from the egg, laid in the cell of a wasp, does not develop until the host larva has consumed a large part of the food prepared for it and has reached almost its fully-grown state. Only then the chrysidid larva crawls on it and sucks it out little by little.
Some years later, chiefly thanks to the investigations of DU BUYSSON, 1892, it became known that LEPELETIER was right. Having placed, in a glass tube, in the corresponding order, all content of the nest cells of the wasp Odynerus, infected by a chrysidid (cuckoo wasp), the investigator could easily observe the behavior of the larvae of the latter. It turned out that the cuckoo wasp larva was not able to use for itself the prepared food store (consisting of caterpillars) even in the case when its surroundings did not contain any other food, and perished amidst this store, if indeed the Odynerus-larva would, by one reason or another, not develop [This larva will be the food of the chrysidid larva]. Thus, in order not to die of hunger, the cuckoo wasp larva does not, and cannot destroy, the egg of the host. Soon after having hatched it directs itself toward the just emerged wasp larva, and only after about a week begins to suck it out. In this, the host larva may reach its fully-grown state, but may also die a little earlier, which will then be expressed [have its effect] in the size of the emerging adult cuckoo wasp.
Regarding the in the cell of a Bembex developing cuckoo wasp Parnopes grandior Pall., FABRE showed that the mentioned chrysidid penetrates into the nests of Bembex rostrata L., when the wasp brings flies into them, but that the chrysidid larva develops only at the expense of the fully-grown larva of the wasp, which already succeeded in making a cocoon. Inside the latter the Parnopes-larva erects its own cocoon. Clearly, also in this case there is no sign of destruction of the egg or of the young larva of the host wasp, nor is there any sign of consuming its store by the chrysidid larva.
Also FERTON, 1905, observed that Chrysis dichroa Dahlb. deposited its egg in the cell of the bee Osmia rufohirta Latr. yet still to be supplied with honey dough. Also in this case the cuckoo wasp larva attacked the already fully grown larva of the host bee. In another, although not well known, case (MANEVAL, 1932) the young larva of Pseudochrysis neglecta Shuck. was found in a state of not completely sunken into the body of the half-grown larva of Osmia villosa Schnck. and then concluding its development in it.
In the just presented cases the chrysidids, although depositing their eggs in foreign nests, do not consume the foreign provisions, and are therefore in fact not inquilines, but metaparasites, standing in the delayed-parasitic phase expounded in an earlier document. Among the Chrysididae there exist nevertheless also typical inquilines. Thus, according to observations of the author [Malyshev, 1911] the newly-born larva of Chrysis cyanea L. first sucks out the egg of [the wasp] Trypoxylon figulus L., deposited onto a paralyzed spider, an then starts to consume spiders prepared in the cell of the Trypoxylon. There is also an indication (BORDAGE, 1913) that the young larva of Chrysis lussa var concinna Grib. only develops when it, as a preparation, destroys the newly-born larva of the solitary wasp Sceliphron, and then lives at the expense of the spiders that were prepared in the cell of the host wasp [And these indeed are cases of true inquilinism : the host is destroyed, and after that the inquiline can consume the food originally belonging to the host (whether this is animal food, or vegatable food [gall-walls and the like].]
In North America it is also observed how the cuckoo wasp Chrysis verticalis Patton. developed at the expense of paralyzed spiders, gathered in the cells by Trypoxylon. The Chrysis deposits its about 1 mm long egg beside the lower wall of the cell while the larger egg of the host wasp is placed onto the abdomen of the spider which lies beside the cap in the same cell. This, one must think, indicates something about the time when it was brought in -- before the closure of the cell, when the wasp flies off in search of building material. On the dorsal side of almost all segments of the body of the newly-born Chrysis-larva one can see paired tubular appendages projecting upwards. A pair of similar projections is situated posteriorly from the last body segment. Alternating projection and contraction of these appendages make it possible for the minute chrysidid larva, having held itself initially at one or another point, to move forward. So, it moves restlessly until it finds the egg of the Trypoxylon, which it consumes completely, and then starts to feed on the spiders. During its whole period of growth it molts 3 times [So this species of cuckoo wasp is also clearly an inquiline].
There is still a large group of Chrysididae the representatives of which deposit their eggs already directly onto that fully-grown larval stage of the host at the expense of which also their larvae develop. This, apparently, in the chrysidids widely distributed method of development was thoroughly described by ADLERZ, 1906, for Chrysis viridula L., which penetrates into the nest of Hoplomerus ( Odynerus ) spinipes L. and deposits its egg on the prey which had already stopped feeding and was transformed already into a prepupa. In order to get to the prey, the cuckoo wasp brushes its way to the cell [i.e. clears the entrance] using its jaws and forelegs. The investigator observed how this work was continued for about an hour, after which the chrysidid went outside, turned around and went back again to the nest where it remained during oviposition. Then it filled up the entrance of the cell, and began to open up the second cell next to it. Upon inspection of the cells it turned out that in both cocoons an opening had been gnawed through which the cuckoo wasp had laid an egg and which it had then sealed off. Also Pleurocera viridis Guer. and Tetrachrysis carinata Guer. deposit their eggs through the firm mineral walls of the cells of Odynerus after the cocoons in them were already made.
All the cases presented above basically belong to Chrysididae of Europe and North America. Meanwhile there are indications that among Chrysididae of other regions there are [apart from species having the usual features] also [species that have] somewhat different features of their life and behavior. According to data of PIEL, 1933, and PARKER, 1936, Chrysis shanghaiensis Sm. develops at the expense of fully-grown caterpillars of the eastern moth Monema flavescens Wlk., already enclosed in hard cocoons. In order to gain access to the caterpillar the chrysidid gnaws a small opening in the cocoon, leads its ovipositor into it and paralyzes the caterpillar. This is possible because in this chrysidid, as turned out, there is a sting, and the venom glands are well developed [as an exception among chrysidids]. As a result of the stinging further transformation of the caterpillar [into a pupa] is halted. After having deposited its egg onto the caterpillar, the chrysidid collects dirt with its jaws from the surface of the cocoon, and having mixed this dirt with its saliva, closes the opening which it had made in the cocoon.
In comparing the behavior of the shanghaiian chrysidid with the above described behavior of Chrysis viridula, the difference is not great : In both cases the chrysidids deposit their eggs onto concealed preys that have prepared for pupation. Only in one case it was a caterpillar of a moth, and in the second case it was a wasp larva. Therefore, both chrysidids are essentially orthoparasites, that is, they stand in the Immediately-parasitic Phase of development, discussed in some earlier document.
So, the Chrysididae form a morphologically very isolated group of semi-wasps, biologically being metaparasites, orthoparasites, and inquilines [i.e. some of them belonging to the Delayed-parasitic (Part XXXIX), others to the Immediately-parasitic (Part XLIV), and again others to the Archaic Inquilinoid (Part XXXIV) Phase of evolution]. It is remarkable that typical inquilinism is found in them only in those cases where in the cells of their hosts spiders are prepared (provisions), and no other preys, and, moreover, no vegetarian food as in the Sapygidae.
It is therefore not surprising that also the with the Chrysididae closely related relict group Cleptidae, which counts about 40 species, stands in the Orthoparasitic Phase of evolution. Thus, one, not more precisely identified species of Cleptes, observed in the Korean peninsula, is a solitary external parasite of prepupae of various saw-flies enclosed in their cocoons. The methods applied by it in oviposition into the cocoon and in the closure of the [earlier made] hole in the cocoon, are very similar to those of the just described shanghaiian chrysidid. In addition, there is an indication that one cleptid in South America was obtained from eggs of stick insects, Phasmidae. One may think that in this case a kind of "error of instinct" had taken place, causing the cleptid to deposit its egg not in the cocoon, as usual, but in some other thing, not so much alike a usual insect egg though, but alike a cocoon or gall. But here the question comes up : Is oviposition by the cleptid into the eggs of the phasmid a primary phenomenon, and into cocoons of saw-flies a secondary one? In the light of what has been expounded this might well be possible.
In the Orthoparasitic Phase of development also stand the antwasps, Mutillidae, armed with a strong sting. See next two Figures for Mutilla europaea, another for Smicromyrme rufipes, and one depicting a mutillid from Trinidad :
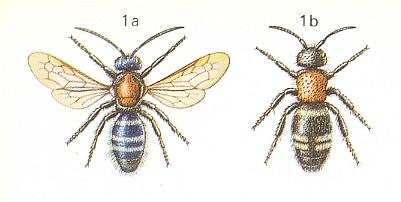
Figure 11 : Mutilla europaea L. (Mutillidae). 1a - male. 1b - female. 14 mm.
(After CHINERY, in Elseviers Insektengids voor West-Europa, 1983)
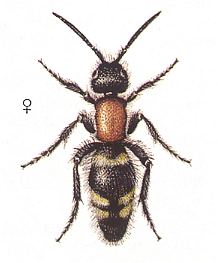
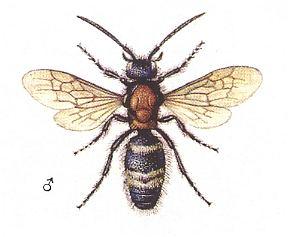
Figure 12 : Mutilla europaea. Mutillidae. Left - female. Right - male. 10-15 mm.
(After SEVERA, in ZAHRADNIK, Thieme's Insektengids voor West- en Midden-Europa, 1977)

Figure 13 : Smicromyrme rufipes. Mutillidae. Female. 4-6 mm.
(After SEVERA, in ZAHRADNIK, Thieme's Insektengids voor West- en Midden-Europa, 1977)

Figure 13a : Female of an antwasp [in German : Spinnenameise], Mutillidae, from Trinidad with characteristic colored coat of hair.
(After, or under responsibility of, KÖNIGSMANN, in NEUE GROSSE TIER-ENZYKLOPÄDIE, Volume 4, Insekten.)
The Mutillids specialized chiefly in parasitizing in cells of bees (Bombus, Nomia, Halictus, Tetralonia) and of wasps (Chalybion), and sometimes also in nests of ants and even in cocoons of the tsetse-fly (Glossina). In all these cases they develop at the expense of fully-grown larvae, prepupae, and pupae of the hosts. Their strong sting, able to bring about very painful stings also in humans, they usually direct not so much against their totally helpless preys, but rather against adult bees and wasps, in the nests of which they penetrate, in order to feed on their saps or to penetrate to their cells. Stinging the prey, in this latter case, possibly only delays the transformations of the prey into its fully-grown stage. Anyway, sometimes, after removal of the mutillid egg from the body of the host the development of the prey proceeded normally.
According to the observations of JANVIER, 1933, Mutilla attenuata Spin. deposits its egg on the pupa of Halictus chloris Ill. where, externally, its larva develops. Another species, Mutilla lonata Spin. attacks the fully-grown larva of Tetralonia tristrigata Spin. It is interesting that Mutilla glossinae Turn. gnaws a hole in the wall of the cocoon of the tsetse-fly, into which it leads its ovipositor and lays an egg, after which it seals off the hole with a slimy mass.
The antwasps of the subgenus Photopsis Blkae, widely distributed to the west from the Mississippi, live in dry places amidst colonies of solitary bees. When one, at a midsummer night, inspects the mentioned settlements of the bees in the light of a lamp, one may see the wingless females of Photopsis walking around, like large ants, on the surface of the soil, while their winged males, provided with large lateral eyes and convex forhead eyes [ocelli], fly at the light. From lumps of soil taken from the nesting places of bees were obtained some tens of females of different species of Photopsis. In laboratory conditions it became clear that the female Photopsis bores a small hole in the wall of a cell or cocoon, for instance of the anthophorine Diadasia vallicola Timb. or of Antidium collectum Huard., and deposits through it usually 3 eggs, of which only one develops. The period of incubation of the small egg covered with a granular chorion lasts about three days. The head capsule of the first-stage larva not having begun feeding is broad, as is the body, of which the segments are devoid of projections -- lateral, dorsal, and abdominal. The whole development of the Photopsis-larva takes place outside the body of the prey, which it consumes completely near the end of its feeding (in the 3rd and 4th instar), and then twines a white cocoon.
Regarding the palaearctic Mutillidae, special ecological investigations were carried out (NONVEILER, 1960).
So, as to their habits, the Mutillidae, like the Sapygidae, Chrysidae, and Cleptidae, never have been typical wasps. They never built cells, and did not bring in them their preys, but, having reached the Orthoparasitic Phase, merely show a behavior corresponding to it -- they sealed off the opening which they themselves had made [as being their only 'building' activity].
As a closest preliminary stage leading up to a series of typical vespine behavioral patterns can be taken those cases when the morphologically vespiform hymenopterans began to search for freely moving preys, generally living in a hidden way : In the soil, in disintegrating wood, in various burrows and hiding places. These states of affairs are, with some gradations, also observed among semi-wasps of a series of other families, namely the Tiphiidae, the Thynnidae, the Pelecinidae, the Dryinidae, and the Bethylidae.
Tiphiidae.
See next Figures for some of the family's representatives :
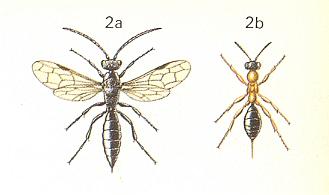
Figure 14 : Methocha ichneumonoides Latr. Tiphiidae.
2a - male. 2b - female. 12 mm.
(After CHINERY, in Elseviers Insektengids voor West-Europa, 1983)
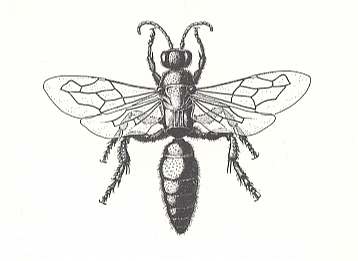
Figure 15 : A female of the genus Tiphia. Tiphiidae.
(After EVANS and EBERHARD, 1973)
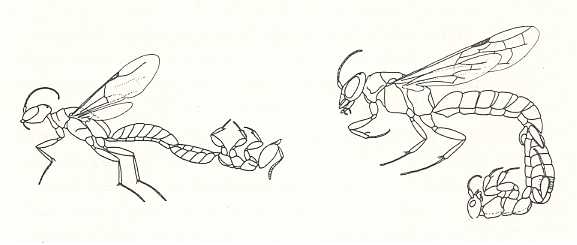
Figure 16 : Phoretic copulation in a bethylid wasp [that is, a wasp of the family Bethylidae] Apenesia nitida (left), and in a tiphiid wasp [that is, a wasp of the family Tiphiidae] Dimorphothynnus haemorrhoidalis (right). In each case the male is on the left, the wingless female on the right. Note that in the left image the female is inverted, while in the right the female is dorsum-up, like the male.
(After EVANS, 1969, in EVANS and EBERHARD, 1973)
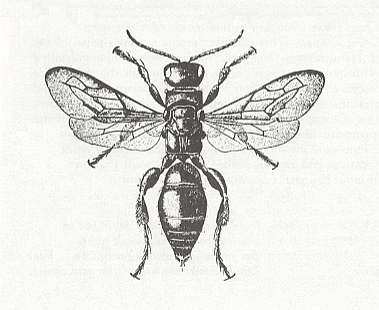
Figure 17 : Tiphia transversa. Female. Tiphiidae.
(After DAVIS, in RICHARDS and DAVIES, Imms's General Textbook of Entomology, 1977)
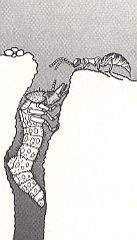
Figure 18 : A female Methocha (Tiphiidae) approaching the burrow of its prey, the larva of a tiger beetle. Methocha is a tiphiid wasp, although the females are wingless and superficially antlike.
(After WILLIAMS, 1919, in EVANS and EBERHARD, 1973)
One of the representatives of this family (Tiphiidae) -- Tiphia femorata F., usually digs in sandy soil in search of the larvae -- living not deep below the surface -- of the summer chafer Rhizotrogus solsticialis L., the cereal beetle Anisoplia austriaca Hbst. and others. According to ADLERZ the chafer-larva, found by the wasp at its feeding place, is paralyzed by a sting in the thorax. The duration of the state of paralyzation is short and lasts precisely as long a time as is needed in order for the Tiphia to undisturbedly deposit its egg on the ventral side of the prey. The egg is adhered along its complete length to the transverse fold between two successive segments (from 2nd up to 5th) with the head-end toward the lateral line of the prey. After this, more or less complete mobility of the chafer-larva is restored, in every case such that the larva continues feeding on the roots of the grasses all the time, while the parasitic larva grows. The egg, and later the larva, of the Tiphia is not only firmly attached to its prey, but is also relatively well protected against being sqeezed [the latter] as a result of the habit of the chafer-larva to hold its body a bit bent. However, this protection of having the tiphiid egg carried in the bend of the abdomen is not absolute though. Thus sometimes, on the body of a chafer, sqeezed and darkened tiphiid eggs are found in the adjoining folds of the segments. Consequently, we may say that the method of the Tiphia, consisting of only temporarily paralyzing the prey, does not exclude danger to its offspring. With this, totally harmonizes the fact that the larvae, paralyzed by these insects, in very different degrees regain mobility. The method of Tiphia, is, as ADLERZ assumes, in a lesser degree worked out than that of the Scoliidae (which will be dealt with below), and it is not unlikely that this method represents one stage of development once common to all Scoliidae.
Detailed investigation of the behavior of the female of that same Tiphia femorata F., held, together with its prey (the larva of the beetle Amphimalon), in a glass container with a layer of soil, showed a series of complex acts. After a number of attacks, vigorously countered by the prey, the wasp grabs it at the dorsum of the thorax, bends its abdomen and deeply drives the sting between the middle legs of the prey, holding its sting for about one minute, until the prey stops moving. Then the wasp inspects the intersegmental folds of the ventral side of prey. If a foreign egg is present there, the Tiphia consumes it together with its shell. The hatched larva is not touched by the Tiphia. Also it does not oviposit again on a prey that has been already infected by it. Then a new series of actions follows : The Tiphia, in a controlled fashion, bites off the legs of the prey, that is, successively the femora, the tibiae, the tarsi. And at this time it slips with its abdomen to the dorsal side of the prey and applies 20-30 light stings which still more weaken the prey. Beside the in this way paralyzed prey, the wasp digs out a small depression in which it places the prey in an arched position, with the back downward, and then adheres its egg in one of the folds on the prey's abdomen (usually between the 4th and 5th segments, if there are no signs of a foreign egg). After this, gradually, in the course of an hour, the mobility of the prey is restored. Let us add that the larva of Tiphia femorata consumes its prey in no less than three weeks. This comparatively long period of feeding can be explained by the fact that the Tiphia-larva up to the last days only sucks blood from its prey, and only against the end of its feeding period devours its internal organs. Generally, as to its way of feeding (five times changing its point of adherence), the Tiphia-larva in many respects comes close to terebrants (Paniscus !), and not to higher forms.
The habits of other species of Tiphiidae generally are similar to those described, but among them nevertheless were observed also essential deviations. And also some new features have been found. Thus, the North American Tiphia papilliavora Roh. stings a chafer-larva repeatedly in one and the same place, namely between the first two thoracic segments at the ventral side, until the prey becomes quiet. After this, the wasp performs a special operation -- kneading with its jaws over all the ventral side of the larva, beginning from the 1st [i.e. the anterior] segment to the last one. When this is done, the Tiphia grabs the larva with its jaws from the side and bends itself transversely over the prey's dorsum [its back] forming an almost complete ring. The end of the abdomen of the wasp is held against the furrow between the 5th and 6th segments of the prey and is rhythmically moved backwards and forwards for some minutes, as if preparing that place where its own larva will begin feeding. At this time any accidental egg or larva from a foreign earlier oviposition the wasp will begin to clean off or damage. Then, in this position, the Tiphia lowers its egg and firmly adheres it with slimy secretion. After 20-40 minutes the paralyzed condition of the chafer-larva ceases. In natural conditions, in all known cases, the Tiphia's leave the chafer-larvae after having paralyzed them in the same cavity where they lived and fed on roots, and do not show any attempts to bury them deeper. It is interesting that the female of the mentioned T. papilliavora sometimes, after oviposition, chews one of the legs of the chafer-larva and may even completely amputate it. The sap that oozes out is licked up by the wasp. Generally, the adult Tiphiidae feed on nectar of flowers (umbellifers, buckwheat, and the like) or lick up honeydew excreted on leaves by aphids, and other sucking insects.
Thynnidae
Fairly close to the Tiphiidae, as to their primitive vespine habits and organization, stand the exotic wasps of the family Thynnidae, chiefly (with about 40 genera and 400 species) occurring in Australia and partly in South America. The small, as compared to males, wholly wingless females of them (the males are winged) search for larvae of chafer-like (lamellicornious) beetles (Scarabaeidae) living in disintegrating wood or in the soil. The prey is paralyzed forever [i.e. paralyzation will not eventually fade away] (with rare exceptions) and is left there where it lived. No displacement from the place where paralyzation took place occurs. In some species the egg is deposited longitudinally along the lateral side of the thorax, and in other species along the mid-line of the abdomen, head-end oriented forwardly. The young larva gnaws a large split in the skin of the host and sinks down into it its head. But it still possesses significant mobility and is able to change its point of feeding.
Regarding the feeding of adult Thynnidae, their males feed on flowers or on sap of trees, oozing out at the place of damage inflicted by boring insects. Their females, which are apterous [wingless] may independently feed only on these saps. Usually, however, they are grabbed by the males and carried, during the long lasting nuptial flight, often repeated, to flowers where they also feed. The female of the Chilean Elaphroptera atra Guer., chews, serving the purpose of feeding, the posterior end of the host-larva, having it not paralyzed.
Pelecinidae
It is interesting that the Pelecinidae, which assume as it were an intermediate place between the terebrants and the aculeates, also, insofar as it is known, develop at the expense of larvae of lamellicornious beetles (chafer-like beetles), which live in the soil. Meanwhile, the females of the pelecinids (next two Figures), reaching a length of up to 6 cm, cannot dig into the soil as can the tiphiids, and in attacking their prey use their unusually extended abdomen as an ovipositor, which lets us remember the cuckoo wasps (Chrysididae).
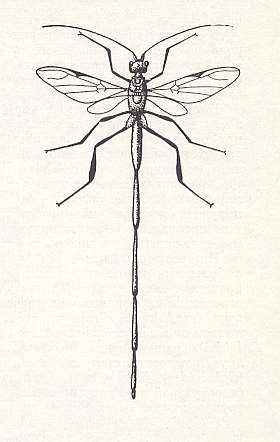
Figure 19 : Pelecinus polyturator Drur. Pelecinidae.
(From CLAUSEN, 1940, in MALYSHEV, 1966)

Figure 20 :
Female of Pelecinus apicalis from Peru, with the characteristic elongated abdomen.
(After, or under responsibility of, KÖNIGSMANN, in NEUE GROSSE TIER-ENZYKLOPÄDIE, Volume 4, Insekten.)
We already saw that various t e r e b r a n t s in the metaparasitic, as well as in the orthoparasitic phase of their evolution, developed as external parasites on larvae living in wood of trees -- chiefly on larvae of Buprestidae (buprestid beetles) and Cerambycidae (long-horned beetles). From here a connection is easily defined with the parasitism of such primitive w a s p s like Thynnidae, Tiphiidae, and Pelecinidae, on larvae of precisely lamellicornious beetles (chafer-like beetles). The majority of the latter lives in the soil, feeding in some cases on living, in other cases on dead, parts of plants. Only some of them develop under bark or at the expense of wood of still standing and even living trees. Ecologically there could not be any serious impediment to such a transition -- the anatomical features of the larvae of the lamellicorns, with their unusual concentration of abdominal ganglia [nerve centers], being more favorable than ever to this.
After all, the orthoparasitic terebrants, as we know, parasitize not only on larvae of various beetles, but also on caterpillars of moths and butterflies, larvae of saw-flies, and others. It is remarkable that, while developing at the expense of larvae of higher insects (Holometabola), these terebrants, and equally also the semi-wasps standing in the same phase of evolution, do not attack insects with incomplete metamorphosis (Hemimetabola). This indicates, of course, that chasing hemiptera (bugs and the like), orthoptera (grasshoppers and the like), and other insects with incomplete metamorphosis, by wasps, has a different history, which we will now expound :
Bethyloidea
The evolutionary phase, now under discussion, having not brought with it great changes of maternal instincts, was preserved by the representatives of bethyloids (Bethyloidea). Until the beginning of the 20th century the bethyloids were placed [as a family I presume] into the serphoid terebrants (Serphoidea Kieffer, Proctotrypoidea Ashm.). Later they were placed in the wasps, and now [1966] they are placed into an independent superfamily of wasps (Bethyloidea), in taxonomic position equivalent to the two other superfamilies of wasps -- the vespoids (Vespoidea) and the sphecoids (Sphecoidea) (BERLAND and BERNARD, 1938). The representatives of this peculiar, and by a number of features undoubtedly very primitive group (the Bethyloidea), have, so to say, remained at the lower stage of the development of aculeates (Aculeata). With about 1200 species and more than 200 genera, the bethyloids are distributed all over the world, but are especially abundant in hot countries. Generally having minute or small sizes, they are, in their general [external] appearance and [morphological] structure, so diverse, that the systematists divide them up into 5 or 6 families, and point to a number of structural features that allow to place the bethyloids close to various related groups -- Tiphiidae, Thynnidae, Mutillidae, Chrisididae, Ampulex (from the sphecoid wasps), and others (WHEELER, 1928).
Regarding the affinity of the bethyloids with terebrants, the opinions of investigators somewhat diverge : some investigators again place them close to the "terebrants having a borer at their posterior end" (Serphoidea), others hold these characters of similarity to be purely superficial. However, in the light of the often being repeated view about the existing affinity -- on morphological grounds -- of the bethyloid wasps with the serphoid terebrants, we may say that also on biological grounds these two groups had, apparently, a common ancestor that stood in that very oophagous [egg-eating] phase from which the wasps already went their separate ways : The Bethyloidea -- along the line of external parasitism, whereas the Serphoidea -- along the line of internal parasitism. Of the latter only the Calliceratidae, placed by BERLAND in the lower Serphoidea, are external parasites, although not on free-living hosts, but on larvae of other terebrants (various braconids and chalcids) parasitizing on aphids and coccids [if my rendition of the latter word is correct]. Moreover, one representative of this same family Calliceratidae, Calliceras abnormis Perk., attacks larvae of bethyloid wasps of the family Dryinidae in their cocoons. This latter fact -- parasitism on such peculiar hosts -- is very remarkable. We should think that it directly points to phylogenetic affinity of serphoid terebrants with bethyloids. And let us remember that among the Serphoidea a complete family, the Scelionidae, consists of true oophags [egg-eaters] developing at the expense of eggs of various insects and spiders. Here, consequently, we stand at the root of that serphoid line of evolution which had given off branches in the direction of bethyloid wasps. Therefore, we indeed see this affinity between the two groups (Serphoidea, Bethyloidea), noted by the authors.
Regarding the biological features of the bethyloids themselves, they have several common features with the terebrants. Although the biology of many bethyloids is still not known, we can now already say that not only the inquilinoid, but also the oophagous phase, at least in their typical form, is not represented in them anymore. But there are, nevertheless, definite indications that they have passed through the oophagous line of development. These indications are given by the peculiar features of the life of certain representatives of the bethyloid wasps, first of all the Dryinidae. The preys of the dryinids are, as in many typical oophags, mentioned earlier, cicadas. Only in this case it is not their egg clutches, but the very adult females of the cicadas not yet having deposited eggs. Having attacked the cicada, and holding it by its jaws and tarsi (see next Figure), the dryinid paralyzes the prey with the sting for a short period, and then deposits an egg inside its abdomen, and sometimes also outside it.
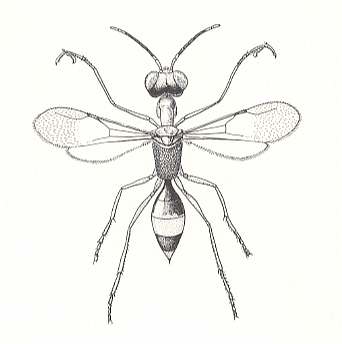
Figure 21 : A female dryinid wasp (Dryinidae). These are very small wasps which attack leafhoppers and planthoppers, grasping them with the pincers formed by their front claws and tarsi. (After PERKINS, 1912, in EVANS and EBERHARD, 1973)
The first-instar larva remains in the abdomen of the prey, but later it projects outside like representing a "fracture", remaining externally protected only by its skins that were shedded at its molts. The larva develops at the expense of the reproductive glands of the prey, so the ovaries of the latter become atrophied ("parasitic castration"). When the larva is fully grown it becomes mobile and leaves the prey. Somtimes in a single cicada two or more dryinid larvae develop, as a result, presumably, of repeated infection. Cases of oviposition on more or less early stages of cicadas or on their males evidently represent a further deviation. See next Figure.
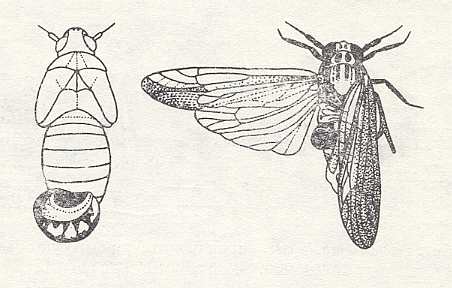
Figure 22 : Larvae of Dryinidae, projecting from the abdomen of cicadas.
Left - from a nymph [i.e. larva]. Right - from an adult.
(From CLAUSEN, 1940, in MALYSHEV, 1966)
Parallel with this, let us note that the larva of one Australian bethyloid, from another group of them (Mesitiinae of the family Bethylidae), also develops as an ectoparasite, as if enclosed into a pouch, but not in a cicada anymore but in a cricket. Also developng as an ectoparasite on the abdomen of the tree cricket is the larva of the enigmatic Rhopalosoma poeyi Hood., sometimes placed in a special family [of its own] of bethyloid wasps.
All this leads to those primitive terebrants that went over from the consumption of eggs in the egg clutches of the hosts to solitary ectoparasitism on host females ready for oviposition. From here it follows that all mentioned bethyloid wasps are essentially still standing on the Imaginally-parasitic Phase expounded earlier.
To the present group of phenomena we must reckon also the peculiar habits of the wasps of the genus Larra [by some authors placed in the family of digger wasps, the Sphecidae]. See next Figure.
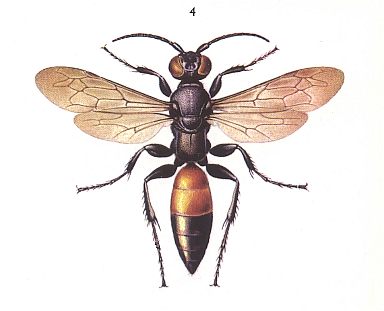
Figure 23 : Larra anathema. 10-25 mm. Sphecidae.
(After SEVERA, in ZAHRADNIK, Thieme's Insektengids voor West- en Midden-Europa, 1977)
The single European species of this genus, Larra anathema Rossi., chases, according to observations of the author [Malyshev, 1941], the common mole cricket Gryllotalpa gryllotalpa L. of the family Gryllotalpidae (Insect Order Orthoptera). The wasp chases the mole cricket out of its subterranean channels to the light of day and paralyzes it by three stings done in a definite order : Into the mesothorax [= second thoracic segment], into the prothorax [= first thoracic segment], and under the throat. See next two Figures.
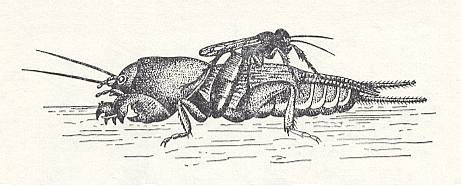
Figure 24 : Larra anathema Rossi. applies the first sting between the middle legs of the mole cricket. (After MALYSHEV, 1966)
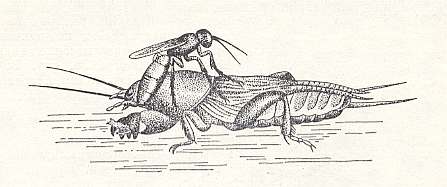
Figure 25 : Larra anathema Rossi. applies the third sting -- under the throat of the mole cricket. (After MALYSHEV, 1966)
The metathorax [= third and last thoracic segment] is not hit, and the posterior pair of legs of the mole cricket is thus still mobile. Immediately after paralyzation the Larra-wasp firmly attaches its small egg into the deep furrow under the base of the foreleg (usually the left) of the mole cricket, and, not making any attempt to conceal the prey, leaves it. See next Figure.
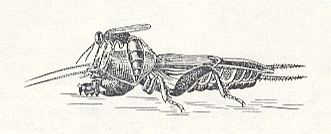
Figure 26 : Larra anathema Rossi. directs its abdomen under the foreleg of the mole cricket, in order to lay under it its egg. (After MALYSHEV, 1966)
The paralysis of the mole cricket is total but very short : After 5-6 minutes the cricket regains mobility, and it hides itself in its subterranean channels, carrying with it the egg of the wasp. See next Figure.

Figure 27 : In the deep furrow under the base of the left foreleg of the mole cricket an egg of Larra anathema Rossi. is present. (After MALYSHEV, 1966)
After this the mole cricket continues its normal free-moving life, and meanwhile the Larra-larva develops on it as an ectoparasite. See next Figure.

Figure 28 : Almost fully-grown larva of Larra anathema Rossi. on the mole cricket. (After MALYSHEV, 1966)
In connection with this, one should note that the North American Larra analis F. does things a little differently : The first sting it applies at the place connecting the abdomen with the thorax. At this, the hindlegs of the mole cricket are paralyzed, the middle legs remain mobile. From here it is clear that precisely the inherited character of behavior, and absolutely not the accidental pose at the moment of struggle or features of the integument of the prey, as is sometimes thought, determines the method of paralyzing of the wasps. In addition, after paralyzing the mole cricket, L. analis turns around with the head toward the abdomen of the prey, and with the end of its abdomen at the third main part of the prey's body precisely behind one of the hindlegs, where the wasp lays an egg and leaves the prey. In the same way, but with some characteristic deviations, also -- according to data of WILLIAMS, 1928 -- exotic species of Larra go their way. Thus, according to his observations, Larra sp. sometimes attempts to drag the (by it) paralyzed mole cricket from place to place.
Here we must again consider the phenomenon of "kneading" with the jaws precisely (of) that place where earlier an egg of this or of a closely related species of the wasp (Larra) might have been laid. This last operation is directed, evidently, to destroy the foreign egg or the hatched larva. Therefore, such an operation, noted above also with respect to the Tiphiidae, takes place essentially only in cases of paralyzation of the prey for a short period, after which the prey will regain complete mobility. Precisely only in these cases, on the body of the prey, again subjected to an attack, a foreign egg may be present. In this way one should interpret the Larra's processing (L. anathema) with the help of the jaws (of) the thin skin under the base of the forelegs of the (by it) paralized mole cricket. This operation is directed, consequently, not to any feeding by the wasp on the saps of the prey, but to the destruction of a foreign egg. Licking up of saps possibly oozing out is only a mere accompanying phenomenon. The small characteristic scar, that is formed here at the place of the wasp's operation, is the result of the unusually firm adherence of the egg of the Larra on the body of the prey along the whole of the egg's length.
It is interesting that L. analis manipulates also under the base of one of the forelegs of the mole cricket, although it adheres its egg at another place -- behind the hindleg. Apart from that it inspects also this place with the tip of its abdomen, as if looking for a foreign egg there.
From here it is clear that the presence of the ability in Tiphiidae, and in Larra and its relatives, of "kneading" with the jaws (of) the place of oviposition -- gives a direct indication of a certain primitiveness of these wasps, that is, an indication pointing to the fact that they also earlier in their history never concealed their prey in a cell.
Another way was taken in the development of other groups of bethyloids, namely along the line of either solitary, or semi-family, or family ectoparasitism on more or less hidden larvae of beetles or butterflies. With them we shall deal later (see below, "The Origin of the Ants"). As an example here we can only indicate that Pristocera armifera Say. -- a representative of the family Bethylidae -- develops as solitary ectoparasite on the larva of the beetle Limonius agonus Say. ( Elateridae) which larva lives freely under the soil. The larva of the mentioned wasp, may, after having sucked out one prey, independently move to another, as it is in the above mentioned larvae of the evanoid Gasteruption and the ichneumonid Habrocryptus.
The bethyloid Allepyris microneurus Kief., developing on larvae of hide-eater beetles (Dermestidae) of the genus Anthrenus, shows totally different habits. Having reached the place where several of its preys live nearby each other, it, before oviposition, paralyzes them in a stronger degree than necessary for [being able to execute the action of] oviposition. It is interesting that the primitive habits in which the connection is found with development at the expense of egg-clutches of the host, are seen also among typical wasps of the family of spider wasps, the Psammocharidae [= Pompilidae]. Thus, the females of Homonotus (Wesmaelinus) sanguinolentus F. and H. iwatai Yasum. attack a female spider in its nest where it is about to lay an egg-cocoon, paralyze it, and then lay an egg upon it. The most remarkable effect of stinging, even in the case of paralyzing ony for a short period, as in H. iwatai, is the complete cessation of oviposition by the spider, where the eggs are also eaten by the wasp larva.
Primitive instincts, not yet having been complicated by dragging the prey and building activities, are sometimes observed in other representatives of the spider wasps. Thus, the female of Pompilus lactuosus Mocs. attacts the enormous, ten times its own weight, spider Lycosa bi-impressa Luc. in its deep burrow, paralyzes it, and, after oviposition leaves it where it was.
The maternal instinct of the wasp also here, consequently, consists of two chief acts -- looking for and finding the prey, leading to its paralyzation, and oviposition. The wasp larva develops on this prey, as before, as a solitary ectoparasite.
Thus we see this phase of the evolution of the Aculeata, in which the, already by their morphological features, vespiforms possessed still rather primitive instincts, very close to the maternal instincts of their terebrant ancestors. A new, as compared to that of the foregoing forms, so to say purely vespine element in the behavior of some of them consists in the fact that they now, in this semi-vespine phase, came into complete and immediate contact with their preys, that live concealed in the soil, various burrows, and hiding places, which [contact] earlier was not possible when preys were looked for that lived concealed in the thickness of plant tissues, in cocoons, cells, and the like, accessible only to the ovipositor of terebrants. This is the case only in those semi-wasps, though, that were in the orthoparasitic phase of evolution, such as the Tiphiidae and the like. Regarding the other group of semi-wasps, remaining in the imaginally-parasitic phase, this difference from the terebrants did essentially not exist, because also their ancestors came in direct contact with their preys.
Both of these groups of primitive wasps originated along different paths, as well as continued to develop further : some of them [had their individual development] at the expense of larvae of higher insects (except larvae of flies), and others of insects with incomplete metamorphosis and of spiders. Transition from one line of evolution to the other did not take place here. Only in the higher stages of evolution, in the behavior of vespoid as well as sphecoid wasps, an unusual placticity was found in virtue of which some of them began to bring to their cell not only various insects, but in cases also pieces of meat of [dead] vertebrates. This whole complex path of transformation of instincts deserves our full attention.
Thus, the just discussed primitive wasps, having hit the prey with their sting, having brought about temporary or constant paralysis, deposited an egg upon it, and thereafter behaving as terebrants, not showing further any sign of care for their offspring. Their larvae, as before, developed as solitary ectoparasites on their preys.
Thus, the whole maternal activity of these primitive wasps essentially consisted of two basic acts :
A -- The hunt, consisting of looking for, finding, and paralyzing the prey, and
D -- Oviposition onto this prey.
Both these acts were repeated in correspondency with the number n of obtained individual preys, which compactly may be written as : n(A + D).
This still represents the semi-vespine, "bethyloid" phase.
The chief insufficiency in the behavior of the primitive wasps (especially the orthoparasitic group) is quickly found in the fact that once-only-hit-by-them preys, as before, remained accessible to attacks by other individuals of the same or of related species. As a response to this situation, among the given wasps habits were [evolutionarily] worked out to oviposit on various parts of the body of the prey, characteristic of given species of wasps, and to develop special methods of cleaning the integument of the prey from accidental eggs [foreign eggs that happen to be there already]. However, all these acts were insufficient and did not avoid useless ruin of eggs layed on the prey. Substantial progress was not yet achieved.
With all this we have concluded our exposition of the semi-vespine (bethyloid) phase of hymenopterous evolution. From here on, that is, along this particular direction of hymenopterous evolution, a number of different vespine phases will follow, eventually leading to the social wasps. In the next document we will discuss the first such vespine phase, viz the primary-vespine (pompiloid) phase.
e-mail :  ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XLVIII.
Back to Homepage
Back to Contents
Back to Evolutionary Part XIV
Back to Evolutionary Part XV
Back to Evolutionary Part XVI
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XIX
Back to Evolutionary Part XX
Back to Evolutionary Part XXI
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XL
Back to Evolutionary Part XLI
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLV
Back to Evolutionary Part XLVI


































 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )