

Above we have seen that the analysis of instinctive acts on the way to the [evolutionary] establisment of the vespoid wasps definitely leads to the point of origin among them of apoine instincts of the type as seen in the Masaridae. But here on the evolutionary path of the sphecoid wasps, as we now know, it does not give final results. This fact forces us to follow a different path of investigation : To further clarify what precisely the nesting instincts are in the most primitive true bees, primitive that is, as to their morphological and biological features. It is, after all, very important to know whether there are, in their nesting habits, such specific instincts which might clarify the most obscure moment of their evolutionary history -- the transition from the instincts of sphecoid wasps to the instincts of sphecoid bees.
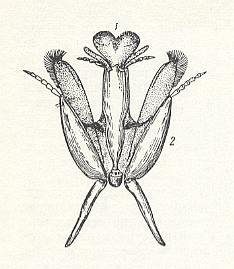
Figure 1 : Mouth-organs of the collete bee Colletes cunicularius L.
1 -- lower lip, ending in a split-up tonguelet.
2 -- lower jaw with palpus (upper jaws not drawn).
(After MALYSHEV, 1966)
The remaining bees possess a narrowing or more specialized tonguelet.

Figure 2 :
Left image -- Schematic section of the filmy cell of Colletes cunicularius L., still empty, with a threshold at the entrance.
Right image -- A cell of Colletes cunicularius L., provisioned with honey-food. The egg is adhered to the ceiling of the cell.
The threshold is elevated and transformed into a cap of the cell.
(Left image after MALYSHEV, 1927, in MALYSHEV, 1966. Right image after MALYSHEV, 1966)
Preparation of the filmy coating is noted also in Australian species of the genus Paracolletes. But no one of the investigators refers to the construction by the colletes of a "threshold" seen by the author in the cells of Colletes cunicularius.

Berland, 1951, assumes that the filmy coating of the cells of the colletes is a secondary adaptation, but does not say anything what then the original coating was in the ancestors of the Colletidae. We cannot, however, agree with such an assumption. First of all there is no such coating of the cells in the case of any other bees, apart from Colletidae and Hylaeidae, but only a layer of varnish in the cells, impregnating in this or that degree the surrounding vaults.
The indication of Berland about the similarity of the filmy coating of Colletes to that of Melitta (placed by the mentioned author into the group of higher bees) is based on the wholly incomplete observations of Perez, 1889, and Ferton, 1909. In reality, as special investigations have demonstrated (Malyshev, 1923), the coating of the cells of melitta (M. leporina Pz.) is of a totally different nature. It is absolutely not elastic, melts upon light heating, dissolves in chloroform, and, consequently, belongs to [a class of] coatings of the wax-type, being a product not of saliva glands, but of the accessorial sexual gland ("alkaline"), which is very well devoloped in Melitta.

Figure 5 : Opened nest of Colletes cunicularius L.
Through the filmy wall of the cells (especially the lower left one) shines the aggregated stock of honey-provisions in them. The upper cell (the one that was last constructed) is still empty, and to it leads the open side-channel.
(After MALYSHEV, 1927, in MALYSHEV, 1966)
Then the whole nest is closed, and next to it the bee may begin with the construction of yet a new supplementary nest turned away from the first. It is certainly of interest that the elaborate, similar to that described, displacement of soil particles in the building of the nest was noted to be the case also in bembexes. The hatched larva of the collete bends arch-like on its ventral side and moves to the edge of the provisions.
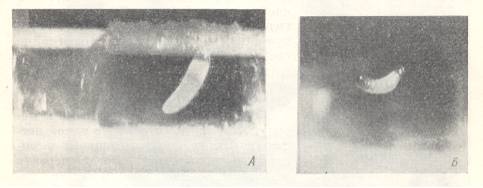
Figure 6 : Nest of Colletes inexpectatus Nosk., constructed in the cavity of a [reed] tube.
Left image -- Egg deposited with the head-end on the wall of the cell. Right image -- Hatching larva with its posterior end touching the provisions.
(After MALYSHEV, 1966)
The egg of it is lightly bent at the ventral side with the narrowed (aboral) end turned to the provisions. From this it follows that at the moment of oviposition the bee, holding itself with its tarsi on the ceiling of the cell, had its back turned down, and its head turned to the exit of the nest. Only in this posture, letting the egg down freely into the air, the bee could fix the egg on the ceiling of the cell with the head-end, having exited from the oviduct, last.
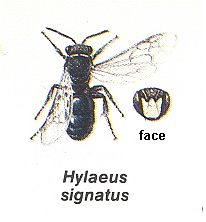
Let us now turn to the representatives of another family of primitive bees, the Hylaeidae. As to their outward appearance, almost or completely lacking a hairy coat, they look more like wasps than bees, and this justifies their popular name -- "masked bees". Study of a number of exotic genera of them significantly relaxed the structural differences between them and the Colletidae, and today the "masked bees" are, together with the Colletidae and other related forms, united sometimes in the one family Colletidae Michiner, in which the hylaeids figure as the subfamily Hylaeinae. According to Michiner it is supposed to be possible to derive from the ancestor, being similar to the above mentioned Australian Paracolletes, the other subfamilies of colletid bees through the loss by them of the brush of collecting hairs on the hindlegs and [through] some other modifications. However, it is ununderstandable how the Hylaeidae, allegedly having evolved from ancestors possessing a collecting brush, could have lost this useful organ, although the corresponding function in them has remained and is today fulfilled already without a special adaptive structure, namely through swallowing the pollen to be collected.
In the light of these typical vespine traits of their organization the hylaeids are not, understandibly, able to collect and transport flower-pollen on the surface of their body or on their legs, but only inside them -- in the crop or in the mouth and on the mouth-parts. Thus, the "masked bees" not only more or less preserved the bald appearance of their related ancestors, but also inherited from them the habits of collecting and transporting of honey-provisions inside their body with them, in order to give it off later from the mouth into the cell. In all remaining independently [i.e. solitarily] nesting bees the provisions are, on the contrary, collected, and in this or another degree, sometimes completely (as in Melitturga), transported to the cell outside the bee's body with the help of specially developed hairs or in special sites of the chitinous integument, in "baskets".
With respect to the selection of a place for the nest, the majority of the "masked bees", in contrast to a number of colletid bees independently building nests in the soil, nests in hollow stalks and in other ready spaces. But their being closely related to the Colletidae they show also here, coating the cavity to become the cell with a silken film given off from the mouth. See next Figure.

Figure 7 : Nest of the "masked bee" Hylaeus communis Nyl., constructed in a narrow [reed] tube opened from the side.
The cells are plastered with a semitransparent film, through which, in the upper cell, the dark provisions shine through. The lower cell is opened laterally. The egg of the hylaeid lies on the provisions.
(After MALYSHEV, 1966)
The stock of honey-provisions to be placed into this filmy cell, given off, as told, by the bee from its mouth, has a fairly liquid consistency and fills the whole posterior part of the cell's cavity. It is interesting that upon opening the masked bees' cells provided with provisions, at least as to the Hylaeus known to the author, a strange smell is detected. The latter is totally unlike flower-smell which comes out of, for instance, the provisions of colletes, and rather is like the smell of cheese or rotten curdled milk.
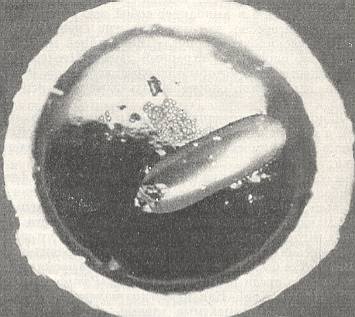
Figure 8 : Cell of Hylaeus communis Nyl., constructed in a straw, anteriorly opened. The egg lies on the provisions.
(After MALYSHEV, 1966)
and the cells usually lie one after the other in a linear order dependent on the shape and size of the cavities occupied by them.
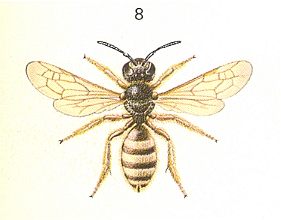
These are the basic facts in the biology of the primitive sphecoid bees. As the most indicative feature of their behavior we must reckon the plastering of the cavity of the cells with a silken film -- a secretion of their mandibular glands, given off from the mouth onto the walls of the cells before they are provisioned, and later during the closure of the cells. It is therefore very remarkable that the appearance of a similar instinct was shown recently also in sphecoid w a s p s, namely among pemphredonid wasps ( Pemphredonidae) of the genus Psenulus Kohl. As is known, the small black pemphredonid- or so-called aphid- wasps are inhabitants of chiefly the Northern Hemisphere. Some of them (Diodontus Curt.) nest, as an exception, in the earth, where they dig shallow branched burrows. The majority, on the other hand, gnaws nest-channels in the pith of dry stalks and branches (Cemonus Jur.), or in weathered wood (Pemphredon Latr. in sp.), and others settle in ready cavities of a different order (Psenulus Kohl.).

In such a great diversity of selection of places for nesting, the selection of prey by the pemphredonid wasps is very similar and with it very peculiar : They hunt mainly for the so-called phytophtires, that is, for aphids (Aphidiidae), and as to their structure and habits closely related to them, for leaf-flees (Psyllidae). Only, as representing only a single known exception to this, they prey upon booklice (Psocidae). The ecological relatedness of all their preys was also engraved in the behavior of the wasps themselves, when, for example, one and the same wasp -- the minute Nitela spinole Latr. -- provisions its cells then with aphids, then with booklice, although a mixture of them in one single nest is not observed. In a similar way three known species of Psenulus hunt for aphids, and one (Psenulus concolor Dahlb.) hunts for leaf-flees. The hunt after such a prey, for which not any other wasp hunts, left a deep trace in the behavior of the pemphredonid wasps. First of all in connection with the small size of the prey, they had to bring into the cell a large number of preys -- usually several tens, as was already partly noted earlier for the Third Vespine Phase. See next Figure.

Figure 9 : Nest of Psenulus fuscipennis Dahlb. Pz. Cells filled with aphids. 1 -- Larva of wasp just hatched. 2 -- Separating wall.
(After MALYSHEV, 1966)
A very important fact is that the pemphredonid wasps hunt not for adult winged preys, but for young individuals -- larvae and nymphs, living in dense colonies on the surface of plants, sucking the saps of them, while not moving. In obtaining such a kind of prey, moreover coated with an extremely delicate integument, totally unsuited to resist, is not in fact an act of catching, but simply collecting provisions, prepared by the wasps in the form of food for their larvae.
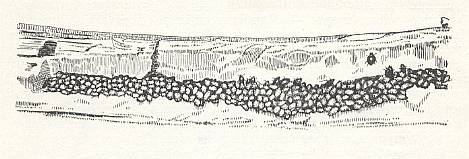
Different from all this, the above mentioned wasps of the genus Psenulus for isolation of the cells with the prepared preys in them use secretions from their mandibular glands. These secretions harden in the air as silken films, similar to what is observed in Hylaeidae and Colletidae. Statements in the literature about the use by the psenuli of filmy material until now were very few. Thus, Hartig, 1932, tells us that in a nest of Psenulus "rubicola" (atratus F.), found by him, the cells were separated by "membranous" separating walls. Then Spooner, 1948, describes and draws a separating wall between the cells in another species, Psenulus concolor Dahlb., as a "smooth secreted membrane", covered on the front side with chunks of pith. Much more definite are the data given thereof by Janvier, 1962. Both species being observed by him -- P. fuscipennis Dahlb. and P. pallipes Pz. (atratus F.) -- have, in contrast to all other wasps, and similar to primitive bees, silk glands, the secretion of which with the help of their tonguelet they apply, in the form of an elastic separating wall, at the mouth of the cells provisioned with aphids. This separating wall, as the investigator tells us, consists of two layers, interiorly divided by scraped-off vegetable particles, whereby the lower (posterior) wall of the partition, being the ceiling of the already provisioned cell, is convex, whereas its upper (anterior) side, forming the floor of the next cell, is concave.

Figure 11 : Opened-up nest of Psenulus fuscipennis Dahlb. The filmy plastering of the middle cell was preserved upon opening almost completely. (After MALYSHEV, 1966)
The caps of the provisioned cells being at the same time transverse partition walls separating adjacent cells of the nest, are, in the psenuli observed by the author, simple (see Figure 9, above ), and thus not double as Janvier, 1962, describes them.
About the second internal half of the partition wall in Psenulus with the concavity turned to the deeper lying cell, also is something said by Spooner, 1948, but he correctly holds it to be a rudiment of a cocoon prepared by the fully-grown larvae of the psenulus. Corresponding with this, the disc-like partition wall of P. atratus Pz. with backwardly bent edges is constructed from "parchment-like" material (and thus not from silk film!). Something similar is noted by Grandi, 1931, and other authors.
The caps closely lie against the aphids packed in the cell, and their edges, adjoining the walls of the cavity, are bent forwardly and form a more or less complete plastering of the new cell.
Thus, the psenulus uses its filmy material in building the cell before beginning to collect in it prey. Oviposition in the still empty cell in psenulus, as generally in pemphredonid wasps, does not take place, as well as in them [does not take place] a step by step nutrition of the larvae. All this fundamentally distinguishes the pemphredonid wasps from primitive masarids, and brings them close to the true, solitary, bees, except the "subsocial" Allodape and its relatives. From here it is clear that the use of an isolation film in the construction of cells originated before the origin of bees, and precisely among pemphredonid wasps, and only among them.
What, then, was the cause, having evoked in the pemphredonid waps the ability to coat the walls of the cells with a silky secretion given off by them? It is very probable that the decisive moment here was the very close contact established between the wasps themselves and their prey. At the base of this contact was the similarity in feeding of the adult individuals and their larvae. The difference here only consists in the fact that the larvae of the wasps feed on the phytophtires [aphids and relatives] prepared for them, chiefly aphids, whereas the wasps themselves, apparently being vegetarians, mainly feed on "honey-dew", that is, on the sweet liquid excreted by the aphids and their relatives in such a quantity that it may drop or even run from the plants inhabited by them. With respect to the pemphredonid wasps this contact was eased by the fact that the wasp here, apprehending its delicate prey with its jaws, evokes with it the appearance of nutritive saps for it. Thus, also here, as in the ancestors of the masarids, we come to the moment when the mother-wasp began to feed her larvae with precisely that food that she hereself fed on. But when in masarids the mother-wasp could hand over her food to the larva from mouth to mouth, here, as we know, this could not take place. Only an interim preparation of honey-provisions could take place with the next oviposition in the cell.
As already told above, the pemphredonid wasps, in their settling in ready cavities, may, possibly as a result of insufficient building material on the walls of the occupied cavity, or as a result of other reasons, totally not make partition walls between the cells, or for this purpose obtain building material from elsewhere. In different conditions, on the other hand, they use for this same need a secretion from their salivary glands. With abundance of this secretion some of them, such as psenuli, distribute their secretion onto the lateral walls of the cell and construct in this way a filmy plastering of their cells, as do the Hylaeidae and Colletidae. Precisely in this way, we must assume, the given group of pemphredonid wasps, having settled in existing cavities, also acquired the possibility to place in the cells instead of their original formed prey, that liquid food which the wasps themselves fed on (that is, in addition to nectar and pollen from flowers, also the sweet excretions of aphids), subsequently replacing completely the bringing-in of preys into the cell.
How natural such a diet is for wasps and bees is clear from the fact that the honey-bee may collect honey-dew excreted by aphids with such diligence as they collect nectar from flowers, eat it and feed it to their larvae. Also many ants eagery visit colonies of aphids on plants and, not attacking the aphids themselves, feed on their excretions. The latter, as is known, do not only contain carbohydrates, but also proteins. For this reason, honey-bees, during the winter months not having the possibility to fly out of the hives and clean their guts, suffer from this for them little suitabe food in winter conditions.
The change of consistency of the provisions -- replacement of the juicy prey, coated with a delicate integument, by honey liquid -- demanded corresponding adaptations from the side of the mother-wasp. To judge from primitive bees, in some cases, as in Hylaeidae, thanks to the shortened and thickened shape of their eggs, it turned out to be possible to deposit the eggs directly onto the surface of the honey-provisions (Figure 8, above ). In others, on the other hand, as in colletes, special methods originated, methods of oviposition on the wall of the cell, as was described above (see Figure 2, right image ).
Thus, from a biological viewpoint the direct path becomes clear, leading from pemphredonid wasps to the primitive bees. This path, however, takes its beginning not directly from psenulus-like wasps of the tribe Psenini, but from their ancestors, having had not a petiolate but hanging abdomen, similar to such wasps of the tribe Pemphredonini [see Figure 8a, above]. Such difference as seen from the morphological viewpoint can hardly be so great if we take into account not only the small size of the abdominal petiole of psenulus, but mainly the simplicity of its structure : It lacks the upper half (tergite) of the corresponding segment, and consists only of its lower part (sternite).
From the biological viewpoint only the consecutive order in provisioning the cells distinguishes the true, sphecoid bees from the higher masarids re-placing their egg onto the provisions, this egg being laid interim on the wall of the cell. Accordingly we see that, as a result of the long parallel development, these and other superfamilies of the aculeates reached a remarkable similarity in their nesting behavior. The existing difference in their behavior is not so great as to not reckon these and others as being bees. It merely consists in the fact that the masarids preserved a characteristic trait of their vespine ancestors -- oviposition before preparing provisions, whereas the "true bees", apart from some exceptions of a higher order, preserved a basic trait of their wasp-like ancestors -- oviposition onto the interim prepared provisions. Thus it becomes clear that the masarids are also bees, but just vespoid bees -- Apoidea vespiformia Mal., in contrast to the other group, having developed parallel with them, of true, or sphecoid bees -- Apoidea spheciformia Mal.
To this we must add that the vespoid wasps, having abandoned the original feeding of the larvae with a whole prey, and passing over to the bringing-in of it in a chewed form, subsequently reached the highest development of vespine instincts -- the social way of life of wasps. On the other hand, the sphecoid wasps, not having, as a result of historically coming together of circumstances, at their disposal the corresponding methods of oviposition and the bringing-in of prey, could not pass over to a step by step feeding of the larvae with chewed prey, and in the result of their evolutionary development did not give forms living socially. But, it is remarkable that after the sphecoid bees had originated, they [i.e. the sphecoid wasps], in this way, through the bees, nevertheless succeeded, from their side, in [developing] a "social" way of life [namely the social bees].
And thus the beginning was set [leading] to the instincts of bees in the broad sense -- Apoidea s. lat. Mal., having played a grandiose role in the appearance and development of the flower plants of the Earth.
Clarification of the further evolution of the sphecoid bees (Apoidea spheciformia Mal.) is beyond the limits of the present investigation [of Malyshev's], although relevant material is already accumulating in science.
With all this we [Jaap Bax] have come to the end of Malyshev's book on the evulution of instincts in the insect Order Hymenoptera. Indeed, in this book, organic species are seen as embodying strategies to exist and persist. And especially in Hymenoptera the instincts to raise the young (and with it to perpetuate the species) are the main ingredients of such strategies. And while following Malyshev's expositions we have further developed our noëtic theory of evolution (see parts LXc and LXd), in which organic strategies play a major role.
But our exposition of Hymenoptera and their evolution is not yet finished. In the documents to come we will deal with fossil Hymenoptera mainly from the Mesozoic, described (in Russian) by A.P.Rasnitsyn. We will learn to see in what temporal order the major groups of Hymenoptera appeared in the geological history of our, no, of the animals' world.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part LXVI.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII
Back to Evolutionary Part LVII
Back to Evolutionary Part LVIII
Back to Evolutionary Part LXII
Back to Evolutionary Part LXIII
Back to Evolutionary Part LXIV