

[As done earlier, in these enquiries into the wing-venation and its transformations in Diptera-Nematocera we will consider the subcostal vein (Sc) at most only occasionally. This vein, especially its terminal branches (Sc1, Sc2), is -- in Diptera -- often difficult to discern.]
Introduction
In Part XXVIII and XXVIII-A we have established the phylogenetic relationships -- cladogenesis -- between all the subgroups of the Bibionomorpha (= Rhyphiformia + Bibioniformia + Fungivoriformia). Of course the result is far from certain. Many phylogenetic relationships so established might turn out to be wrong. Indeed, in the previous document we have shown the inherent problems of phylogenetic systematics including its basic philosophy concerning the general nature of organic evolution. However, for developing a general theory of evolution including a theory of the metaphysics underlying organic evolution these uncertainties are not so important, that is, having to change certain details of the established cladistics will not affect the content of the general (metaphysical) theory. So when elucidating the general coexistence of 'clades' and 'types', that is, of monopyletic groups and groundplans, we can use -- as an example -- the phylogeny-as-it-could-be, or (equivalently) the cladistics-as-it-could-be, of the Bibionomorpha. As has been argued, the cladistics of the Bibionomorpha (as does that of any monophyletic group of organisms), that is the pattern of bifurcating genealogical lines within the group, resides, not in the Explicate Order, but in the Implicate Order. The cladistics of the group (here of the Bibionomorpha) is in fact the bifurcating noëtic trajectory which visits the stability fields of organic groundplans and makes them --together with the appropriate existential conditions -- project into the Explicate Order. Such a projection, from the trajectory, of groundplans (ultimatly genus-groundplans) will only take place if the accompanying existential conditions fully comply with the actual ecological situation extant in the Explicate Order, that is, the actual existence of the ecological niche to which the mentioned existential conditions are geared. Upon the first projection, resulting in the appearance of certain organisms carrying the projected set of superimposed (genus- family- etc.) groundplans [this appearance seen in the Explicate Order in the form of adaptation and/or migration to a certain ecological niche], the existence of these organisms lasts as long as the alternating projections and injections of their noëtic contents continue. If they stop, the organisms will disappear, which we 'experience' as their (evolutionary) extinction. So it is the ecological conditions prevailing in the Explicate Order that determine whether or not groundplans, (the stability fields of which are) having been visited by a noëtic trajectory, will be projected into the Explicate Order and continue to do so. In this way evolution is governed by ecology. All purely formal aspects of evolution ultimately lie in the Implicate Order.
Noëtic interpretation of the cladistic pattern of the Bibionomorpha
Because the cladistic system of the Bibionomorpha is quite extensive, we divide our exposition in several parts.
Rhyphiformia
To begin with, the cladistics we had found ( Part XXVIII-A ) in the Rhyphiformia is here reproduced :
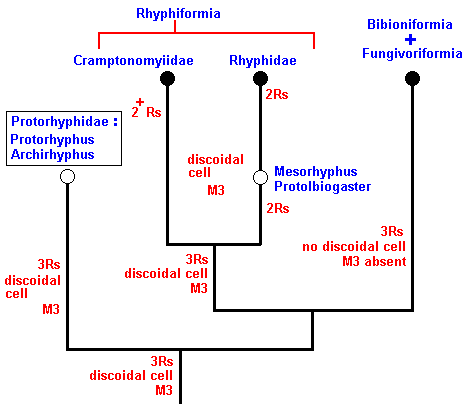
This cladistic pattern, established by phylogenetic systematics, can now be 'translated' into the noëtic picture, that is, the genealogic lines and bifurcations depict in fact the course of the noëtic trajectory that, in the Implicate Order, visits the noëtic stability fields of organic groundplans :
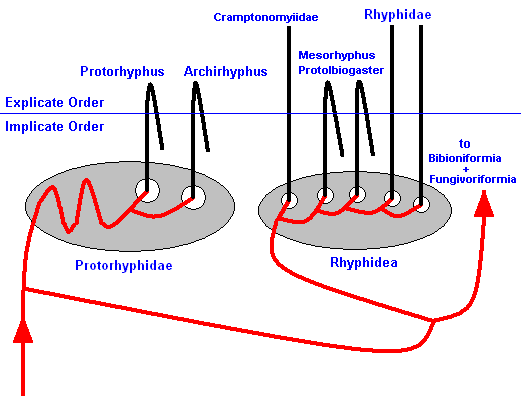
Figure 2 : Noëtics of the Rhyphiformia and the Protorhyphidae. The noëtic trajectory (red) successively visits the stability fields of the organic groundplans of the Rhyphiformia (and Protorhyphidae). After that it continues its course to those of the groundplans of the Bibioniformia and Fungivoriformia.
Projection into the Explicate Order only takes place after the noëtic trajectory having visited the genus-groundplans. From each genus one or more projection lines (black verical lines) ascend into the Explicate Order (details not drawn) resulting in the evolutionary appearance of species in their proper ecological niches. Projection in fact consists of an alternation of projection and injection. As long as this alternating projection and injection continues the species persists in the Explicate Order. When it stops, the species will disappear from the Explicate Order, it becomes extinct. When all species of a given taxon become extinct, the taxon itself becomes extinct. In the diagram extinction is depicted by a 'returning' projection line.
As regards the Rhyphiformia and the Protorhyphidae there is no discrepancy between monophyletic groups and types (groundplans).
Bibioniformia
Also in Part XXVIII-A we found the phylogenetic cladistics of the Bibioniformia :
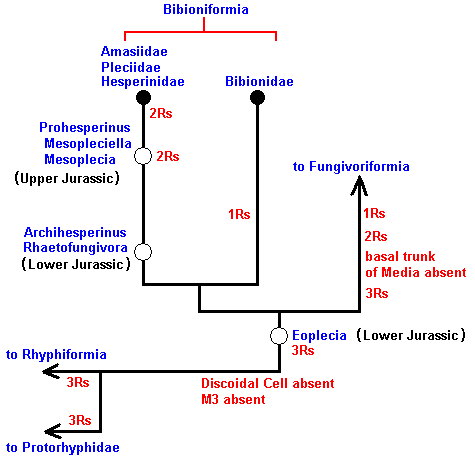
Figure 3 : Phylogenetic tree of the Bibioniformia, and the places in this tree where the fossils should belong.
Also this cladistic pattern, established by phylogenetic systematics, can now be 'translated' into the noëtic picture :
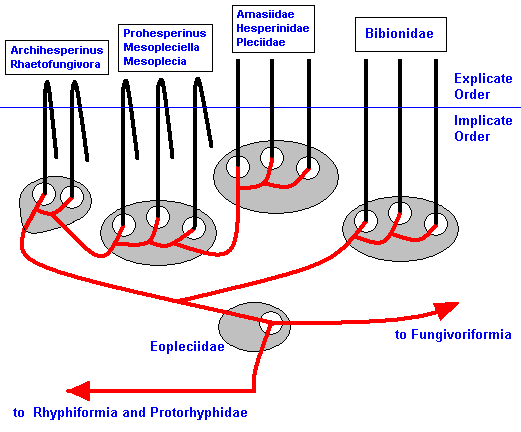
Figure 4 : Noëtics of the Bibioniformia. Also here there is no serious discrepancy between monophyletic group and type. The cladistic relationships drawn between the fossils Archihesperinus, Rhaetofungivora, Prohesperinus, Mesopleciella, and Mesoplecia and also between the recent Amasiidae, Pleciidae, and Hesperinidae, are purely hypothetical. Like the previous and following diagrams, the present diagram only serves to expound our general theoretic construct.
Fungivoriformia (including Parafungivoriformia)
The Fungivoriformia (and Parafungivoriformia) form a large group of Diptera. Moreover, many fossils that belong to this group are found (see Part XXVIII-A).
While in the Rhyphiformia and Bibioniformia there was virtually no discrepancy between monophyletic group and type (groundplan), such a discrepancy appears to exist in the Fungivoriformia + Parafungivoriformia. However, to distinguish types (especially more or less high-level, or high-order types, types, that is, above the family-groundplan), is difficult and uncertain here. ROHDENDORF, as we know, works, not from a genuinely hennigian phylogenetic, but from a typological viewpoint (although not precisely defined, as, alah, in all authors working typologically). Therefore, his (1964) family-groupings -- superfamilies -- (of recent families) might be helpful : In the superfamily Fungivoridea he places most of the fungivoroid families (such as the Ditomyiidae, Zelmiridae, Fungivoridae, Lycoriidae, etc). In the superfamily Cecidomyiidea (= Itonididea) he places the families Lestremiidae, Cecidomyiidae (= Itonididae), and Heteropezidae. In the superfamily Scatopsidea he places the families Hyperoscelididae (= Corynoscelidae), Synneuronidae, and Scatopsidae. In the superfamily Bolitophilidea he places the family Bolitophilidae. And while all these insects belong in the infraorder Bibionomopha, the family Pachyneuridae belongs -- as the superfamily Pachyneuridea -- in the infraorder Tipulomorpha. That is, while phylogenetically the family Pachyneuridae belongs to the Fungivoriformia (Bibionomorpha), it belongs typologically in the Tipulomorpha (the latter according to ROHDENDORF, 1964, based on the structure of its wings) (see Figure 18, in Part XXIX).
In the many fossils belonging to the Fungivoriformia and Parafungivoriformia it is difficult -- if not, virtually impossible -- to distinguish different types ( This is generally true of fossil insects, because in most cases they consist in wing (fragments) only). In the Jurassic fossils ROHDENDORF, 1946 and 1964, does not recognize recent families (except for the Allactoneuridae, which is insufficiently argued). All are typically Jurassic (lower and upper Jurassic) families typologically conceived. The most important are : Pleciofungivoridae, Pleciomimidae, and Allactoneuridae. From what we can say of these fossils is that they all form one single higher-order type (above family level). And we suppose this broad type to be identical to that which comprises all recent families of the Fungivoriformia and Parafungivoriformia, except the Pachyneuridae, the Heteropezidae, the Itonididae (but not the Lestremiidae), the Corynoscelidae, Synneuronidae, and perhaps a few others (such as the Scatopsidae).
The Fungivoroid Type ( Yellow area in Figure 23 )
In order to get a good image of this broad type we depict a number of its typical representatives :
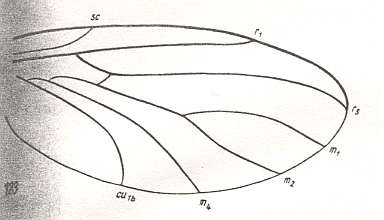
Figure 5 : Wing of Pleciomima sepulta ROHD. Upper Jurassic of Karatau (southern Kazachstan). Family Pleciomimidae. Length of wing 2.5 mm. Radial Sector unbranched, replacement of the medial trunk just begun (deepening of the cubital fork, stretching of the cross-vein tb1). See also next diagram.
(After ROHDENDORF, 1938, from HENNIG, 1954)
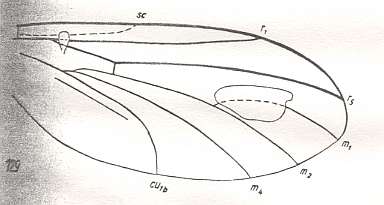
Figure 6 : Wing of Mimallactoneura vetusta ROHD. Upper Jurassic of Karatau (southern Kazachstan). Family Pleciomimidae. Length of wing 3.00 mm. Radial Sector unbranched, replacement of the medial trunk just begun (deepening of the cubital fork, stretching of the cross-vein tb1).
(After ROHDENDORF, 1946, from HENNIG, 1954)
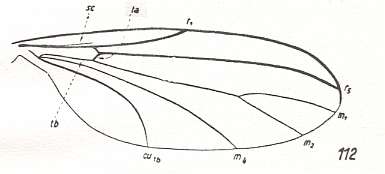
Figure 7 : Wing-venation of Catocha spec. Recent. Family Lestremiidae (Itonididea). The cross-vein ta is still in its original position and orientation, that is, it makes a clear angle with the Radial Sector. The cubital fork is deepened, causing the cross-vein tb1 to assume a horizontal orientation. The deepening of the cubital fork in effect dissolves this fork. (After HENNIG, 1954)
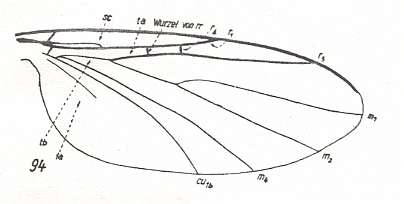
Figure 8 : Wing-venation of Ectrepesthoneura hirta WINN. Recent. Family Sciophilidae. The upper branch of the Radial Sector is very short and cross vein-like. It ends up in R1. Also the root of the Radial Sector is cross vein-like. The Subcosta ends up in R1. The Sciophilidae belong to one of the fungivoriformian families that lead from the Diadocidiidae, via the Bolitophilidae, to the Lycoriidae + Fungivoridae.
(After HENNIG, 1954)
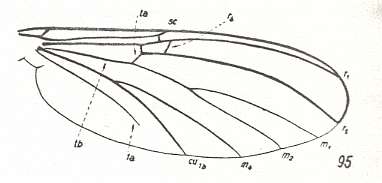
Figure 9 : Wing-venation of Mycomyia limbata WINN. Recent. Family Sciophilidae. For yet another drawing see HERE, number 9 . (After HENNIG, 1954)
For the wing-venation of Sciophila, see HERE, number 20 .
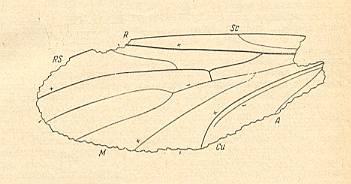
Figure 10 : Left wing of Palaeohesperinus longipennis ROHD. Family Pleciofungivoridae. Lower Jurassic of Issyc-Kul, central Asia. Holotype Coll. PIN Nr.358/95. Length of remains 3.13 mm. Well-preserved wing (ROHDENDORF, 1964, p.180).
ROHDENDORF writes in the description of the genus : M3 (which we -- with HENNIG -- interpret as being M4 ) almost straight and branches off from the common stalk of M [= Media] a little distad of the level of the R-Rs forking. There are no m-cu cross-veins [In many fossils of this period there are more than one m-cu cross-veins, one of them is the tb1 cross-vein].
In the description of the present species longipennis ROHDENDORF writes : M3 [M4] along all of its length comes nowhere nearer to CuA than to M1+2.
(After ROHDENDORF, 1964)
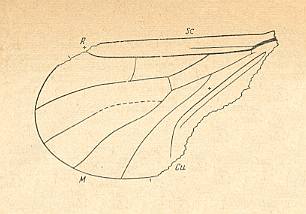
Figure 11 : Positive impression of left wing of Archipleciomima obtusipennis ROHD. Family Pleciofungivoridae. Lower Jurassic of Issyc-Kul, central Asia. Holotype Coll. PIN Nr.358/125. Length of remains 1.9 mm., length of whole wing 1.875 mm. Well-preserved wing (ROHDENDORF, 1964, p.179).
ROHDENDORF writes in the description of the genus : M3 (which we -- with HENNIG -- interpret as being M4 ) branches off from the common stalk of M [= Media] at the level of the R-Rs forking, curved and not connected with CuA by cross-veins.
(After ROHDENDORF, 1962, from ROHDENDORF, 1964)

Figure 12 : Wing of Pleciofungivora major ROHD. Upper Jurassic of Karatau (southern Kazachstan). Family Pleciofungivoridae. Length of wing 3.00 mm. Radial Sector 2-branched. Replacement of the medial trunk well under way -- nearly completed -- (deepening of the cubital fork, stretching of the cross-vein tb1).
(After ROHDENDORF, 1946, from HENNIG, 1954)
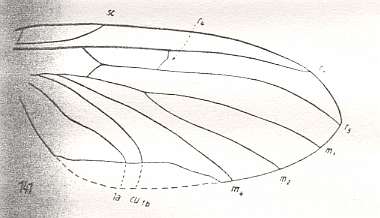
Figure 13 : Wing of Mesosciophila venosa ROHD. Upper Jurassic of Karatau (southern Kazachstan). Family Allactoneuridae. Length of wing 4.5 mm. Radial Sector 2-branched. It is evolutionarily under way to become unbranched. The branch of the Radial Sector is short and ends up in R1. Replacement of the medial trunk well under way -- nearly completed -- (deepening of the cubital fork, stretching of the cross-vein tb1).
(After ROHDENDORF, 1946, from HENNIG, 1954)

Figure 14 : Wing of Pleciomima sepulta ROHD. Upper Jurassic of Karatau (southern Kazachstan). Family Pleciomimidae. Length of wing 2.5 mm. Radial Sector unbranched, replacement of the medial trunk just begun (deepening of the cubital fork, stretching of the cross-vein tb1). See also next diagram.
(After ROHDENDORF, 1938, from HENNIG, 1954)

Figure 15 : Wing of Mimallactoneura vetusta ROHD. Upper Jurassic of Karatau (southern Kazachstan). Family Pleciomimidae. Length of wing 3.00 mm. Radial Sector unbranched, replacement of the medial trunk just begun (deepening of the cubital fork, stretching of the cross-vein tb1).
(After ROHDENDORF, 1946, from HENNIG, 1954)
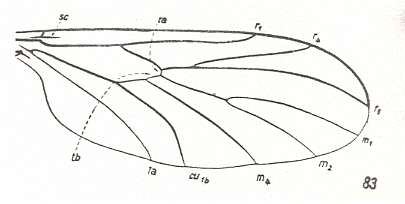
Figure 16 : Wing-venation of Ditomyia fasciata MEIG. Recent. Family Ditomyiidae. (After HENNIG, 1954)
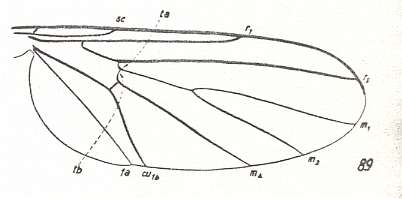
Figure 17 : Wing-venation of Diadocidia ferruginea MEIG. Recent. Family Diadocidiidae. (After HENNIG, 1954)
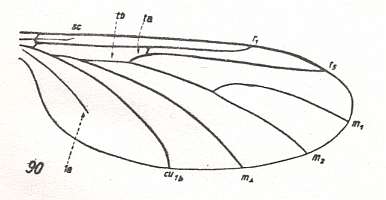
Figure 18 : Wing-venation of Heterotricha relicta EDW. Recent. Family Diadocidiidae. The replacement of the vanished medial trunk has begun : The cubital fork deepens, pulling the cross-vein tb1 in the horizontal direction. The Radial Sector is already unbranched. (After EDWARDS, 1925, from HENNIG, 1954)
Before we give the noëtic interpretation of the cladistic pattern (phylogenetic tree) of the Parafungivoriformia and Fungivoriformia, we first reproduce the phylogenetic results obtained in earlier documents :
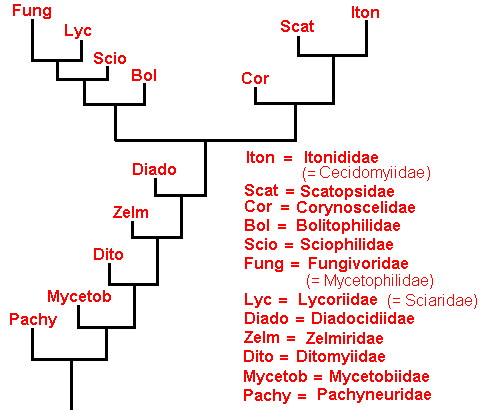
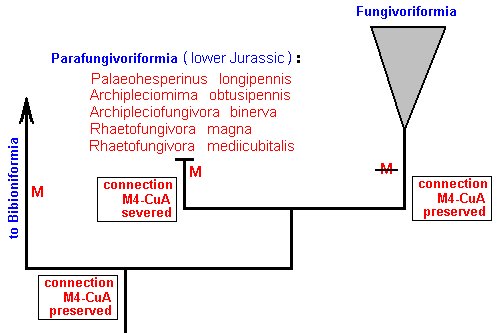
Figure 20 : The place of the mesozoic Parafungivoriformia in the phylogenetic tree of the Bibionomorpha.
The synapomorphous character determining the Parafungivoriformia to be a monophyletic group is the severance of the connection between M4 and CuA. In the Bibioniformia, as well as in the Fungivoriformia, this connection is preserved. The synapomorphous character determining the true Fungivoriformia to be a monophyletic group is the loss of the basal trunk of the Media. In the Bibioniformia and Parafungivoriformia this basal trunk is still present.
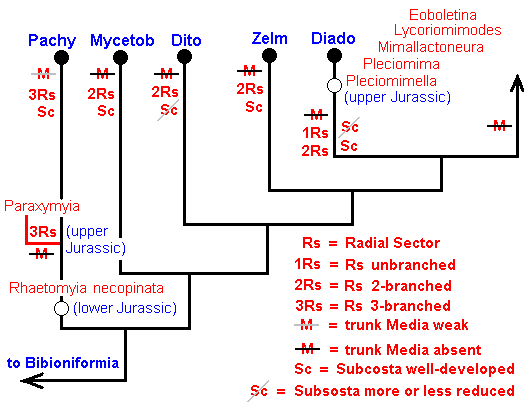
Figure 21 : Phylogenetic tree of the first series of true Fungivoriformia with fossils included.
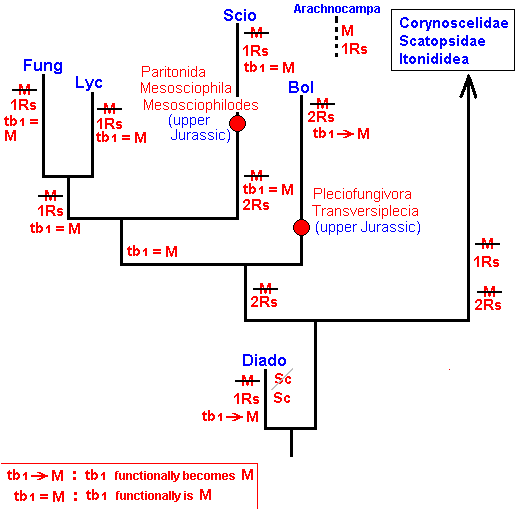
Figure 22 : Phylogenetic tree of the first terminal series of fungivoriformian families with mesozoic fossils.
There are no mesozoic fossils to place phylogenetically into the second terminal series of fungivoriformian families.
We will now present (but not in a detailed fashion) the noëtic interpretation of the cladistics obtained by phylogenetic systematics.
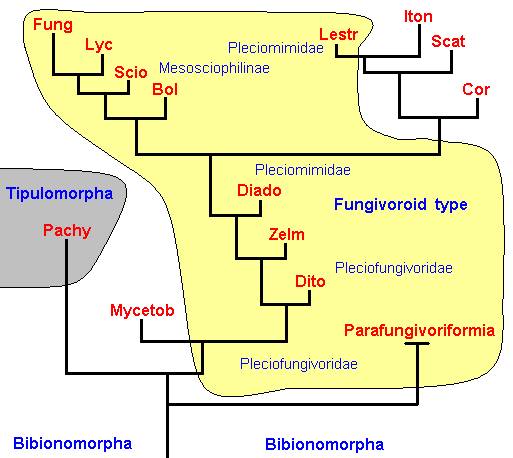
Figure 23 : Noëtic interpretation of the cladistics of the Fungivoriformia.
Superimposed upon the cladistics are (in the present diagram) some major types (family-groundplans and higher-order groundplans) : The two major types are the Tipulomorpha (brown) and the Bibionomorpha (white + yellow).
The Bibionomorpha consist of a broad (higher-order) type, the Fungivoroid Type (yellow) (including many fossil and recent families), and a number of family-types (only indicated by the family names [for the abbreviations see Figure 19] ). The fossil sister-group of the Fungivoriformia, the Parafungivoriformia, is indicated. The black bifurcating lines must now be interpreted as the noëtic trajectory (as it follows its course in the Implicate Order) visiting the stability fields of the types (groundplans). Upon (finally) having visited the (stability fields of the) genus-groundplans (not indicated) projection into the Explicate Order takes place and continues in the form of alternating projections and injections (i.e. projection from the Implicate Order into the Explicate Order, and then injection from the Explicate Order back into the Implicate Order again, and so on). In the case of all fossil groundplans this projection / injection sooner or later stops, resulting in the extinction (in the Explicate Order) of the relevant taxa. The reason that the projection / injection sequence stops is the disappearance in the Explicate Order of the corresponding ecological existential conditions of the relevant taxa.
The noëtic trajectory is supposed to visit first the stability fields of the several jurassic family- and subfamily-groundplans -- lying within that of the Fungivoroid Type : those of the Pleciofungivoridae, Pleciomimidae, Mesosciophilinae (Allactoneuridae), and possibly some other subtypes -- resulting in the appearance of the representatives of those families in jurassic times. Later they will become extinct, but some of them have evolved into recent families, which noëtically means that the trajectory leaves the stability field of the jurassic groundplan and enters (not within time, but as a formal derivation) that of the cenozoic groundplan, that is, the groundplan now, as a result of projection, possessed by recent forms. After this noëtic interpretation of the cladistics of the Fungivoriformia (and Parafungivoriformia) the present diagram must be imagined to look similar to that in Figure 4 which was concerned with the noëtics of the Bibioniformia
Having now noëtically interpreted the Rhyphiformia, the Bibioniformia and the Fungivoriformia, we have completed the noëtic interpretation of the cladistics of the whole Bibionomopha (as representing a concrete example of evolutionary noëtics), and with it (we have) concluded the study of the Diptera-Nematocera (midges, gnats, mosquitoes).
Conclusion of the evolutionary study of the Order Diptera.
Having concluded our evolutionary study of the Nematocera, it would be natural now to engage in the next suborder of the Diptera, the Brachycera-Orthorrapha (and after that in the Brachycera-Cyclorrapha).
However, this website -- I mean this total website consisting of First Part, Second Part, Third Part, Fourth Part, and Fifth Part -- is not primarily concerned with how precisely things go in the natural world. That is the task of natural science. Although we in fact did develop some theories that purport to explain how, for example the shapes of snow-crystals develop, or in what way certain insect groups have developed from other such groups, these theories only have served to develop and test our much more general ideas about the ontological structure of Reality as such, that is, whether or not Reality consists of several different domains of Being (say, a material-physical one and an immaterial noëtic one), and if so, what these domains precisely are and whether or not they interact. That is to say, the ultimate subject of this website is Metaphysics in the sense of ontology (doctrine of ways of Being). All more or less specific physical or biological theories developed on this website are geared to an understanding, not of the special things and events with which these theories are concerned, but to develop an understanding of the general and fundamental structure (or nature) of Reality as such.
After many studies, laid down in First to Fourth Part of Website, we had finally developed a metaphysical theory about the ultimate structure of Being or Reality. It combines elements of Aristotelian metaphysics [doctrine of Substance] (mainly dealt with in First Part of Website) with some ideas of David BOHM about the Implicate Order (dealt with in Third Part of Website). This metaphysical theory is laid down in the present Part (i.e. Fifth Part) of Website after having studied (there) Intentional Logic (which logic is based, i.e. dependent on, Being -- [dependent on, not as a doctrine, but as how Being as such really is, determining whether, and if so, how it can be known] -- as it is assumed to be according to Aristotelian metaphysics).
As will be clear from all the foregoing documents, our (metaphysical) theory assumes the presence, and interaction, of two domains of Being, viz., the so-called Explicate Order (in which things and events exist within time and space [i.e. unfolded along space and time dimensions] ), and the Implicate Order, in which things exist immaterially, 'noëtically' (i.e. enfolded (back) along the space and time dimensions, implying that in this Order things do not exist in time or space). In the Implicate Order the 'microscopic' structure of Causality is formed by so-called If-Then constants, seen in the Explicate Order as a necessary succession of states of some dynamical system. But in addition to these immaterial If-Then constants there exist in the Implicate Order also 'macroscopic' immaterial structures such as co-called 'chreodes' that direct the sequence of If-Then contants, (this directing) seen in the Explicate Order as the generation of individual crystals and also of individual organisms (that is, of genuine [aristotelian] substances).
In that form our theory thus could well accommodate facts as the generation of crystals and the morphogenesis of organisms. But the greatest challenge to this theory is the fact of organic evolution, and especially the fact of the origin of the so-called 'smart adaptations' in which evolution abounds. Of them, the morphological ones are covered by our concepts of 'noëtic reaction' and subsequent 'projection' of noëtic reaction products into the Explicate Order. Also we have succeeded to find a place for each of the two main aspects of evolution, namely its ecological aspect (adaptations) and its formal aspect (groundplans, types). Here the analogy with the thermodynamic stability fields of mineral and aggregational phases, combined with the idea of the 'noëtic trajectory' visiting (the stability field of) one groundplan after another (= formal derivation of one groundplan from another), have played an important role in developing our general theory of organic evolution.
This (further) development (of the general theory) took place during, and, more importantly, as a result of, our findings in the study of the various evolutionary features of the Diptera-Nematocera. In Diptera, after the Nematocera come the Brachycera (Orthorrapha and Cyclorrapha). However, except for the family Tachinidae (endoparasites of other insects), and for the epizoic Diptera (already dealt with), and perhaps for some other families (such as Bombiliidae), we do not expect to find in Brachycera any additional essentially instructional and inspiring features that have a bearing on the further development of our general theory of evolution, and with it, finally, of metaphysics. And, after all, we have dealt with the origin of the Brachycera (orthorrapha and cyclorrapha) in earlier documents (from Part XXI onwards).
REMARK : Despite all this, we have, beginning with Part LXIX, Origin and Evolution of Diptera revisited picked up the Order Diptera again, including the Brachycera (orthorrapha and cyclorrapha), but concentrating on wings and their venation only (functional wing-types and formal venational derivation). In all that, many new elements could be added to our noëtic theory of evolution.
Things will be quite different when we turn to H y m e n o p t e r a, that is, to another insect Order which is, although in some respects comparable to, in others, however, fundamentally different from the Order Diptera. The Order Hymenoptera ( 'membranously-winged insects' ) consists of the wasps (saw-flies, parasitic wasps, solitary and social wasps), bees (solitary and social), and ants. While studying them, the many extraordinary facts present in this insect Order will add much to our general theory, especially the facts that have to do with the evolutionary development and diversification, not of morphological structures, but of i n s t i n c t s. Also in Diptera we will find a development of instincts, but in Hymenoptera their development surpasses that of all invertebrate and vertebrate animals, except perhaps that of the higher mammals and man (According to me, the s o c i a l instincts of ants are higher developed than they are in man, where it is virtually absent ['social' behavior in man is determined almost entirely by the prospect for individual benefit, while in ants it is fully intrinsic.] ).
While in Diptera (and in most other insect orders, or most other animals for that matter) we generally have to say that a species or higher-taxonomic group is able to exist and persist (in the Explicate Order) as a result of special a d a p t a t i o n s to some (already) existing ecological situation, adaptations that is, obtained by their members, -- in Hymenoptera the existential conditions more and more (as we ascend in the Order) take the form, not so much of an adaptation (to an existing ecological situation), but more of a s t r a t e g y to exist and persist. The stronger this is so expressed the lesser it is an adaptation : while in most animals we can speak of "f i n d i n g an appropriate ecological niche already existing somewhere" (not as a special locality, but as a web of existing special ecological relations), the higher Hymenoptera (and especially the social Hymenoptera) in a strong degree c r e a t e their appropriate ecological niche (each group a different one). This independent creation of the niche is best called "s t r a t e g y to exist and persist". In Diptera and most other animals projection of some qualitative content representing a species or family (from the Implicate to the Explicate Order) largely depends upon whether the 'co-projected' existential conditions of that particular content match with actually existing ecological situations in the Explicate Order. In many groups of lower Hymenoptera this is certainly also the case, meaning that they too adapt to existing ecological situations (occupy new ecological niches). But higher up into the Hymenoptera it is strategies, not adaptations, that count. Up to a certain degree here too we can speak of triggering projection (so triggered) by the existence of a certain ecological situation. But here it is a strategy that is projecting. And this strategy largely creates a new ecological niche in some existing more generally appropriate ecological context. Further, this strategy should either be identified with the (morphological and physiological) groundplan or type (as it is defined in earlier documents of the present Series), or (it should be identified) with the ecological existential conditions co-projected with the groundplan as its adaptations. The first suggestion seems best to me. The term "strategy" emphasizes the independent nature of the existential condition in the sense that it largely creates this condition, in contrast to a mere adaptation.
Let us work this out a little more.
Thereby we compare the existential conditions of inorganic substances ( 'substance' taken in the metaphysical sense) with those of living beings ( 'organic substances' ). In order to exist and persist, an inorganic substance, such as a crystal, must be thermodynamically stable. Indeed, crystals are, thermodynamically, equilibrium structures (they contain the least energy, that is, they exist in some energy minimum). On the other hand, a living being (an organic substance), thermodynamically is a far-from-equilibrium structure. And only as such it can be a complex and functional (material) structure. But it needs energy to persist in this state of non-equilibrium. And because for any given something to absorb energy is working against the 'thermodynamic stream' if it leads to a non-equilibrium with its environment, which is the case in all organisms, the absorption does not take place spontaneously, but must be forced. This the autotrophic organism, such as a green plant, realizes by trapping solar energy and chemically storing it, while the allotrophic organism, such as all animals, does this by feeding, that is by taking-in substances (in the chemical sense) that contain energy (that can be released). All these activities geared to maintain the organic body (to prevent its break down, i.e. to prevent its thermodynamic equilibrium with the surroundings) are in fact what the living condition really is. Indeed, crystals and the like can exist simply when the thermodynamic conditions are within a certain range. But organisms, because they are thermodynamically far-from-equilibrium structures, need so to say more : they must themselves embody s t r a t e g i e s in order to be able to exist and persist. In lower organisms it is in fact only their physiology and chemistry that constitute a strategy for them to exist, and can be called 'passive strategy'. In higher organisms the strategy they have for them to exist and persist might be called 'active strategy' for obvious reasons.
And a s t r a t e g y, whether passive or active, i n o r d e r t o e x i s t, is in fact l i f e i t s e l f.
In the course of the evolution of animals, and especially in that of the Order Hymenoptera, we see active strategy coming from passive strategy, reaching its climax in the ants.
In fact we can roughly equate "passive strategy" with (morphological or physiological) "adaptation", and thus having in Hymenoptera an evolution from mere adaptation (to some existing ecological niche) to (active) strategy (i.e. creating their own ecological niche [insofar as we can still call such a thing an ecological 'niche'] ). The strategy (and also the adaptation) is, of course, not only geared to preserve the individual organism but also (to preserve) its type, i.e. the species. Thus, all reproductive activities too belong to the strategy. And in Hymenoptera the evolutionary development of active strategy culminates in the origin of what we could call teleological instincts, that is, instincts leading to actions that are, in a way, not driven by local and direct causes or stimuli but by some remote state of affairs, remote in time and place (the 'goal'). In the case of lower organisms (including the lower Hymenoptera) the strategy is passive : they are 'attracted' to, and by, existing food sources (of a certain type) or by elements constantly accompanying such sources. Also the phenomenon of 'seeking food' belongs hereto. It is all within the domain of physics. On the other hand, there are many species of Hymenoptera (and other animals) whose members collect food, not for themselves but for their young. In Hymenoptera this often takes place before there are yet any young. In addition, it often is the case that wasps of certain species prepare for some accommodation or shelter for the coming offspring, and provide it with food (often sedated insects). Ants built nests for future purposes. Such activities are driven by special instincts, which lead to teleological actions, that is actions set in motion, not by direct and local causes, but by remote states of affairs, that is, states of affairs not yet existing. It is clear that such actions cannot form a part of the physical world, because there 'actions at a distance' (cause and effect separated in space) cannot occur (only seemingly so in the case of long causal chains -- where the end lies far away from the beginning -- in which the elementary cause-effect pairs, together constituting the chain, are each 'local actions'). 'Action at a distance' in fact means that although the cause is not directly at hand the effect nevertheless emerges. Something comparable takes place in teleological actions of certain animals. Also here the cause is not at hand, while the effect nevertheless emerges. While the individual elementary actions, together constituting the one single teleologic action, are physical events such as movement, repulsion and attraction, the genuine cause of the whole action, that is, directing the whole action, is nowhere to be found in the chain of the elementary constituent actions. So we must conclude that teleological actions (triggered by corresponding instincts) cannot take place in the Explicate, that is, Physical Order. Evidently this poses a tremendous problem in our general theory of evolution and of the metaphysical context in which evolution is supposed to take place or is directed.
And it is the study of the various behavioral patterns in Hymenoptera from which we expect the solution of the mentioned problem, which solution will certainly involve a further development of our theories. In the evolution of Hymenoptera we will distinguish -- in the next document -- the various more or less consecutive strategies (in the sense explained above) that can be observed in them, and follow their evolution from passive to active strategies.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXXI.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX