

[As done earlier, in these enquiries into the wing-venation and its transformations in Diptera-Nematocera we will consider the subcostal vein (Sc) at most only occasionally. This vein, especially its terminal branches (Sc1, Sc2), is -- in Diptera -- often difficult to discern.]
Introduction.
After having finished our "theoretical intermezzo" in which our noëtic theory of evolution was worked out further, but still in a more or less general fashion (especially in part XXV-A. XXV-B, and XXV-C), we are now at the place where we continue our investigation of the wing-venation in Nematocera, that is, in all the lower groups of Diptera, as to its evolutionary significance. But having now acquired some insight into the relationship that exists between the theory of phylogenetic systematics (in the sense of HENNIG) and our own noëtic theory of evolution, we are ready to temporarily leave the typological point of view -- as we had had it with respect to the other Nematocera -- and apply HENNIG's phylogenetic point of view to our remaining Nematocera, that is all the families together making up the infraorder Bibionomorpha. And when we have done that we have at last arrived at the end of our investigation of the Nematocera and will then proceed with the rest of the Diptera (Brachycera-Orthorrapha and Brachycera-Cyclorrapha).
In what follows we expound in what way we are going to investigate the evolution of the Bibionomorpha. And, as we had done with the superfamily Rhyphidea and with the infraorders 'before' the Bibionomorpha, we will concentrate on the wing-venation. The Rhyphidea already belong to the Bibionomorpha, but our treatement of their wing-venation ( Part XXI ) was concerned mainly with the typological and evolutionary connections of the Rhyphidea with the Brachycera.
Now we will deal with the Bibionomorpha (including the Rhyphidea) as to the phylogenetic significance of their wing-venation, and the bearing of the latter on the noëtic evolution of the Bibionomorphous groundplans. For this to get started a number of preliminaries must be considered.
In earlier documents, especially (sub)parts XXV-A, XXV-B, and XXV-C, we have found out that while seen in the Explicate Order, the evolution of organisms, and thus also that of the Diptera, has proceeded polyphyletically. But this polyphyletic evolution turned out just to be the expression in the Explicate Order of a formal monophyletic evolution of groundplans in the Implicate Order. In the latter Order the organic groundplans are present as noëtic patterns present in their respective noëtic stability fields in the Implicate Order. The successive projection from the Implicate Order into the Explicate Order of (copies of) the groundplans -- ultimately of genus-groundplans -- depends on (1) whether the noëtic trajectory in the Implicate Order has actually visited the stability fields of these groundplans, and (2) whether the relevant ecological niches are actually present in the Explicate Order. For what is, in each separate case, actually projected is the reaction-product resulting from the noëtic reaction between the noëtic content of the genus-groundplan and the noëtic content of one of the genus' potential ecological niches. And, as has been said, this projection will only take place if (1) the noëtic trajectory actually has visited the stability field of the genus-groundplan (and with it that of the family-groundplan) and if, (2) together with that, the appropriate ecological niche does actually exist in the Explicate Order, that is, exactly that biological configuration to which the noëtically evolved adaptation (as a result of the mentioned noëtic reaction) precisely points. The projection of the genus-groundplan + adaptive content -- which is the mentioned reaction-product -- is followed by an injection of a copy of it back into the Implicate Order and immediately followed again by the projection of a copy of it into the Explicate Order, etcetera, etcetera. This alternation of projection and injection continues, and as long as it does so the adapted species will continue to exist in the Explicate Order. When it stops, we experience this as the extinction of that species. We can call the totality of projections and injections of such a reaction-product (genus-groundplan + adaptive content) a projection line.
The successive 'projection lines' of different reaction-products as they follow the course of the noëtic trajectory (going from the stability field of one groundplan to that of the next, and in this way noëtically deriving the latter from the former), are experienced in the Explicate Order as the polyphyletic appearance of groups of organisms (one genus after, not from, another, one family after, not from, another). But, as has been said, this appearance in the Explicate Order is just a reflection or expression of the successive visits (derivations) of the noëtic trajectory in the Implicate Order. And it is precisely the -- branched -- course of this trajectory that is revealed by the methods of phylogenetic systematics in the sense of HENNIG. All this was explained in the foregoing series of documents.
And after having discussed all this in a general fashion we wish to explicitly apply all this to a concrete animal group. And this animal group will, as has been said, be the infraorder Bibionomorpha.
The typological group of Diptera drawn up by ROHDENDORF 1964 as the infraorder "Bibionomorpha" coincides with the monophyletic group "Bibionomorpha" as drawn up by HENNIG 1954. Only the family Pachyneuridae is included by HENNIG in the Bibionomorpha while ROHDENDORF places it in the Tipulomorpha.
The group Bibionomorpha consists of the following dipteran families :
Nutritive environments of the larvae of Diptera.
Woodland zone
The highest abundance and diversity Diptera attain in the woodland zone. The significant amount of deposit, the large stock of rotting vegetable matter (woodland litter [podstilka], wood), the sufficient dampness during the course of the whole summer season, the absence of excessive insulation, the relatively high humidity of the air under the cover of the forest, create favourable conditions for the development of Diptera. The presence of utterly diverse biotopes provides for the possibility to species to (individually) develop, species that is, that possess different ecological potentials.
When characterizing climatic and undergrowth conditions of this or that region we must bear in mind that the abundance of Diptera in different zones is connected with microclimatological conditions of the several parts of that region and with the ecological features of the different stages of individual development.
The connection of the larvae of Diptera with the substrate.
The development of the pre-imaginal stages of Diptera proceeds in the most diverse substrates. Every solid or loose substrate, dry or damp, originating from plant or animal, rich or poor in organic residues, in short, every type of accessible substrate is capable of [letting it] being colonized by different groups of Diptera.
A large number of [species of] Diptera develops in soils of different mechanical composition.
Typically soil-borne are the larvae of Asilidae [robber-flies, Brachycera-Orthorrapha ( = roughly Asilomorpha)], Tabanidae [horse-flies, Brachycera-Orthorrapha ( = roughly Asilomorpha)], the majority of the larvae of Therevidae [Brachycera-Orthorrapha ( = roughly Asilomorpha)], Empididae [Asilomorpha], many Tipulidae [Nematocera-Tipulomorpha] and Rhagionidae [Asilomorpha].
A significant complex of Diptera is for individual development connected with disintegrating wood and the materials coming forth from it. Members of this complex can be encountered within such wood as well as under its bark, in mouldered wood [present] in hollows, or in a fermenting sap. To this complex belong the larvae of Dictenidia BRUL., Tanyptera LATR., Phoroctenia COQ., Ctenophora MEIG. (family Tipulidae) [Nematocera-Tipulomorpha], Hyperoscelididae [Nematocera-Bibionomorpha], Axymyiidae [Bibionomorpha], Pachyneuridae [Tipulomorpha or Bibionomorpha], Hesperinidae [Bibionomorpha], Clusiidae [(suborder) Brachycera-Cyclorrapha or, more or less equivalently, (infraorder) Myiomorpha], Lonchaeidae [Myiomorpha], many Bibionidae [Bibionomorpha], Sciaridae [= Lycoriidae] [Bibionomorpha], Ceratopogonidae [ = Heleidae] [Nematocera-Tipulomorpha], and, finally, Stratiomyidae [Asilomorpha].
With rotting wood are connected different fungi, where fruiting bodies and films of mold harbour their characteristic complex of Diptera ( Mycetophilidae [ = Fungivoridae] [Bibionomorpha] and related families, further, Limoniidae [Tipulomorpha], Platypezidae [Myiomorpha], and others).
Diverse is the content of the inhabitants of forest-litter [podstilka]. Here develop Bibionidae [Bibionomorpha], Tipulidae [Tipulomorpha] (mainly species of the genus Tipula L.), Cecidomyiidae [ = Itonididae] [Bibionomorpha], Ceratopogonidae [Tipulomorpha], Chironomidae [ = Tendipedidae] [Tipulomorpha], Lauxaniidae [ = Sapromyzidae] [Myiomorpha], Fanniinae [Myiomorpha-Muscidae], Lonchopteridae [Brachycera, Musidoromorpha], and others.
A large group of larvae (Cecidomyiidae [Bibionomorpha], Agromyzidae [Myiomorpha], Chloropidae [Myiomorpha], some Drosophilidae [Myiomorpha], Ephydridae [Myiomorpha] ) lives in tissues of living plants.
There exists a whole complex of Diptera that are inhabitants of different rotting vegetable and animal residues, especially excrements. These are representatives of the families Scatopsidae [Bibionomorpha], Psychodidae [Tipulomorpha], Muscidae [Myiomorpha], Calliphoridae [Myiomorpha], Scatophagidae [ = Cordyluridae] [Myiomorpha], Sarcophagidae [Myiomorpha], Phoridae [Brachycera-Cyclorrapha, Phoromorpha], some species of Syrphidae [hover-flies, Myiomorpha]. Many species from these families go into living in bird nests, in burrows and nests of rodents (Psychodidae [Tipulomorpha], Sphaeroceridae [ = Borboridae] [Myiomorpha], Calliphoridae [Myiomorpha]. Among the latter, living in bird nests Protocalliphora HOUGH are known as blood-suckers.
Representatives of a whole series of families (mainly of Brachycera-Cyclorrapha like Sarcophagidae [Myiomorpha], Milichiidae [Myiomorpha], Calliphoridae [Myiomorpha], Muscidae [Myiomorpha] ) develop in corpses of different animals including those of insects. From these groups are immediately derived those representatives that live in living tissues of various animals. Thus, the larvae of Nemestrinidae [Asilomorpha], Acroceridae [Asilomorpha], Bombyliidae [Asilomorpha], Pyrgotidae [Myiomorpha], Conopidae [Myiomorpha], Tachinidae [Myiomorpha], and others, develop in various insects. The larvae of warble flies [Myiomorpha-Oestridae] live in the subepidermal cell-tissue and in the body-cavity of various vertebrates. The larvae of the flesh fly (Sarcophagidae [Myiomorpha] ) feed on living tissues of animals and of man.
A peculiar group is formed by symbiotic larvae, for example the larvae of Microdon MEIG (Syrphidae [Myiomorpha] ) live in ant nests.
A special group is formed by larvae-commensals [that is, eating at the same table]. For example, the not-specialized larva of Smittia ephemerae KIEFF. [Tipulomorpha-Chironomidae] lives on the legs, abdomen, and gills of Ephemera vulgata L. [Mayflies], where it feeds on particles of detritus, which sticks on the hairs of the body and legs of the [aquatic] larva of the mayfly. The larvae of some species of Smittia HOLMG. and Orthocladius v. d. WULP live under the wing-sheaths of [larvae of] mayflies. Some larvae colonize the mantle-cavity of molluscs from where they can penetrate into the body.
Many Diptera are for their individual development connected with various liquid environments : principally with water, but also with sap which flows from wounds in trees, and with liquefied disintegrating fungi. In sap flowing from damaged spots on trees we see developing many larvae of Ceratopogonidae [Tipulomorpha], Limoniidae [Tipulomorpha], Anisopodidae [ = Rhyphidae] [Bibionomorpha], Trichoceridae [ = Petauristidae] [Tipulomorpha], Syrphidae [Myiomorpha], and Dolichopodidae [Asilomorpha]. Some species of Diptera are able to develop in drippings of resin on tree trunks. Thus, on pine-trees representatives of Cecidomyia pini de GEER [Bibionomorpha-Cecidomyiidae], on spruce representatives of Stelechodiplosis magna MöHN [Bibionomorpha-Cecidomyiidae] develop. On silver fir we see developing Wichmaniella crassa MöHN [Bibionomorpha-Cecidomyiidae], Cheilosia morio ZETT. [Myiomorpha-Syrphidae], species of Brachyopa MEIG. [Myiomorpha-Syrphidae], some species of Forcipomyia MEIG. [Tipulomorpha-Ceratopogonidae]. The colonization of parts of resin is connected with a preventive change of it. Especially around the larva a cradle is formed consisting of solid non-sticky substrate.
In rivers, brooks, and sources, with well-aerated flowing water develop groups which need an increased content of oxygen in the water and which are adapted to conditions of a constant, sometimes fast, stream. In such conditions at the bottom, sometimes under stones or on various objects under water, develop the larvae of Deuterophlebiidae [ Deuterophlebiomorpha (Nematocera) ] and Blephariceridae [ Blephariceromorpha (Nematocera) ]. Here one can encounter larvae of Simuliidae [black-flies, Tipulomorpha], usually clinging to plants or stones. In simular conditions, in moss covering stones, often develop some Limoniidae [Tipulomorpha].
Rather common in small sources in deposits of mud are the large grayish larvae of Pedicia rivosa L. [Tipulomorpha-Limoniidae]. In sources and brooks, in accumulations of wet wood develop many Chironomidae [Tipulomorpha] and some Limoniidae [Tipulomorpha], for example Lipsothrix errans WALK.
Inland water-basins with stagnant water (lakes, ponds, old dryed-up riverbeds, and so on) are also characterized by a definite complex of Diptera that develop in them. On banks and in deposits of mud at the bottom one can encounter larvae of Chironomidae [Tipulomorpha], Limoniidae [Tipulomorpha], Tipulidae [Tipulomorpha], Tabanidae [Asilomorpha], Dolichopodidae [Asilomorpha], some Stratiomyidae [Asilomorpha], Ceratopogonidae [Tipulomorpha]. In aquatic plants (that is, mining their stems) develop larvae of Ephydridae [Myiomorpha].
A whole series of species develop in salty inland waters or at marine coastal zones (some Stratiomyidae [Asilomorpha], Coelopidae [Myiomorpha] ).
Whatever, even insignificant, accumulations of water, for example in depressions on roads, imprints of animal ungulae [hoofs], in hollows, also become a habitat of mainly minute forms with a short summer developmental cycle (many Chironomidae [Tipulomorpha], Ceratopogonidae [Tipulomorpha], Mycetobiidae [Bibionomorpha]. The larvae of certain Ceratopogonidae [Tipulomorpha], Cecidomyiidae [Bibionomorpha], develop even in micro-water-basins in axils of leaves. With vaginas of leaves are connected larvae of Dicerura scirpicola KIEFF. [Bibionomorpha-Cecidomyiidae] and D. kaltenbachi RüBS. Among the rhizoids (roots) of moss one can often encounter larvae of Chironomidae [Tipulomorpha], some Ceratopogonidae [Tipulomorpha] (genus Bezzia KIEFF), Limoniidae [Tipulomorpha].
Rare cases are observed where larvae live in substrates totally alien for insects, for example larvae of Helaeomyia petrolei COQ. [Myiomorpha-Ephydridae] develop in oil wells.
Character of the distribution of the larvae in their substrates.
It is necessary to succinctly dwell on regularities of distribution of larvae of Diptera in different types of substrates.
In soils [such as a forest floor] the main mass of dipterous larvae is concentrated in the litter layer [podstilka] and in the upper coarse-humus [vegetable mould] layer at 0-40 cm depth, which [latter layer] is most rich in plant roots, pervaded by tunnelings and well-aerated.
Larvae of the representatives of few families are encountered in the soil at a depth until 1 m (larvae of Asilidae [Asilomorpha], Therevidae [Asilomorpha], sometimes Tabanidae [Asilomorpha], Bombyliidae [Asilomorpha].
We can distinguish a definite zonality of the distribution of dipterous larvae in the soil. In tundras, where the accumulative horizon [where, I assume, humus will be produced and accumulate] is not developed, the high-level of groundwater results in the fact that groups of animals, which in other zones are characteristic of the [content of the] soil, concentrate in moss patches. In the zone of the northern and middle taiga the main mass of dipterous larvae is concentrated in the litter layer [podstilka] and in the upper coarse-humus layer [ The Taiga are the dense forests between tundra and steppe]. The concentration of the basic colonization in the upper layers of the soil is the rule, chiefly connected with the high-level humidity of the ground. In the southern regions of the woodland zone, but also in the woodland-steppe and steppe regions, the main animal colonization of soils changes over to within the soil horizon.
Important for the distribution of larvae in rotting wood is its state of disintegration and the present colonization of it by other invertebrates. Questions about the succession of invertebrate animals at different stages of the wood's disintegration is meticulously discussed by B.M. Mamajev.
Analysis of data about the distribution of larvae in aquatic environments indicates that the majority of species is adapted to the shoreline part of water-basins and only a few live at the bottom at larger depths, or right in the water. The main mass of dipterous larvae (Chironomidae [Tipulomorpha], Ceratopogonidae [Tipulomorpha], Tipulidae [Tipulomorpha], Limoniidae [Tipulomorpha], and others), is concentrated in a damp substrate at the bank [shore] and in deposits of mud at a depth of not more than 1 meter. Larvae of Ceratopogonidae [Tipulomorpha], for example, live at a depth down to 40 cm. The width of the shoreline band that can be colonized is significantly dependent on the angle of the slope and the degree of moistness of the substrate lying above the water level.
A comparatively small number of species can develop at the bottom of water-basins, like the above mentioned Blephariceridae [Blephariceromorpha] and Deuterophlebiidae [Deuterophlebiomorpha]. At the bottom of lakes and ponds many Chironomidae [Tipulomorpha], Ceratopogonidae [Tipulomorpha] (Sphaeromias CURT.) develop. One encounters significantly less larvae right in the water where they concentrate mainly at the surface film. Such are the larvae of Culicidae [Tipulomorpha] and some Stratiomyidae [Asilomorpha]. Sometimes larvae make use of various plants and aquatic growths floating on the surface (Ceratopogonidae [Tipulomorpha], Chironomidae [Tipulomorpha].
Nutritive connections of the larvae.
Depending on the way of feeding and the nature of the substrate to be used [consumed] we can distinguish different categories of nutritive regimes. Larvae of Diptera are able to feed on various disintegrating materials and on living tissues of plants and animals. Among dipterous larvae we can distinguish such groups as saprophags, phytophags, mycetophags, coprophags, necrophags, and zoophags.
A whole series of factors must be taken into account when studying nutritive regimes of larvae. Larvae can in different ways utilize the substrates which are at their disposal. They can eat them directly or eat the bacteria and fungi that grow on them. The way of processing the food mass can also be different dependent on the structure of the mouthparts and the particular features of digestion. Finally, one must take into account the fact that in many cases a mixed diet is observed. Many questions concerning the feeding habits of individual groups are still [1969] not clarified.
We will now successively discuss the mentioned nutritive regimes.
S a p r o p h a g o u s larvae (scavengers).
The group of (vegetable) scavengers (saprophags) includes a very wide range of organisms that feed on different decaying remains of higher plants and fungi, and rotting wood [Animals that feed on decaying animal remains are also scavengers, which we subsume, however, under the nutritive regime of necrophagous animals].
Typical saprophagous insects are the larvae of the majority of species of the genera Bibio GEOFFR. [Bibionomorpha-Bibionidae], Sciara MEIG. [Bibionomorpha-Sciaridae], Scatopse GEOFFR. [Bibionomorpha-Scatopsidae], many species of Tipula L. [Tipulomorpha-Tipulidae], for example T. transbaicalica ALEX., T. scripta MEIG., T. excisa SCHUMM., T. nubeculosa MEIG., T. rubripes SCHUMM., and others.
Saprophagous larvae live in the most diverse environments : in humus-rich soil, in woodland litter [podstilka], in deposits of mud in water-basins. They represent the dominating faunistic element in all soils with woodland litter well-expressed or with a developed accumulative humus horizon. Saprophagous larvae play an important role in the disintegration of vegetable remains. And here we must first of all point to the larvae of Bibionidae [Bibionomorpha]. The larvae of Bibio marci L. in the hornbeam forest of the Moldavian Kodr claim the second place in biomass after the earthworms, but in their role of disintegrating woodland litter they surpass the latter. Already by a number of 200 individuals pro 1 m2 the larvae of this species consumes about 15 percent of leaf fall-out, and after 100 days of activity produce about 100 g excrements. The larvae of Bibio marci L. are able to process leaves of oak, beech, hornbeam, and other leaf-carrying species. The larvae of B. clavipes MEIG., B. nigriventris HAL. and others, intensively process the litter in pine tree forests.
The significant role played by the larvae of Bibionidae [Bibionomorpha] in the intensification of the disintegration of woodland fall-out is connected with features of their ecology. The larvae are gregarious and their density in some cases reaches mythical magnitudes. The density of larvae of Bibio pomonae FABR. in the pine forests of the Archangel region [in the North of european Russia] in certain parts reaches 200-300 individuals pro 1 m2. But a most large number of larvae of Bibio GEOFFR. is observed in the woodland litter of leaf-carrying forests. Such in the litter of oak forests on the slopes of the hills in the nature reserve "Kedrovaja padj" in the autumn of 1964 on an area of 10 m2 were found 12 colonies of larvae of B. nigriclavipes HARDY & TAKAHASI. In each colony were found up to 300 individuals. In Hungary on an area of 72 m2 were found 35 colonies of B. marci L., while in each of them were found 500 up until 2000 larvae.
Comparatively intensively, minute larvae of Sciaridae [Bibionomorpha], also living gregariously, process woodland litter transforming it into humus. The number of larvae in the litter in certain plots in the Kadnikovski woodland of the Vologodski region reached 100 individuals pro 1 m2. As to the significance of the larvae of this family in the process of the formation of humus was also pointed to by several other authors. The larvae of Neosciara modesta STAEG. are capable of processing leaves of different sorts of trees and bushes but prefer leaves of elder, spindle tree, and elm. The larvae coming forth from 20 pairs of midges were, in the course of three weeks, able to process 30 g leaves as to their dry weight.
In large numbers one encounters the saprophagous larvae of the subgenus Vestiplex BEZZI [Tipulomorpha-Tipulidae] in the fall-out in forests. In the dense forests of the Kursk nature reserve their abundance during the summer months reaches 3.8 thousand [in the original tekst the latter word is absent] individuals pro 1 m2.
In simular conditions in leaf-containing composts one encounters in large numbers larvae of certain species of Scatopse GEOFFR. [Bibionomorpha-Scatopsidae] -- up until 14.7 thousand individuals pro 1 m2 layer of 10 cm thickness, which play an important role in the disintegration of vegetable remains.
Actively process woodland litter the larvae of certain species of the genus Limonia MEIG [Tipulomorpha-Limoniidae], for example L. nubeculosa MEIG., and L. macrostigma SCHUMM., which feed on freshly fallen leaves of alder, hazel, and ash.
Interesting observations have been made of the larvae of some species of Rhagionidae [Asilomorpha], which feed on fallen leaves in spite of the fact that the larvae of the majority of the representatives of this family are carnivorous.
For processing the fall-out by various insects not only its content has significance, but also the stage of disintegration of the leaves.
Among dipterous larvae an important place is claimed by that group of which the members feed on strongly disintegrated, but still possessing its structure, bark and wood -- saproxylophagous insects. Typical representatives of this group are larvae of Tipulidae [Tipulomorpha], Limoniidae [Tipulomorpha], Bibionidae [Bibionomorpha], Hesperinidae [Bibionomorpha], Pachyneuridae [Tipulomorpha], Ditomyiidae [Bibionomorpha], Sciaridae [Bibionomorpha], and others.
In pale, relatively coarse and damp wood of leaf-carrying tree sorts, large semi-transparent larvae of Phoroctenia vittata MEIG. [Tipulomorpha-almostcertain-Tipulidae] and Ctenophora tricolor LOEW. make tunnels.
In coarse dark wood live the larvae of Symmerus annulatus MEIG. [Bibionomorpha-Ditomyiidae] and Epiphragma ocellaris L. [Tipulomorpha-Limoniidae].
In totally soft wood, in wood rot of hollows [in wood] or of tree-stumps develop larvae of Dictenidia bimaculata L. [Tipulomorpha-Tipulidae]. In damp dark-brown pine wood develop larvae of Sciaridae [Bibionomorpha] : Trichosia sp. (in wood of fir-tree) and Scythropochroa quercicola WINN. (in wood of silver fir). In pieces of damp dark wood or in tree-stumps live larvae of Pachyneura sp. [Tipulomorpha-Pachyneuridae].
The larvae of the majority of the considered groups live a solitary life. In large aggregations live the larvae of Hesperinus WALK. [Bibionomorpha-Hesperinidae] penetrating by, circular in cross section, tunnels pieces of rather solid and hard wood.
Also living gregariously in wood are larvae of certain species of Bibionidae [Bibionomorpha], for example Bibio marci L., B. venosus MEIG., Dilophus febrilis LOEW., Pleciidae (Plecia nigra LUND.).
In some cases in logs a great abundance of larvae of Pachyneura sp. [Tipulomorpha-Pachyneuridae] is observed.
A comparatively large group of larvae, for their development connected with wood, are not genuine xylophags [consumers of wood] but feed on a bacterial flora and on fungi that develop in it. Most interesting in this regard are the ambrosian xylomycetophags [larvae that feed on fungi that have invested wood]. The adults or larvae of the insects of this subgroup in the typical case build tunnelings in wood, free from wood rot, on the walls of which mycelium of fungi grows. Their spores usually are carried along by the female and the mycelium serves as the main food source for the larvae. This phenomenon is widely distributed among insects, especially many beetles grow fungi on the walls of the tunnelings and also the larvae of certain Diptera.
Strongly damp wood, such as tree trunks lying on the ground near various brooks and sources, is colonized by larvae of Temnostoma LEPEL. et SERV. [Myiomorpha-Syrphidae] and larvae of Axymyiidae [Bibionomorpha].
The larvae themselves build the tunnelings and empty them of wood rot.
Certain larvae, for example Brachyopa MEIG. [Myiomorpha-Syrphidae] utilize old tunnelings of other ambrosian insects, settling themselves, for example, in tunnelings of Lymexylonidae (wood-boring beetles).
To ambrosian xylomycetophags [living in wood but feeding on fungi investing it] one should also reckon those larvae of Diptera that live in fermenting sap flowing from wounded trees and freshly cut tree-stumps. As regards feeding they differ little from typical representatives of this subgroup but do not live in tunnelings but directly in the fermenting liquid. The species-composition of Diptera living in such sap-producing wood is very diverse. Numerously present are usually the long snake-like larvae of Anisopodidae [ = Rhyphidae] [Bibionomorpha], Mycetobiidae [Bibionomorpha] and Ceratopogonidae [Tipulomorpha], further, the larvae of Libnotes undulata MATS. [Tipulomorpha-Limoniidae], and some larvae of Syrphidae [Myiomorpha].
The larvae of the majority of the discussed groups of Diptera actively grind rotting vegetable residues, transforming them into strongly reduced -- with respect to size -- [particles of] material, that has lost its original structure, and that is now subjected to a more intensive bacterial action [I assume that this action is not meant to be that of symbiotic bacteria living in the larva, but that the mentioned bacterial action is meant to be an action on what had resulted from the larva's feeding activities such as spillings and what had been excreted by it]. No less significant for the intensification of bacterial processes of disintegration are the mining larvae. For example, larvae of Sapromyza basalis ZETT. [Myiomorpha-Lauxaniidae ( = Sapromyzidae) ] mine fallen leaves that have endured the winter, and leaving them before pupation ["Mining leaves" means that the larva lives inside leaves eating through them while staying between the upper and lower leaf skin, and in this way forming 'mines'.]. The mining of leaves contributes to a rapid entry of different bacteria into the leaf.
Many larvae of Diptera feed on various rotting aquatic plants, on spores of plants, as do for instance the larvae of Culicoides LATR. [Tipulomorpha-Ceratopogonidae]. In the same way larvae of Chironomidae [Tipulomorpha] feed. Various particles of rotting plants, various aquatic growths, and so on, are eaten by larvae of Culicidae [Tipulomorpha] that live in water-basins. These groups affect the speed of disintegration of vegetable residues only indirectly. They slacken or quicken certain microbiological processes.
P h y t o p h a g o u s larvae
The second nutritive regime as it occurs among dipterous larvae consists in feeding on living higher (green) plants [feeding on living fungi is yet another nutritive regime observable in Dipterous larvae which will be discussed farther below].
The existing phytophagous larvae are very diverse with respect to the way of utilizing the substrate and the way of feeding.
The larvae of Tipula paludosa MEIG. and Tipula oleracea L. [Tipulomorpha-Tipulidae], the majority of the species of the genus Nephrotoma MEIG. [Tipulomorpha-Tipulidae], and some Bibionidae [Bibionomorpha], gnaw the roots of plants and in some cases cause significant damage. Thus, in coastal regions of Latvia the quantity of Tipula paludosa MEIG. and Tipula oleracea L. sometimes reached 300-400 individuals pro 1 m2, while as alarming is already reckoned a number of 30-50 individuals pro 1 m2. The density of larvae of Dilophus femoratus MEIG. [Bibionomorpha-Bibionidae], which gnaw roots of plants, in open meadow plots in the Archangel region reached 50-100 individuals pro 1 m2. The large larvae of Bibio hortulanus L. are widely known as damaging of sugar turnips and potatoes. Sometimes the damage caused by them is very significant -- up until 90 percent of damaged tubers, because in several cases the larvae are encountered in the fields in enormous numbers.
There exists a whole group of specialized phytophagous larvae which can be considered to be parasites of plants. Penetrating into the tissue of stems and leaves they mine them while they develop within the plants usually in the course of the whole vegetational period. Pupation takes place, as a rule, within the dead stems and leaves. Larvae of Chloropidae [Myiomorpha] and Opomyzidae [Myiomorpha] [in Rhodendorf 1964, p. 108 : Opomyzinae, a subfamily of the family Anthomyzidae] mine mainly stems of grasses. The larvae of Agromyzidae [Myiomorpha] develop in leaves. Larvae of Ephydridae [Myiomorpha] mine stems of various aquatic plants.
M y c e t o p h a g o u s larvae
The group of larvae representing the third type of nutritive regime includes larvae that feed on fungi. This group evermore grows in correspondence with the accumulation of known facts principally at the expense of larvae which were considered to be saprophags. Such are the majority of the larvae of gall midges (Cecidomyiidae) which are until now placed by many authors in the category of saprophagous larvae. In fact they are mycetophagous larvae feeding on threads of fungi. The larvae of certain species of Sciara MEIG., which develop in wood invested with dry rot, feed on fungi of this rot, and not on wood.
Mycetophagous larvae are principally connected with the mycelium of fungi which develop on wood. On the surface of various fallen tree trunks, on parts covered with mycelium, larvae of many species of Platypezidae [Myiomorpha] live. The flat larvae, living gregariously with 10-20 individuals as gray oval spots are well visible against the background of snow-white mold which covers the surface of logs, of various branches, laying on the forest floor. The larvae quickly devour the mycelium and thus destroying the snow-white spots of mold.
In similar conditions develop larvae of Cecidomyiidae [Gall midges] [Bibionomorpha] : Peromyia monilis MAM., Dichaetia pusilla MAM. (on logs of lime tree), Acoenonia europaea MAM. (on beech). On mycelium, which overgrows thin pieces of alder lying on the forest floor, develop Brachyneura fungicola MAM. [still] (Cedidomyiidae). In the thin film of mycelium under bark on tree-stumps and logs of pine trees develop larvae of Aprionus similis MAM., Winnertzia nigripennis KIEFF., Camptomyia maxima MAM., and in tree-stumps and logs of leaf-bearing trees develop larvae of Micropteromyia ghilarovi MAM., Karschomyia aceris MAM., and larvae of other species of gall midges.
A peculiar ecological group form the larvae that develop in the tubular hymenophores of tree-fungi. On the surface of such fungi from the underside often under a thin transparent film develop large greyish larvae of Ceroplatidae [Bibionomorpha] looking like slugs.
In the body of fungi large semi-transparent larvae of Metalimnobia quadrimaculata L. [Tipulomorpha-Limoniidae] are common. Often one encounters in such conditions the larvae of some species of gall midges [Bibionomorpha-Cecidomyiidae]. In the fruiting bodies of Polystictus versicolor, which [fungi] develop on birch, one may encounter the peculiar larvae of Ditomyia fasciata MEIG. [Bibionomorpha-Ditomyiidae], and Platypeza infumata HAL. [Myiomorpha-Platypezidae].
Larvae of Diptera form the main group among the invertebrates inhabiting fruiting bodies of edible mushrooms [ edible, I assume, not particularly for man, but [also] for certain animals] (Order Agaricales). Milky-white larvae of Mycetophilidae [ = Fungivoridae] [Bibionomorpha], minute oval larvae of Drosophilidae [Myiomorpha], yellowish larvae of Sphaeroceridae [ = Borboridae] [Myiomorpha], develop in masses in the caps (including the hymenophor) as well as in the stem of the fruiting bodies. In non-edible mushrooms or mushrooms with a solid fruiting body larvae usually are absent.
C o p r o p h a g o u s larvae
Larvae connected with the fourth nutritive regime -- the coprophags -- feed on animal (whether vertebrate or invertebrate) excrements.
N e c r o p h a g o u s larvae
Larvae connected with the fifth nutritive regime -- the necrophags -- feed on dead animals.
Concerning Coprophagia and Necrophagia
As distinct independent categories of nutritive regimes coprophagia and necrophagia are listed in KRIVOSJEINA 1969, p. 26 and 28.
Myself I [JB] think that, at least in insects, and at least under natural conditions, coprophagia and necrophagia are not independent categories of nutritive regimes. In both coprophagia and necrophagia decaying organic matter is fed upon. They stand out only because this decaying matter originates from animals, that is, not from plants. So both categories should be better subsumed under saprophagia sensu lato. They are, so to say, extensions of the basic habit of feeding on decaying vegetable matter (saprophagia sensu stricto) to feeding on decaying animal matter. Vegetable matter, it is true, differs from animal matter, but, although there still remains a difference, decaying vegetable and animal matter are becoming more similar as the process of disintegration progresses. And ecologically, with respect to finding them, excrements, dead animals, and rotting vegetable matter (such as dead trees), are similar.
Z o o p h a g o u s larvae (carnivores and internal parasites)
The last type of nutritive regime is shown by larvae that feed on living animals. For dipterous larvae this means that they either hunt and devour other invertebrate animals, mainly insect larvae, or that they parasitize them, that is, being internal parasites. The difference from feeding on, and parasitizing in, living plants lies, of course, in the biochemical nature of the food (because in both cases it is fresh), calling for a different feeding apparatus and a different digestion. Also ecologically they differ : finding the right food plant, and hunting or parasitizing the right animal, demand different adaptations (morphological and behavioral).
Clearly expressed carnivores are the larvae of the majority of families of the Brachycera Orthorrapha [largely belonging to the infraorder Asilomorpha, such as horse-flies, robber-flies, and the like, that is, true flies, but not the 'truest' flies (such as fruitflies or the house-fly and its allies)], among which many or all species live at the expense of different invertebrates.
Larvae of Rhagionidae [Asilomorpha] feed mainly on earthworms, the larvae of Tabanidae [Asilomorpha] and Therevidae [Asilomorpha] feed mainly on larvae of insects. Actively attacking earthworms are larvae of Rhagio tringarius L. [Asilomorpha-Rhagionidae].
Not less energetic behave larvae of Therevidae [Asilomorpha], which have their habitat in soils of light mechanical content or in wood rot. Very agile, they easily move in the soil and change places in order to find food over significant distances. Such a way of life is also characteristic of larvae of Tabanidae [Asilomorpha].
The larvae of Empididae [Asilomorpha] are in their appearance and behavior less agressive, but that does not mean that they do not hunt various invertebrates, especially earthworms. In the soil one can often encounter larvae, of which the intestine is filled with blood of these invertebrates.
Under bark, in burrows of bark-eaters one can almost always encounter larvae of Medetera FISCH. [Asilomorpha-Dolichopodidae], Lonchaea FALL. [Myiomorpha-Lonchaeidae]. Larvae of Medetera signaticornis LOEW., Medetera stackelbergi PAR. and others, very actively and eagerly feed on living larvae of bark-eaters or kill larvae of Sciaridae [Bibionomorpha]. It is reported that sometimes these larvae also attack young beetles.
In tree-stumps or logs, where larvae of long-horned beetles develop, one can almost always encounter larvae of Laphria MEIG. [Asilomorpha-Asilidae]. The large yellowish larvae with neatly expressed bumps that go around the segments easily move throug tunnelings looking for larvae of long-horned beetles.
In laboratory conditions they accept as food larvae of many other insects [meaning that they are, at least physiologically, not specialized in eating larvae of long-horned beetles].
To the category of virtually omnivorous carnivores belong the larvae of Xylophagus MEIG. [Asilomorpha-Xylophagidae].
They eagerly eat larvae of bark-eaters, namely of long-horned beetles, of crane-flies, of gall midges, of fungus gnats, and even larvae of click beetles which possess a solid integument. Very rarely, but nevertheless recorded, are cases of feeding on earthworms and cases of cannibalism. The larvae pierce the integument of the prey, discharge digestive saps into the body, and then suck the content such that only an almost empty skin is left. The larvae of some species, for example Xylophagus cinctus de GEER. [Asilomorpha-Xylophagidae] are able to feed on remains of dead insects.
Carnivorous larvae among Nematocera [midges, mosquitoes, crane-flies, black-flies, etc.] is a rare phenomenon. Carnivorous Cecidomyiidae [Bibionomorpha] are comparatively little agile, and as a result of their minute sizes cannot pierce integuments of insects which are solid. Therefore, naturally the adaptation was to feeding on little agile invertebrates living in colonies and with delicate integuments (such as aphids and ticks). There is a whole series of species, for example of the genus Lestodiplosis KIEFF. [Bibionomorpha-Cecidomyiidae] which develop at the expense of larvae of other species of gall midges. Carnivorous are further some larvae of Bezzia KIEFF. [Tipulomorpha-Ceratopogonidae], Ceroplatidae [Bibionomorpha], and Macroceridae [Bibionomorpha].
Among larvae of Diptera-Cyclorrapha [the 'truest' of flies] genuine carnivores are rarely observed. Such are larvae of Sciomyzidae [Myiomorpha], known as predators of gastropods. Larvae of various insects are eaten by larvae of Scatophaga stercoraria L. [Myiomorpha-Cordyluridae] [Cordyluridae = Cordiluridae = Scatophagidae] which live in various rotting organic remains. In the majority of cases larvae, for example of Phaonia goberti MIK. and other representatives of the family Muscidae [Myiomorpha], many Calliphoridae [Myiomorpha], and Sarcophagidae [Myiomorpha], turn out to be at the same time carnivorous, coprophagous, and necrophagous.
Larvae of Lonchaea scutellaris RIED., L. zetterstedti BECK. [Myiomorpha-Lonchaeidae] are able to feed on living larvae, but they do this not so eagerly than in cases where they feed on corpses [of insects]. The larvae have a hard time to tear open the integument of the prey feeding not only on the liquid content but also on pieces of tissue. The larvae of Lonchaea carticis TAYLOR are able to develop while they only feed on various remains in the tunnelings of Pissodes larvae [beetles]. It is clear that for many larvae of this family [Lonchaeidae], to which point observations of a series of authors, carnivorous behavior is not obligatory. Larvae of Palloptera usta MEIG. [Myiomorpha-Pallopteridae], which are related to this group, mainly feed on corpses of larvae and beetles, and rarely prey upon other animals, in these cases devouring minute larvae of bark-eaters. [ The Pallopteridae are in ROHDENDORF, 1964, considered to be a subfamily (Pallopterinae) of the family Lonchaeidae, itself belonging to the superfamily Sapromyzidea ( = Lauxanioidea) ].
A special form of zoophagia can be considered internal parasitism, and also is the habit of certain dipterous larvae. Even [at least] a whole family, the Tachinidae, is such that their larvae live as internal parasites in other insects, mainly caterpillars. It is clear that such endoparasitic groups (but the same can be said of ectoparasites which also occur in Diptera) as such represent a special ecological type, comparable to that of the parasitic wasps (Hymenoptera). In KRIVOSJEINA, 1969, little attention is given to parasitic Diptera (the scope of the book is evidently already broad enough!). For the family Tachinidae a lot is expounded in HERTING, B., 1960, Biologie der westpaläarktischen Raupenfliegen, Dipt., Tachinidae.
Let us continue with KRIVOSJEINA, 1969, p. 30.
Larvae of parasitic Diptera as a rule parasitize various invertebrates. The host, which is infested by the parasite, keeps on developing. It does not show features of strain. Pupation and flying-off of the parasites usually coincide with the conclusion of the infested larva's development. The parasitic larvae find themselves in the body of the host during the whole period of the parasite's development, and leave it before pupation. Only in some cases, where massive infestation has taken place, for instance in the infestation of earthworms by larvae of Onesia ROB.-DESV. [Myiomorpha-Calliphoridae], the latter may migrate out of one worm into another. This happens in the case of death of the earthworms which were the initial hosts.
There are larvae of Diptera, Pipunculidae [Myiomorpha], that develop in cicadas, Conopidae [Myiomorpha] in bumblebees, wasps, and bees, Pyrgotidae [Myiomorpha] in the body of scarabeid beetles, Sarcophagidae [Myiomorpha] in the body of earthworms, and Calliphoridae and Tachinidae in isopods, earthworms, and in various insects.
Insofar as earthworms are known to actively process the soil, the question concerning larvae as parasites of worms constantly drew the attention of investigators. Thus, the larva of Pollenia rudis FABR. [Myiomorpha-Calliphoridae], develops in the body of the worm from its first to last instar. Presently P. rudis FABR. is recorded as parasite of Allolobophora chlorotica SAV., Eisenia rosea SAV., Lumbricus terrestris L., A. caliginosa SAV. Also parasitizing earthworms are Sarcophaga carnaria L. [Myiomorpha-Sarcophagidae], Onesia ROB.-DESV. [Myiomorpha-Calliphoridae].
After having given this list of nutritive regimes as they are encountered in larvae of Diptera, KRIVOSJEINA (p. 31) concludes with some general observations.
The listed trophic groups [categories] do not cover all diversity of the nutritive connections that are observed in Diptera. Much remains unclear. The biology of a series of groups is still totally unknown. With respect to the ecology of certain individual groups there does not as yet exist one definite opinion. Some reckon, for instance, the soil-dwelling larvae of Asilidae [Asilomorpha] as being phytophagous, others as predators. The larvae of Stratiomyidae [Asilomorpha] are considered to be predators by some authors. Unclear is the nature of feeding of many larvae of Empididae [Asilomorpha] and others.
Resolving the question concerning the nature of feeding of this or that group is often hampered by the fact that nutritive connections usually do not present themselves in such a pure way that one can, without errors, determine their category. The larvae of many species of Stratiomyidae [Asilomorpha] develop under bark (for example Pachygaster minutissima ZETT., P. orbitalis WAHLB., and others), are mainly saprophagous, they feed on various minute particles of wood, [namely] wood rot. But it can often be observed that they concentrate around dead bodies of various insects and eat them. The same can be observed also in the aggregations of Solva WALK. [Asilomorpha-Stratiomyidae], in many Lonchaeidae [Myiomorpha] and Pallopteridae [Myiomorpha, see ABOVE ]. In laboratory conditions the larvae-xylophags of Tanyptera LATR. [Tipulomorpha-Tipulidae] and Ctenophora MEIG. [Tipulomorpha-Tipulidae] pupate sooner when they are offered as food not only pure wood but also protein-containing food in the form of dead bodies of insects. The same phenomena can be observed not only in Diptera. Among the click beetles Corymbites LATR. and Selatosomus STEPH. there is a group of omnivores with strongly expressed phytophagia of the larvae, while for a normal development of the latter food originating from animals is also required.
Sometimes, in cases of the absence of appropriate objects [to feed on], cannibalism is observed, which is intrinsic to larvae of many species of Stratiomyidae [Asilomorpha], Rhagionidae [Asilomorpha], Xylophagidae [Asilomorpha], Lonchaeidae [Myiomorpha], and others. It is possible that such behavior is clarified by the fact that it is necessary for larvae developing in a meagrely nutritive substrate for their final development to obtain some quantity of protein-containing food.
The dual character of feeding is observed in many saprophags. In some cases typical saprophags are, in conditions of insufficient wetness, able to feed on living tissues of plants (for example Bibio hortulanus L.). Similar phenomena are characteristic to many groups of insects.
There exists a definite dependency between the systematical position of [any] given Diptera and the way of feeding of their larvae. The larvae of the majority of families of Nematocera [midges, gnats, mosquitoes, crane-flies, etc] are saprophagous. Cases of being carnivorous are rare (some species of Cecidomyiidae [Bibionomorpha], Ceratopogonidae [Tipulomorpha], Limoniidae [Tipulomorpha] ). A comparatively small group form the phytophagous Diptera larvae, namely some Dilophus MEIG. [Bibionomorpha-Bibionidae], Tipula L. [Tipulomorpha-Tipulidae], Nephrotoma MEIG. [Tipulomorpha-Tipulidae], and many species of Cecidomyiidae [Bibionomorpha].
Very compact regarding its nutritive connections is the group of Brachycera-Orthorrapha [horse-flies, robberflies, and the like]. The larvae of the majority of families are clearly expressed zoophags (predators or parasites). Literally unique are the saprophags among them (larvae of some species of Stratiomyidae [Asilomorpha], Tabanidae [Asilomorpha], and Rhagionidae [Asilomorpha].
The larvae of Cyclorrapha [hover-flies, fruit flies, house-flies, bluebottles, and the like] are connected with diverse habitats, but coprophags and zoophags predominate. Phytophags are a little less represented among them, and only an insignificant part of them are saprophags.
With all this we conclude the part concerning feeding habits and habitats of dipterous larvae, as it is taken from KRIVOSJEINA, 1969, p. 14-32. We will use this account when separately considering dipterous families. The Diptera Nematocera are in this respect the most diverse and will offer much to understand the evolutionary process as driven by the colonization of new habitats and by the occupation of new ecological niches in an existing or new habitat.
Of the Nematocera the infraorder Bibionomorpha is very promising in this respect because of the presence of the very diverse and special family of gall midges (Cecidomiidae) and of a number of relict families. But the infraorder Tipulomorpha is also interesting, especially as a result of the presence of the extensive and diverse family Limoniidae which shows many evolutionary 'excursions' to most diverse larval habitats, and of the presence of the very ancient family Tanyderidae.
The habitats of the Bibionomorpha
From the above survey we will now single out the habitats of the representatives of the Bibionomorpha :
A significant complex of Diptera is for individual development connected with disintegrating wood and the materials coming forth from it. Members of this complex can be encountered within such wood as well as under its bark, in mouldered wood [present] in hollows, or in a fermenting sap. To this complex among Bibionomorpha belong the larvae of Hyperoscelididae, Axymyiidae, Pachyneuridae, Hesperinidae, many Bibionidae, Sciaridae [= Lycoriidae].
With rotting wood are connected different fungi, where fruiting bodies and films of mold harbour their characteristic complex of Diptera. Among Bibionomorpha these are : Mycetophilidae [ = Fungivoridae] and related families.
Diverse is the content of the inhabitants of forest-litter [podstilka]. Here develop some Bibionomorpha : Bibionidae, Cecidomyiidae [ = Itonididae].
A large group of larvae lives in tissues of living plants. Among Bibionomorpha these are : Cecidomyiidae [= Itonididae].
There exists a whole complex of Diptera that are inhabitants of different rotting vegetable and animal residues, especially excrements. Among Bibionomorpha these are representatives of the family Scatopsidae.
Many Diptera are for their individual development connected with various liquid environments : principally with water, but also with sap which flows from wounds in trees, and with liquefied disintegrating fungi. In sap flowing from damaged spots on trees we see developing many larvae, among which the Bibionomomorpha are represented by Anisopodidae [ = Rhyphidae].
Some species of Diptera are able to develop in drippings of resin on tree trunks. Thus, on pine-trees among representatives of Bibionomrpha we see Cecidomyia pini de GEER [Cecidomyiidae], on spruce representatives of Stelechodiplosis magna MöHN [Cecidomyiidae] develop. On silver fir we see developing Wichmaniella crassa MöHN [Cecidomyiidae].
Whatever, even insignificant, accumulations of water, for example in depressions on roads, imprints of animal ungulae [hoofs], in hollows, also become a habitat of mainly minute forms with a short summer developmental cycle. Among the Bibionomorpha we can expect here : Mycetobiidae. The larvae of certain Cecidomyiidae develop even in micro-water-basins in axils of leaves. With vaginas of leaves are connected larvae of Dicerura scirpicola KIEFF. [Cecidomyiidae] and D. kaltenbachi RüBS.
The group of (vegetable) scavengers (saprophags) includes a very wide range of organisms that feed on different decaying remains of higher plants and fungi, and rotting wood [Animals that feed on decaying animal remains are also scavengers, which we subsume, however, under the nutritive regime of necrophagous animals].
Typical saprophagous insects among the Bibionomorpha are the larvae of the majority of species of the genera Bibio GEOFFR. [Bibionidae], Sciara MEIG. [Sciaridae], Scatopse GEOFFR. [Scatopsidae].
Among dipterous larvae an important place is claimed by that group of which the members feed on strongly disintegrated, but still possessing its structure, bark and wood -- saproxylophagous insects. Among the Bibionomorpha typical representatives of this group are larvae of Bibionidae, Hesperinidae, Pachyneuridae, Ditomyiidae, and Sciaridae.
In coarse dark wood live the larvae of Symmerus annulatus MEIG. [Ditomyiidae].
In damp dark-brown pine wood develop larvae of Sciaridae : Trichosia sp. (in wood of fir-tree) and Scythropochroa quercicola WINN. (in wood of silver fir). In pieces of damp dark wood or in tree-stumps live larvae of Pachyneura sp. [Pachyneuridae].
The larvae of the majority of the considered groups live a solitary life. But some do not. For Bibionomorpha we can say the following : In large aggregations live the larvae of Hesperinus WALK. [Hesperinidae] penetrating by, circular in cross section, tunnels pieces of rather solid and hard wood.
Also living gregariously in wood are larvae of certain species of Bibionidae, for example Bibio marci L., B. venosus MEIG., Dilophus febrilis LOEW., and of Pleciidae (Plecia nigra LUND.). In some cases in logs a great abundance of larvae of Pachyneura sp. [Pachyneuridae] is observed.
A comparatively large group of larvae, for their development connected with wood, are not genuine xylophags [consumers of wood] but feed on a bacterial flora and on fungi that develop in it. Most interesting in this regard are the ambrosian xylomycetophags [larvae that feed on fungi that have invested wood]. The adults or larvae of the insects of this subgroup in the typical case build tunnelings in wood, free from wood rot, on the walls of which mycelium of fungi grows. Their spores usually are carried along by the female and the mycelium serves as the main food source for the larvae. This phenomenon is widely distributed among insects, especially many beetles grow fungi on the walls of the tunnelings and also the larvae of certain Diptera. As for Bibionomorpha : Strongly damp wood, such as tree trunks lying on the ground near various brooks and sources, is colonized by larvae of Axymyiidae.
To ambrosian xylomycetophags [living in wood but feeding on fungi investing it] one should also reckon those larvae of Diptera that live in fermenting sap flowing from wounded trees and freshly cut tree-stumps. As regards feeding they differ little from typical representatives of this subgroup but do not live in tunnelings but directly in the fermenting liquid. The species-composition of Diptera living in such sap-producing wood is very diverse. Among Bibionomorpha : Numerously present are usually the long snake-like larvae of Anisopodidae [ = Rhyphidae] and Mycetobiidae.
The Bibionomorpha are, as it seems, not so strongly connected with living plants : The larvae of some Bibionidae gnaw the roots of plants and in some cases cause significant damage, such as those of Dilophus femoratus MEIG. [Bibionidae]. The large larvae of Bibio hortulanus L. are widely known as damaging of sugar turnips and potatoes.
M y c e t o p h a g o u s larvae
The group of larvae representing this type of nutritive regime includes larvae that feed on fungi. Here we only consider representatives of the Bibionomorpha. The ecologically defined group -- the mycetophags -- evermore grows in correspondence with the accumulation of known facts principally at the expense of larvae which were considered to be saprophags. Such are the majority of the larvae of gall midges (Cecidomyiidae) which are until now placed by many authors in the category of saprophagous larvae. In fact they are mycetophagous larvae feeding on threads of fungi. The larvae of certain species of Sciara MEIG., which develop in wood invested with dry rot, feed on fungi of this rot, and not on wood.
Mycetophagous larvae are principally connected with the mycelium of fungi which develop on wood.
In similar conditions develop larvae of Cecidomyiidae [Gall midges] : Peromyia monilis MAM., Dichaetia pusilla MAM. (on logs of lime tree), Acoenonia europaea MAM. (on beech). On mycelium, which overgrows thin pieces of alder lying on the forest floor, develop Brachyneura fungicola MAM. [still] (Cedidomyiidae). In the thin film of mycelium under bark on tree-stumps and logs of pine trees develop larvae of Aprionus similis MAM., Winnertzia nigripennis KIEFF., Camptomyia maxima MAM., and in tree-stumps and logs of leaf-bearing trees develop larvae of Micropteromyia ghilarovi MAM., Karschomyia aceris MAM., and larvae of other species of gall midges.
A peculiar ecological group form the larvae that develop in the tubular hymenophores of tree-fungi. We mention only Bibionomorpha. On the surface of such fungi from the underside often under a thin transparent film develop large greyish larvae of Ceroplatidae looking like slugs.
Often one encounters in (the body of) fungi the larvae of some species of gall midges [Cecidomyiidae]. In the fruiting bodies of Polystictus versicolor, which [fungi] develop on birch, one may encounter the peculiar larvae of Ditomyia fasciata MEIG. [Ditomyiidae].
Larvae of Diptera form the main group among the invertebrates inhabiting fruiting bodies of edible mushrooms [ edible, I assume, not particularly for man, but [also] for certain animals] (Order Agaricales). We mention Bibionomorpha. Milky-white larvae of Mycetophilidae [ = Fungivoridae] develop in masses in the caps (including the hymenophor) as well as in the stem of the fruiting bodies. In non-edible mushrooms or mushrooms with a solid fruiting body larvae usually are absent.
Carnivorous larvae among Nematocera [midges, mosquitoes, crane-flies, black-flies, etc.] is a rare phenomenon. We mention only Bibionomorpha. Carnivorous Cecidomyiidae are comparatively little agile, and as a result of their minute sizes cannot pierce integuments of insects which are solid. Therefore, naturally the adaptation was to feeding on little agile invertebrates living in colonies and with delicate integuments (such as aphids and ticks). There is a whole series of species, for example of the genus Lestodiplosis KIEFF. [Cecidomyiidae] which develop at the expense of larvae of other species of gall midges. Carnivorous are further some larvae of Ceroplatidae and Macroceridae.
General remarks.
As for the Bibionomorpha, as seen within the Nematocera, and compared with other groups of the Nematocera, we can say that they are the 'terrestrial part', the land-midges, of the Nematocera, contrasting in this respect with many Tipulomorpha, especially with Culicidae, Chironomidae, Simuliidae, and other Nematocera.
Wing-venation and habitat of Bibionomorpha
As can be seen above, within Bibionomorpha the ecological niches are diverse but, broadly speaking, the habitats of the larvae are more or less similar. And because the adults are not predators in the sense of hunting their prey on the wing [as in Asilids, and thus, as such predators, being around in a more or less large area -- the area where their prey-insects live], and the larvae not aquatic, the adults are expected to be closely connected with the larval habitats, that is, they will 'hang about' these same habitats. They emerge there and will lay their eggs there.
Earlier we had considered the wing-venation of Diptera to be some sort of adaptive feature : The insect experiences its habitat by its smell and by vision, and it is natural to suppose that the way of flying of it must be part of experiencing this habitat, that is, the way of flying -- the flight-regime -- must be compatible with the insect experiencing its habitat by its smell and vision. So it may be that different habitats of the adult flies are, among other things, reflected in their correspondingly different flight-regimes. And if it is true that, among other features, the wing-venation reflects the flight-regime, that is, a particular wing-venation realizes a particular flight-regime, then we expect different patterns of wing-venation in Diptera of which the adults hang about in different habitats.
We now think, however, that this is only very generally true : Often different diptera are connected with the same habitat (as can be seen in the above summary of habitats of dipteran larvae [and, consequently of habitats of the adults] ). These diptera include groups with a wing-venation as different from that of the bibionomorphs (having more or less short wings with a relatively poor venation) as it is in Tipulidae and Limoniidae (both having long petiolated wings with a rich venation) for instance. Indeed, some tipuloids have the same habitats as bibionoids generally have (See above, in the summary of dipteran habitats ). Nevertheless tipuloids (that is, mainly Tipulidae and Limoniidae) generally have habitats different from bibionoids and may experience these different habitats differently, co-expressed in their different flight-regimes and thus in their different type of wing-venation. But this cannot be said of the Bibionomorpha taken alone (and perhaps also not of the tipuloids taken alone). As has been said, the habitats of the bibionoid flies are largely identical (with only a number of exceptions among the Cecidomyiidae [gall-midges] ), and the flight-regimes might then be identical too. The latter is also suggested by the overall similarity of wing-venation in Bibionomorpha. So apart from some more or less general features of the wing-venation (and other wing-structures) that are undoubtedly functional, that is, have functional, and therefore adaptive, significance in the tipuloids as well as in the bibionoids, the differences of wing-venation that exist within the Bibionomorpha (to stick with these), must have a f o r m a l origin, instead of an ecologically (adaptive) one. Said in another way : That particular basic general plan of the wing-venation, commonly possessed by all Bibionomorpha, makes possible a flight-regime that is compatible with ('flying around in') the general type of habitat characteristic of virtually all bibionomorphs. But all the many modifications of this basic general plan of wing-venation, modifications that exist among the representatives of the Bibionomorpha, must be considered as being formal changes, changes that is, that have no adaptive significance.
We can say that in the tipuloid families Tipulidae, Limoniidae, and some others, the elongated and petiolated wings with the main venational elements situated in the apical half of the wing are realizing a particular type of flight-regime, and thus these wings, including their venation, are adaptive in character. They are adapted to the way the habitat is, and should be, experienced by the tipuloid fly. But within the group of these tipuloids the differences of wing-venation cannot be considered to be adaptive anymore, they are formal differences. As we have seen, also within the group of bibionoid flies (the whole of the Bibionomorpha) we have to do with a functional aspect present in the wings and their venation, but here, the also existing non-functional aspect is much more pronounced than it is in the tipuloids. And non-functional, but nevertheless constant, features we call formal features. And such features are part of the family-groundplan which, as all groundplans, is also not functional but formal, which means that its origin and subsequent transformations must lie, not in the Explicate, but in the Implicate Order.
Of course, an organic groundplan is not completely devoid of functionality, otherwise it would not be an organic groundplan. Its structure must meet all basic requirements of Life, which here means that it cannot contain structures or qualities that are totally incompatible with Life as such.
In its generality all this was expounded in the foregoing series of documents, especially in parts XXV-A, XXV-B, and XXV-C.
In all this we must realize that the distinction we make between (1) the non-adaptive groundplan of an animal (in Diptera supposedly co-expressed by all non-functional aspects of their wing-venation) and (2) the adaptive structures that are added to that grounplan, is highly speculative. So it is far from certain that the wing-venation in Diptera, supposedly co-expressing the family-groundplan, is largely formal in character. And so also in the group of Bibionomorpha we cannot be completely certain that the differences of wing-venation between its many families reflect a purely formal evolution (as contrasted with an adaptive or functional evolution). In contrast to this, we can be sure that most of the structure of the larvae of Diptera is functional and adaptive. Only a few, if any, larval characters reflect the groundplan of the family. The same is true of the details of the antennae and mouthparts of the adult.
So in the absence of certainty, let us merely s u p p o s e that the differences of wing-venation between the different families of (at least) the Bibionomorpha are the result of a purely formal evolution. If so, they reflect the differences of the respective family-groundplans as they exist in the Bibionomorpha.
Phylogenetic systematics, in the sense of HENNIG, is in fact largely formal because it does not consider explicitly ecology and adaptation as factors of evolution. It just investigates character-transformations, that is, the derivation of character-states from more primitive states of these same characters. It assumes that these transformations have actually taken place in the evolutionary history of the group that is studied by it, without considering the possible functional significance, if there is any, of them. So phylogenetic systematics does not decide that a given, formally possible, transformation of a character must actually have happened because it is part of some functional improvement of the organism, for example representing an improved adaptation to the organism's environment. The possibility of formally deriving one character-state from another is sufficient for assuming the transformation to have actually taken place in the history of the group, provided it is not too big a step, and provided that other features in the organisms of that group do not contradict it. Further, phylogenetic systematics, although acknowledging the possibility that some given character-transformation has taken place independently several times (convergence), it, in most cases, assumes a given character-transformation to be a one-off event, i.e. it having taken place in only a single species, and the result being subsequently inherited by its many descendants. And this view of handling things is legitimate if indeed the transformations studied by it and used to reconstruct the phylogenetic development of the group, are non-functional, that is, are purely formal ( because functional transformations can, and will, take place independently many times; they, together with other such functional changes, create the functional or ecological types, and these types do generally not match with the monophyletic groups of phylogenetic systematics, they cut across the branching-structure of its genealogic system). So if we now assume that the changes of wing-venation in Bibionomorpha are formal changes, then we could legitimately apply the methods of phylogenetic systematics (as they are explained in especially parts XXV-A, XXV-B, and XXV-C) to reconstruct the phylogenetic history of the Bibionomorpha. And, as has been explained in the mentioned documents, this phylogenetic 'history' has essentially taken place in the Implicate Order, where we have a noëtic trajectory successively (but with branchings) visiting the noëtic stability fields of (the noëtic contents of) the groundplans ( It is in this course of the trajectory where the groundplans are noëtically -- that is, formally -- d e r i v e d from each other).
The wing-venation in Bibionomorpha takes part in the expression of the family-groundplan [the Bibionomorpha containing many families], and each family-groundplan itself is further qualitatively determined by supervening structures that sometimes partly 'overwrite' the groundplan-structure, resulting in a genus-groundplan. Projection into the Explicate Order now takes place from the genus-groundplans when the noëtic trajectory has indeed visited these groundplans and when they are noëtically provided with structures (existential conditions) that are adaptations to some ecological niche that actually exists in the Explicate Order. The cladistic course of the noëtic trajectory through the stability fields of the groundplans is revealed by the methods of phylogenetic systematics. And what we see in the Explicate Order is the successive projections along parallel lines.
The cladistic evolution of the Bibionomorpha.
As we have said, this evolution can be revealed by the methods of phylogenetic systematics. For Bibionomorpha this is done, based on wing-venation, by HENNIG already in 1954 in : "Flügelgeäder und System der Dipteren", in BEITRÄGE ZUR ENTOMOLOGIE, 4. Band. Nummer 3/4. Berlin September 1954. But, as he himself indicates, the conclusions are far from complete and far from certain. For the time being I assume that they are still not revised. And I myself am not in a position to undertake such a revision. And, indeed, it is not our aim to reconstruct as precisely as possible the phylogenetic development of the Bibionomorpha. Our true aim is to understand evolution in terms of the interaction between the Explicate and Implicate Orders, that is, to develop the general metaphysical (in the sense of ontological) background of all evolutionary processes. So even when our reconstruction below of the phylogenetic development of the Bibionomorpha, as it will be based on HENNIG's discussions and assumptions, and solely based on wing-venation, will turn out not to be entirely correct, the generalities we had deduced from it will still hold, because we develop our general evolutionary theory from types of evolutionary events, not from individual evolutionary events. Accordingly, when setting up the reconstruction we will not discuss the several possible character-transformations (i.e. whether a transformation went so or perhaps so) and also not discuss the several possible phylogenetic kinships that might exist between discussed taxa, mentioned and discussed by HENNIG. We will simply single out those character-transformations in the wing-venation, and those kinship relations, that follow from the study of wing-venation, that we deem, for the time being, the most probable.
We shall also incorporate the concept of stem-group. It was introduced by HENNIG only after his 1954 work, and is very useful for allocating fossils in the phylogenetic system (as has been explained in part XXV-B).
Let us begin to expound some transformations in the wing-venation insofar as they are important for the reconstruction of the phylogeny of the Bibionomorpha. The most basic venational plan of the Order Diptera is presented in the next Figure.
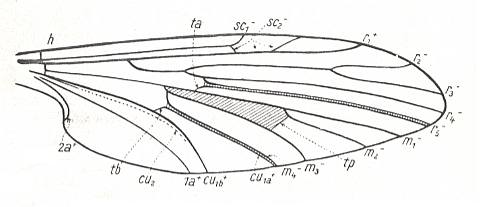
Figure 1 : Scheme of the basic venational plan of the Order Diptera.
Shaded area = discoidal cell.
The 'cross-vein' labelled tb is in fact tb2 or just the basal part of Cu1a . The cross-vein lying directly above it (connecting the discoidal cell with Cu1a) should be called tb1 or just m-cu. (See also Figure 4, below )
The cross-vein labelled ta connects the Radial Sector with the Media, and can also be called r-m.
Whether Sc2, after coalescing for a short distance with the Radius, ends up in the Costa, is not certain.
The vein labelled r5 is considered to be the product of the fusing together of the Anterior Media (MA) and the last branch of the Radial Sector r5 .
The vein labelled m4 cu1a is considered to be the product of the fusing together of these two veins.
h = humeral cross-vein. tp = cross-vein closing the discoidal cell.
Although the main veins are labelled with small letters here, we will indicate them by capital letters.
The structure, here labelled cu2 is in Diptera just a fold, (functionally and morphologically) separating the anal area from the rest of the wing. It is also called the posterior Cubitus (CuP).
(After HENNIG, 1954)
In reconstructing the phylogeny of the Bibionomorpha we begin with fossil forms from which can be derived all recent Bibionomorpha. Such a primitive form has been found in lower Jurassic deposits :

Figure 2 : Wing of Protorhyphus stigmaticus HANDL. Order Diptera, Family Protorhyphidae. Upper Lias [= upper Lower-Jurassic] of Mecklenburg, Germany.
(After HANDLIRSCH, 1938, from HENNIG, 1954)
This form has the Discoidal Cell present and its Radial Sector with three branches.
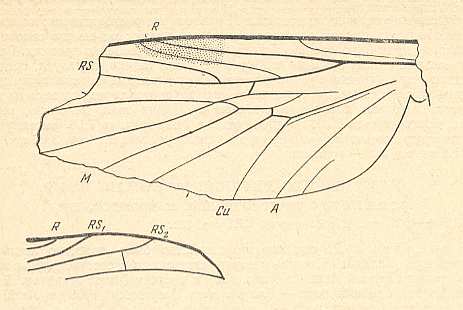
Figure 3 : A representative of the Rhyphidea from the middle Jurassic of Karatau (southern Kazachstan) : Archirhyphus asiaticus ROHD. (Protorhyphidae). Holotype, Coll. PIN No. 2452/334.
Upper image : Left wing, length 2.9 mm, width 1.05 mm.
Lower image : Distal part of right wing.
[The age of the insect-bearing deposits of Karatau (at least of the locations Michailovka and Galkino) always was indicated as being middle Jurassic, but it is almost certain that it must be Malm, that is, Upper Jurassic.]
(After ROHDENDORF, 1964)
The next diagram indicates the most important structures in this primitive wing-venation :

Figure 4 : Diagram of Protorhyphoid wing-venation (Costa, Subcosta, and Radius [R1] not drawn).
The structure formed by CuA, tb2, and M4 can be called the "cubital fork". It may be interpreted as the fork of the anterior branch of Cu, where this anterior branch is CuA (and tb2 the basal part of M4 which should then be called CuA1), and where the posterior branch of Cu is CuP (this latter is in all Diptera -- where it is present at all -- just a fold, and is not drawn here). The cubital fork (CuA, tb2, M4) is present in almost, if not, all, Bibionomorpha. It sometimes becomes shallower or deeper. The position and orientation of the cross-vein tb1 is important, so also the number of branches of the Radial Sector (Rs) and the positions of their origins with respect to the cross-vein ta (= r-m).
In the course of evolution this protorhyphoid venation will undergo certain modifications. First of all : Discoidal Cell remains to be present, but the distal part of R4 coalesces with the distal part of R2+3 resulting in it becoming a crossvein-like connection of R5 with R2+3. This has happened in the recent species Cramptonomyia spenceri :

Figure 5 : Wing-venation of Cramptonomyia spenceri ALEX. Recent. Order Diptera, Family Cramptonomyiidae (or just Rhyphidae), superfamily Rhyphidea, Suborder Nematocera. (After ALEXANDER, 1931, from HENNIG, 1954)
According to HENNIG, 1969, p.389, the family Cramptonomyiidae not only consists of the genus Cramptonomyia but also includes the genus Haruka. Also in the latter genus there is a 'cross-vein' between the two branches of the Radial Sector, and this 'cross-vein' may also be interpreted as the remains of another branch of the Radial Sector.
Another transformation of the protorhyphoid wing-venation, independent of the previous transformation, consists in the fact that R4 has totally vanished, leaving the Radial Sector with only two branches (while the discoidal cell remains present). This has already occurred in Mesorhyphus from the Upper Lias. See next two Figures :
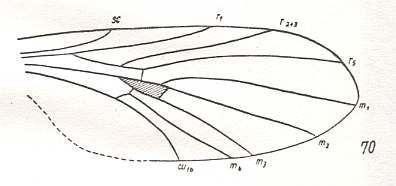
Figure 6 : Mesorhyphus anomalus HANDL. from the upper Liassic [= upper Lower-Jurassic] of Mecklenburg, Germany.
(After HANDLIRSCH, 1938, from HENNIG, 1954)
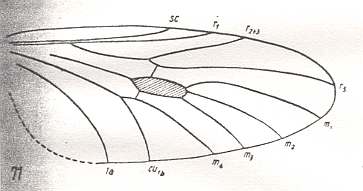
Figure 7 : Mesorhyphus nanus HANDL. from the upper Liassic [= upper Lower-Jurassic] of Mecklenburg, Germany.
(After HANDLIRSCH, 1938, from HENNIG, 1954)
This configuration (discoidal cell still present, Radial Sector 2-branched [R4 vanished] ) can still be observed in recent species of the Rhyphidae. See Figure :
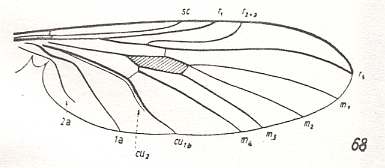
Figure 8 : Wing-venation of Phryne fuscipennis MACQ. Order Diptera, Family Rhyphidae, Superfamily Rhyphidea, Suborder Nematocera. Recent.
(After HENNIG, 1954)
The recent genus Olbiogaster apparently has the same wing-venation as Phryne, but I do not possess a drawing of it. It also belongs to the family Rhyphidae. The Cramtonomyiidae + Rhyphidae can be called the Rhyphiformia. The fossil family Protorhyphidae can, and will, be allocated in the stem-group of the Rhyphiformia (in fact in the stem-group of Rhyphiformia + Bibioniformia + Fungivoriformia). The fossil genus Mesorhyphus can, and will, be allocated in the stem-group of the family Rhyphidae.
Based on these facts we can reconstruct the phylogeny of the Rhyphiformia and Protorhyphidae. See next two Figures.
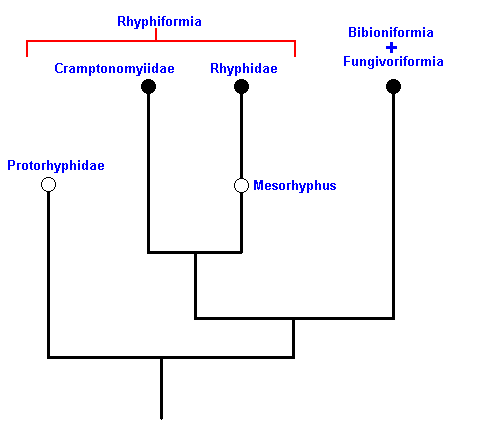
Figure 9 : Phylogeny of the Rhyphiformia and Protorhyphidae. The fossil forms Protorhyphus (Protorhyphidae) and Mesorhyphus have the same age (upper Lias). Archirhyphus (Protorhyphidae, see Figure 3, above ) is younger : Upper Jurassic.
The Cramptonomyiidae are considered to be the sister-group of the Rhyphidae. The Rhyphiformia are considered to be the sister-group of the Bibioniformia + Fungivoriformia.
In the above phylogenetic diagram we can now enter the venational transformations on which it is based. Here, "3Rs" means : Radial Sector is 3-branched, while "2Rs" means : Radial Sector is 2-branched. And "2+Rs" means : Radial Sector with three branches, of which one is very short, that is, almost vanishing. "M3" means : The vein M3 is present.
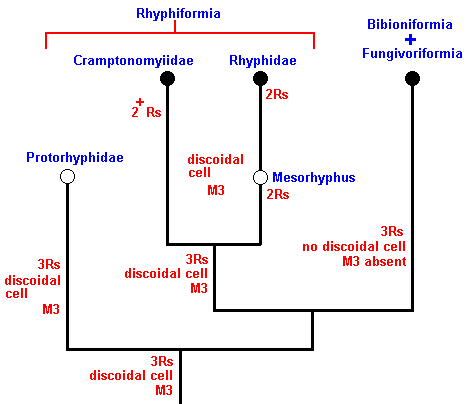
Figure 9a : Phylogeny of the Rhyphiformia and Protorhyphidae. Indicated are the venational transformations on which this phylogenetic system is based. The evolution leading from the Protorhyphidae to the Rhyphiformia consists in the reduction of the number of branches of the Radial Sector (from 3 to 2 [or 'almost' 2] ) while the discoidal cell remains. The evolution that leads to the Bibioniformia + Fungivoriformia consists in the initial preservation of a 3-branched Radial Sector, the disappearance of the discoidal cell, and the vanishing of the vein M3.
As has been said, the sister-group of the Rhyphiformia is the group Bibioniformia + Fungivoriformia. Like the Rhyphiformia the latter has descended from Protorhyphoid forms. The (fossil) forms that initiated the formation of the Bibioniformia + Fungivoriformia center around the genus Eoplecia, known as Eoplecia primitiva HANDL. from the upper Liassic. See next Figure.
Figure 10 : Eoplecia primitiva HANDL. from the upper Liassic [= upper Lower-Jurassic] of Mecklenburg, Germany. Radial Sector 3-branched. No Discoidal Cell. M3 vanished.
(After HANDLIRSCH, 1938, from HENNIG, 1954)
Eoplecia has still the 3-branched Radial Sector of the Protorhyphidae, but has lost the Discoidal Cell and also the vein M3. It has a long second anal vein (2a). In the course of evolution the descendants of Eoplecia (or of one of its close relatives) have reduced their Radial Sector from 3 to 2 branches. Which branch was actually lost is hard to tell. Also the second anal vein was reduced. In this way we arrive at the (recent) genera Hesperinus, Amasia, and Plecia (and Penthetria, if this is not synonymous with Plecia) See next six Figures.
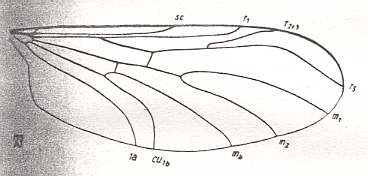
Figure 11 : Wing-venation of Hesperinus imbecillus Lw. Recent. Family Hesperinidae.
(After HENNIG, 1954)
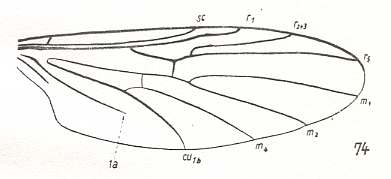
Figure 12 : Wing-venation of Amasia funebris MEIG. Recent. Family Amasiidae
(After HENNIG, 1954)
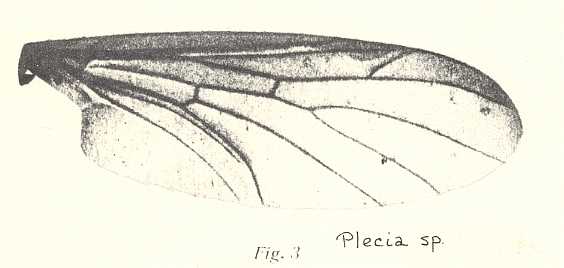
Figure 12a : Wing of Plecia sp. Family Pleciidae (= Penthetriidae).
( From LINDNER, Die Fliegen der paläarktischen Region.)
Figure 13 : Wing-venation of Plecia nigra LUND. Recent. Family Pleciidae (= Penthetriidae). (After KRIVOSJEINA, 1969)
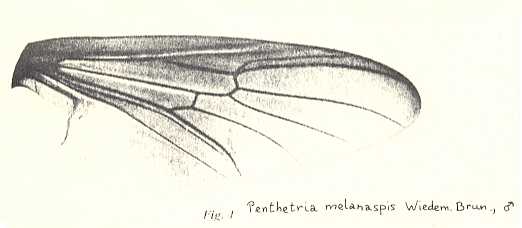
Figure 13a : Wing of Penthetria melanaspis. Recent. Family Pleciidae (= Penthetriidae). In this photograph the vein CuP (posterior cubitus) is clearly visible. It runs closely along CuA (anterior cubitus). The cross-vein ta lands on M1 instead of on the common stalk of M1 and M2.
(From LINDNER, Die Fliegen der paläarktischen Region)

Figure 13b : Wings of Penthetria. Recent. Family Pleciidae (= Penthetriidae). Both wings look much like those of Amasia .
Top : Penthetria motschutskii. Bottom : Penthetria holocericea.
( From LINDNER, Die Fliegen der paläarktischen Region)
But in fact, diptera with such wing-venation were already present in the upper Jurassic :

Figure 14 : Mesopleciella minor ROHD. Upper Jurassic of Karatau, southern Kazachstan. Length of wing 3.75 mm.
(After ROHDENDORF, 1946, from HENNIG, 1954)

Figure 15 : Mesoplecia jurassica ROHD. Upper Jurassic of Karatau, southern Kazachstan. Length of wing 7.0 mm. (After ROHDENDORF, 1938, from HENNIG, 1954)
We may allocate these two fossils in the stem-group of the group Hesperinidae + Amasiidae + Pleciidae, or we may allocate them in the stem-group of the Bibioniformia (Hesperinidae + Amasiidae + Pleciidae + Bibionidae).
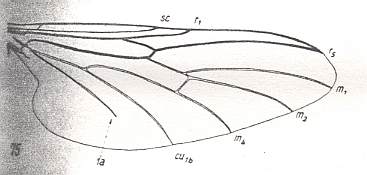
Figure 16 : Wing-venation of Bibio marci L. Recent. Family Bibionidae. The main trunk of the Media is still strongly present, at least until the cross-vein tb1 (which is placed rather distally). The Radial Sector is unbranched. (After HENNIG, 1954)
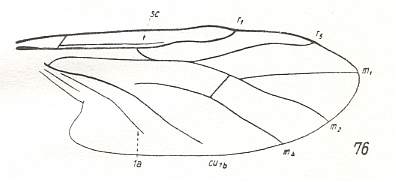
Figure 17 : Wing-venation of Bibiodes spec. Recent. Family Bibionidae. Also here the main trunk of the Media is still clearly present. It has coalesced along a short distance with the Radial Sector which is unbranched. (After CURRAN, 1934 from HENNIG, 1954)
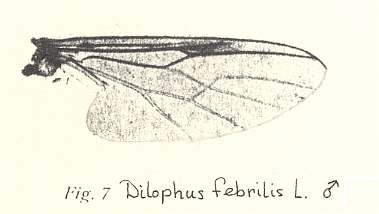
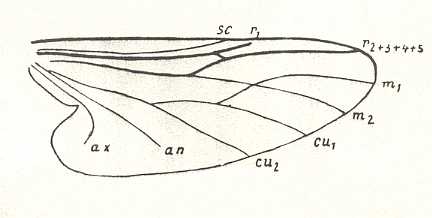
Figure 17b : Wing-venation of Dilophus albipennis MEIG. Recent. Family Bibionidae. While in Bibio the veinlet ta is still clearly manifesting itself as a genuine cross-vein, in Dilophus it lines up with the Radial Sector, making this region of the venation look very specialized. The same configuration is shown in the region of the cross-vein tb1 ( for reference to this vein see Figure 4 ).
( From LINDNER, Die Fliegen der palaearktischen Region)
Within the Bibioniformia, the group consisting of the families Hesperinidae , Amasiidae , and Pleciidae , forms the plesiomorphous sister-group of the group consisting solely of the family Bibionidae (containing the genera Bibio, Bibiodes, and Dilophus). The fossil genera Mesopleciella and Mesoplecia belong in the stem-group of either the mentioned plesiomorphous sister-group or of that of the Bibioniformia. And, as has been said, the sister-group of the latter is constituted by the Fungivoriformia. On the basis of all this we can now present the course of phylogeny in the Bibioniformia :
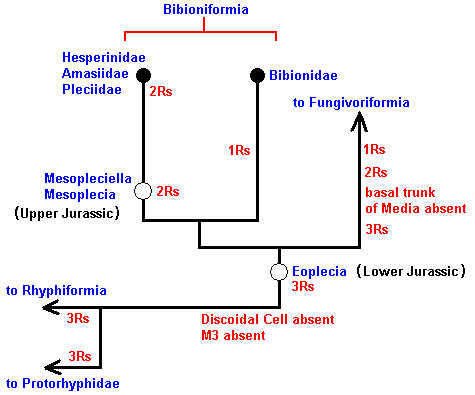
Figure 18 : Phylogenetic tree of the Bibioniformia. "xRs" = x-branched Radial Sector. For Eoplecia see Figure 10 above .
Next we will follow the phylogeny of the sister-group of the Bibioniformia, the group Fungivoriformia. This is a large and complex group, consisting of many families. Its content roughly coincides with the Fungivoridea + Cecidomyiidea + Scatopsidea in the sense of ROHDENDORF 1964.
The fossil form Eoplecia ( Figure 10 above ), or its close relatives at the time (lower Jurassic), did not only give rise to the Bibioniformia, but also to the sister-group of the latter, the Fungivoriformia. While the transition of the venation of Eoplecia (lower Jurassic) into that of the first Bibioniformia took place by the vanishing of one of the branches -- R2+3 or R4 -- of the Radial Sector, resulting in a venation as we see it in Mesoplecia (upper Jurassic) and later in the recent the Hesperinidae and allies, -- the venational transition (from Eoplecia) to the Fungivoriformia took place by the fading-away of the basal trunk of the Media. And if it is true that the Pachyneuridae belong to the Fungivoriformia, then we can say that initially the Radial Sector remained 3-branched (although one branch being already very short), that is, the venational groundplan of the Fungivoriformia has the Radial Sector still 3-branched. In the genus Pachyneura the basal trunk of the Media is, it is true, still present, but in fact is -- according to HENNIG's examination of the wings -- no more than a fold, or merely a pale vein. [This cannot be said of the other genus Axymyia (which could not be examined by HENNIG), which is supposed to also have a 3-branched Radial Sector (and therefore placed in the family Pachyneuridae). In both drawings of the wing-venation of this genus known to me the basal trunk of the Media seems to be normally developed. One of these drawings is given here :
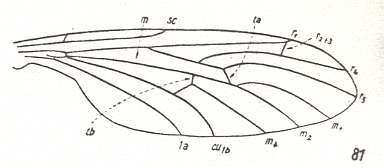
Figure 18a : Axymyia furcata McATEE. Family Pachyneuridae. The basal trunk of the Media seems well-developed. (After CURRAN, 1934, from HENNIG, 1954)
The next Figure is a photograph of a wing of Axymyia :

Figure 18b : Axymyia kertészi. Family Pachyneuridae. Here the basal trunk of the Media seems to be only a pale vein or just a fold. The vein R2+3, if there is any, is difficult to discern in this photograph. See also next Figure.
( From LINDNER, Die Fliegen der palaearktischen Region)
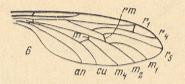
Figure 18c : Mesaxymyia kerteszi DUDA. Family Pachyneuridae (Axymyiidae according to KRIVOSJEINA, 1969). Here the basal trunk of the Media is drawn as a normal vein, and the vein R2+3 is explicitly drawn. (After KRIVOSJEINA, 1969)
For the time being, we will leave this genus (Axymyia or Mesaxymyia) out of our exposition, it could belong to the Bibioniformia instead of to the Fungivoriformia, because from the photograph the status of one of the alleged three branches of Radial Sector is not certain, that is, the Radial Sector might be 2-branched, although the two drawings, of two different species, indicate it to be 3-branched. Further, the status of the basal trunk of the Media remains uncertain..
We can see the evolution of the group Fungivoriformia -- based on their wing-venation -- begin with its very stem-species (having lived somewhere in Jurassic times). It divided into two daughter-species one of which gave rise to the family Pachyneuridae while the other eventually gave rise to all the rest (fossil and recent) of the Fungivoriformia.
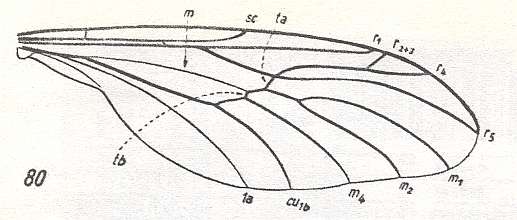
Figure 19 : Wing-venation of Pachyneura fasciata ZETT. Recent. Family Pachyneuridae. Here the main trunk of the Media is no more than a fold or a very weak vein. The Radial Sector is still (seen from Eoplecia) 3-branched, although one of these branches is very short. It is reasonable to assume that R4, from its original position as this was in Eoplecia , shifted its base to R2+3 so that it now originates from it. In the further course of evolution the free piece of R2+3 disappeared (or the short R4 disappeared). (After DUDA, 1930 from HENNIG, 1954)
The next Figure is yet another drawing -- now from LINDNER, Die Fliegen der palaearktischen Region -- of the wing-venation of the same species ( Pachyneura fasciata ZETT.) In contrast to the previous drawing, here the posterior Cubitus (CuP) is indicated (running closely alongside CuA), but Sc2 (second branch of Subcosta) is not drawn :
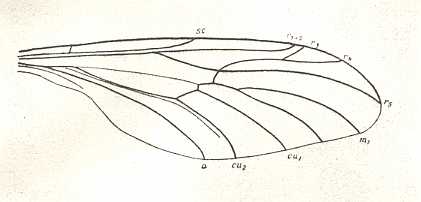
A pre-stage of the venation of Pachyneura might be that of the lower Jurassic Rhaetomyia, in which the fork of the upper branch of the Radial Sector is still rather long, and the basal trunk of the Media apparently still well-developed, but, as ROHDENDORF (1964, p.151) explicitly writes, thin, that is, thinner than the trunks of R and CuA :
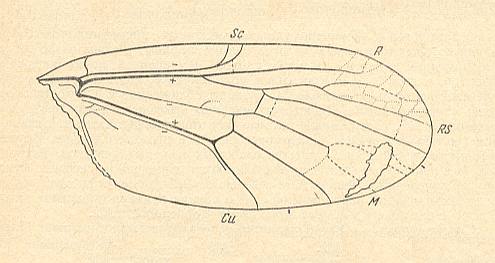
Figure 19a : Positive impression of right wing of Rhaetomyia necopinata ROHD. from the lower Jurassic of Issik-Kul (Central Asia). Coll. PIN No. 358/76. Length 3.44 mm.
The fork of the anterior branch of the Media is explicitly described (by ROHDENDORF) to be present (but undoubtedly being weak). Apart from some extra veinlets and extra, but weak, cross-veins (and of course apart from the type of branching of Rs), the venation is typically 'bibionomorphous', notwithstanding the fact that the main forkings lie almost beyond the mid-point of the wing's length.
(After ROHDENDORF, 1962, from ROHDENDORF, 1964)
It might be instructive to compare with more or less related venations HERE, such as the numbers 2, 11, 20, 14, 13, and others .
Yet another pre-stage of the wing-venation of Pachyneura, that is, a pre-stage in the evolution from Eoplecia to Pachyneura, is that of the upper Jurassic Paraxymyia, in which the basal trunk of the Media has already vanished, but in which the Radial Sector is still 3-branched :
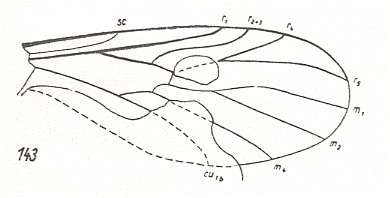
Figure 19b : Wing of Paraxymyia quadriradialis ROHD. Upper Jurassic of Karatau, southern Kazachstan. Length of wing 2.25 mm. Family Paraxymyiidae. Here the main trunk of the Media has vanished apparently completely. But the Radial Sector is still (seen from Eoplecia) 3-branched, where these branches are still long as in Eoplecia . In the further course of evolution of the venation the vein R4 has then severed its connection with R5 and moved over to R2+3, while the origin of R2+3 + R4 has shifted a little in the distal direction, resulting in the venation of Pachyneura except for the total absence of the basal trunk of the Media in Paraxymyia (in Pachyneura this trunk is still a fold or pale vein).
(After ROHDENDORF, 1946, from HENNIG, 1954)
There exists a recent family -- consisting of only two species -- Perissommatidae, based on the genus Perissomma, which, as regards its wing-venation, is more or less closely related to the Pachyneuridae. The Radial Sector is 3-branched with the fork on the anterior branch, like in Pachyneura. The rest of the venation is typical bibioni- or fungivoriformian : M3 absent, no discoidal cell. Wing not elongated. See next Figure.
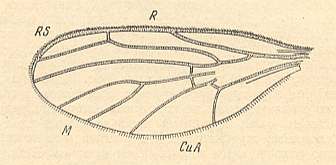
Figure 19c : Wing of Perissomma fuscum COLLESS. Recent. Family Perissommatidae. (After COLLESS, 1962, from ROHDENDORF, 1964)
The next major stage of evolution of the Fungivoriformia is in fact represented by the rest of the Fungivoriformia : Complete disappearance of the basal trunk of the Media, and the reduction of the number of branches of the Radial Sector. This is initiated by the recent genus Mycetobia, belonging to the family Mycetobiidae :

Figure 20 : Wing-venation of Mycetobia pallipes MEIG. Recent. Family Mycetobiidae. Here the main trunk of the Media has totally vanished, and the Radial Sector has become 2-branched. (After HENNIG, 1954)
The next stage of evolution is represented by the recent family Ditomyiidae. Here the fork of the Radial Sector (this fork consisting of the two branches of Rs) has become shorter, and the Subcosta has been reduced to a very short veinlet ending freely in the wing-membrane. See next two Figures.
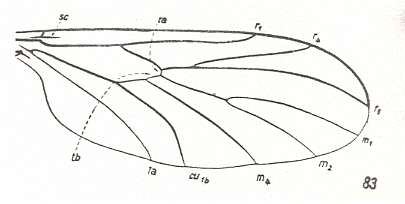
Figure 21 : Wing-venation of Ditomyia fasciata MEIG. Recent. Family Ditomyiidae. (After HENNIG, 1954)
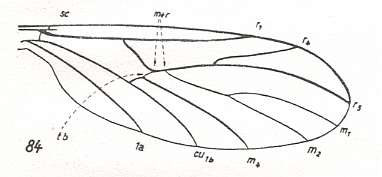
Figure 22 : Wing-venation of Ditomyia conjuncta FREEM. Recent. Family Ditomyiidae. Here the Media has coalesced for a short distance with the Radial Sector. [ This species could, according to HENNIG, just as well be placed into the New Zealand genus Nervijuncta]. (After FREEMAN, 1951, from HENNIG, 1954)
The next stage of this evolution is represented by the wings of the recent family Zelmiridae (= Ceroplatidae + Macroceridae). See next four Figures.
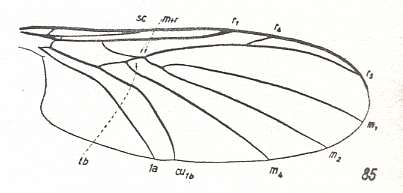
Figure 23 : Wing-venation of Macrocera lutea MEIG. Recent. Family Zelmiridae. (After HENNIG, 1954)
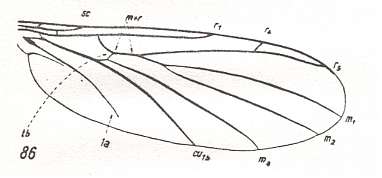
Figure 24 : Wing-venation of Zelmira fasciata MEIG. Recent. Family Zelmiridae. (After HENNIG, 1954)
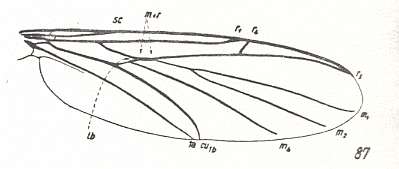
Figure 25 : Wing-venation of Allactoneura nigrifemur ENDERL. Recent. Family Zelmiridae. (After HENNIG, 1954)
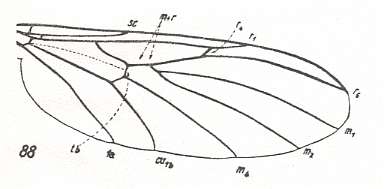
Figure 26 : Wing-venation of Apemon marginata MEIG. Recent. Family Zelmiridae. (After HENNIG, 1954)
For Zelmiridae, see also HERE, number 4, 5, 6, and 7 .
These forms (Zelmiridae) are derived (apomorphous) with respect to the Ditomyiidae in the structure of the Radial Sector : The common trunk of its fork is much longer as in Ditomyiidae and the Fungivoriformian families so far discussed. The anterior branch (R4) of the Radial Sector is (therefore) short. It either ends up freely in the Costa, or in R1 (Apemon, Figure 26). The anal vein (1a) can be shortened (Zelmira). Moreover, as in Ditomyia conjuncta (Ditomyiidae) , the Media is in all members of Zelmiridae for a short distance coalesced with the Radial Sector in the range where normally the cross-vein ta lies.
The Zelmiridae are, however, primitive (plesiomorphous) with respect to the Ditomyiidae in the fact that the Subcosta is still fully developed, having both of its branches, reaching the Costa, respectively R1.
So between the Zelmiridae and the Ditomyiidae there is specialization-crossing, which means that they cannot formally be derived from each other. We assume a specialization-crossing to be found in virtually all cases in the Fungivoriformia and beyond, especially when not only the characters of the wing-venation are examined but other characters as well. In such cases the 'next' family in the evolutionary ascent of the Fungivoriformia does not directly come from the 'previous' family but from one of the members of its stem-group. Of course, a given recent family never can evolve from some other recent family, because 'evolving' is not a formal derivation of one structure from another, but a physico-biological process that takes time, and surely a considerable amount of time. So when a given family is said to have evolved from another, we mean that it evolved from some non-remote ancestors of the latter, ancestors that are still morphologically and biologically identical to it. See next two diagrams.
These next two diagrams dipict two possible cases :
First case :
Figure 26a : In this diagram no specialization-crossing is assumed to exist between the Ditomyiidae and the Zelmiridae. We let the apomorphy of the Zelmiridae be represented by the character transformation z ==> z1. The recent Zelmiridae have evolved from non-remote ancestors of the recent Ditomyiidae. In this process they acquired character state z1 as a result of the transformation z ==> z1. No such transformation has taken place during the ascent of the Ditomyiidae from these non-remote ancestors.
The next diagram assumes the other case :
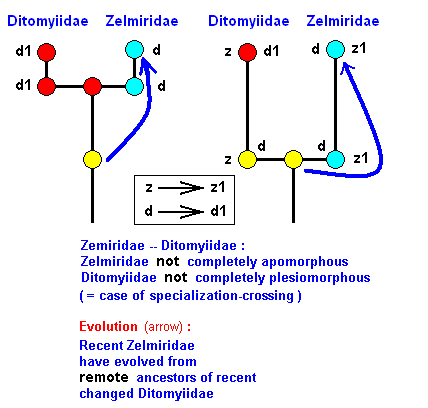
Figure 26b : In this diagram a specialization-crossing is assumed to exist between the Ditomyiidae and the Zelmiridae. We let the apomorphy of the Zelmiridae be represented by the character transformation z ==> z1, and let the apomorphy of the Ditomyiidae be represented by the character transformation d ==> d1.
As a result of specialization-crossing the origin of the new taxon (the Zelmiridae) must be shifted farther back into history. This is preliminarily expressed in the left image. The right image more precisely depicts this new situation.
In this particular case (specialization-crossing) we must assume a species to split up, resulting in two daughter-species (see right image), one of which (still) possessing the plesiomorphous character states z and d, while the other, although also possessing the plesiomorphous character state d, possesses the apomorphous character state z1. And this at the same time forces us to assume that the first mentioned daughter-species (z, d), during the course of evolution, developed still further, resulting in the species (z, d1). The species (z1, d), on the other hand, the second daughter-species, did not develop further, and immediately gave rise to the recent family Zelmiridae. But, for the Ditomyiidae, only the species (z, d1) (and thus not already the species (z, d)) gives rise to this family (Ditomyiidae). The species (z, d) merely belongs to the stem-group of the Ditomyiidae.
And, of course, in this case (specialization-crossing) we can also say that the recent Ditomyiidae evolved from Zelmiridae, namely by acquiring character state d1 in the transformation d ==> d1. And here this means that, only insofar as things depend on this particular transformation, the recent Ditomyiidae could have evolved either from non-remote or from remote ancestors of the Zelmiridae.
The upshot of all this is the fact that, as a result of the so often occurring specialization-crossings, the branches of the forks of the phylogenetic course become longer, that is, the forks become deeper, and deeper so the more primitive the characters involved in the specialization-crossing are (because then the point of origin of a group must revert farther back into history). And often in these long branches the respective groups transform. This means that many related taxa, such as related families, have evolutionarily developed almost completely independently of each other.
Having considered the essence of specialization-crossing, and its effect on the phylogenetic picture, we now continue following the phylogeny of the Fungivoriformia.
The next venational stage in fungivoriformian evolution is represented by the recent family Diadocidiidae :
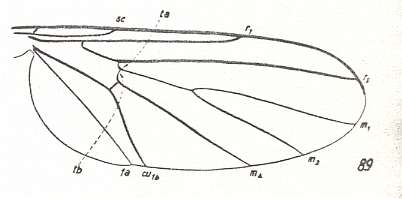
Figure 27 : Wing-venation of Diadocidia ferruginea MEIG. Recent. Family Diadocidiidae. The Radial Sector is unbranched. Replacement of the basal medial trunk by the cross-vein tb1 not yet begun. Subcosta relatively well-developed.
(After HENNIG, 1954)
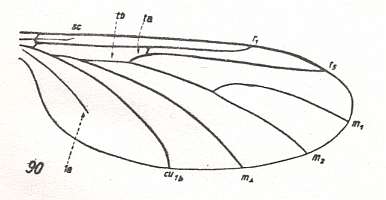
Figure 28 : Wing-venation of Heterotricha relicta EDW. Recent. Family Diadocidiidae. Here a begin is made of the replacement of the medial trunk by the cross-vein tb1. Subcosta more or less reduced. (After EDWARDS, 1925, from HENNIG, 1954)
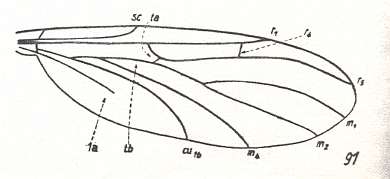
Figure 29 : Wing-venation of Pterogymnus elongata FREEM. Recent. Family Diadocidiidae. Here the Radial Sector is still 2-branched. Its anterior branch ends up, not in the Costa, but in R1. Subcosta well-developed.
(After FREEMAN, 1951, from HENNIG, 1954)
For Diadocidiidae, see also HERE, number 3 .
We are now ready, on the basis of all this, to construct the first part of the phylogenetic course of the Fungivoriformia. And to know the place of that part in the complete phylogenetic tree of the Fungivoriformia, we first present a symplified sketch of this complete tree (without insertion of fossil forms yet) :
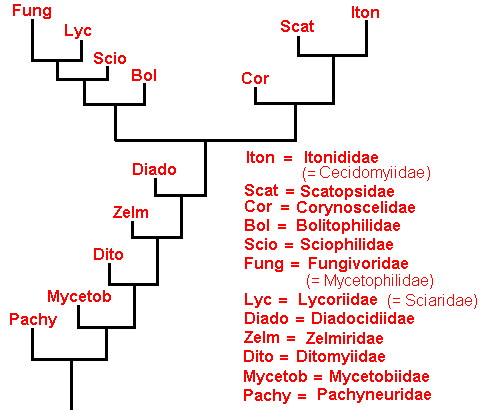
Figure 30 : Phylogenetic system of the recent Fungivoriformia.
The phylogenetic development begins with an ascending series of Families, and then splits up into two such series. In the wing-venation of the members of the first series -- Pachyneuridae, Mycetobiidae, Ditomyiidae, Zelmiridae, and Diadocidiidae -- the basal trunk of the Media has, after becoming a very weak vein, vanished, but still without its being replaced by the cross-vein tb1 . In the two subsequent series -- Bolitophilidae, Sciophilidae, Fungivoridae, Lycoriidae, and Corynoscelidae, Scatopsidae, Itonididae -- the vanished basal trunk of the Media is replaced by tb1 and other veins. However, in the Corynoscelidae the basal trunk of the Media is not so replaced, while it is again so replaced in the other two families of the second subsequent series, in the Scatopsidae and in the Itonididae. So if these two subsequent series together form a monophyletic group, then the mentioned replacement has taken place for a second time in the Scatopsidae + Itonididae.
We now depict the phylogeny of the first series of families (of the Fungivoriformia) [in order to easily refer to the wing-venation in these families the reader can successively click on their names] :
Pachyneuridae Mycetobiidae Ditomyiidae Zelmiridae Diadocidiidae.
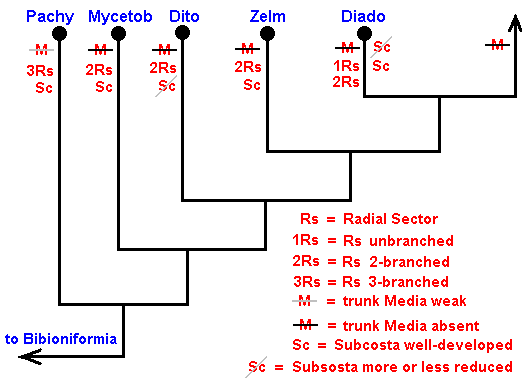
Figure 31 : Phylogenetic system of the first part of the recent Fungivoriformia.
As a result of specialization-crossings (some of them present in the venation, others undoubtedly in the rest of the holomorph of the representatives of every two sister-families), the families cannot be assumed to have evolved directly from each other (i.e. one family from the unchanged proximal ancestors of the other), but every family from the stem-group of its sister-family, that is, from still more primitive members of this stem-group [or just from that stem-group if we define the latter as being composed of members that are all more primitive [in some respects] than are those composing the recent family].
As has been said earlier, this phylogenetic tree (and most others we give) is at most approximately correct, and, moreover, based only on wing-venation.
The phylogeny just sketched continues : Subsequent to the Diadocidiidae there are two series of families. And these series probably are sister-groups of each other. Let us begin with one of them : Bolitophilidae ==> Sciophilidae ==> Fungivoridae + Lycoriidae.
While in the line of Diadocidiidae the Radial Sector goes from 2-branched to unbranched, the 2-branched condition continues in the family Bolitophilidae :

Figure 32 : Wing-venation of Bolitophila hybrida MEIG. Recent. Family Bolitophilidae. See for another drawing HERE, number 2 in which the basal trunk of the Media is apparently fully present. (After HENNIG, 1954)
But in Arachnocampa the Radial Sector has become unbranched. However, its allocation into the family Bolitophilidae is uncertain (see below) :
Figure 33 : Wing-venation of Arachnocampa luminosa EDW. Recent. Family Bolitophilidae (according to HENNIG).
(After TONNOIR and EDWARDS, 1927, from HENNIG, 1954)
And, according to HENNIG, 1954, p.306, the genus Pterogymnus might also belong to the Bolitophilidae. See Figure 29 . Its wings have a 2-branched Radial Sector.
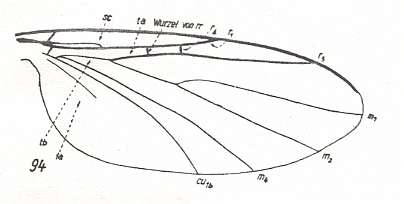
Figure 34 : Wing-venation of Ectrepesthoneura hirta WINN. Recent. Family Sciophilidae. The upper branch of the Radial Sector is very short and cross vein-like. It ends up in R1. Also the root of the Radial Sector is cross vein-like. The Subcosta ends up in R1. The Sciophilidae belong to one of the fungivoriformian families that lead from the Diadocidiidae, via the Bolitophilidae, to the Lycoriidae + Fungivoridae.
(After HENNIG, 1954)
Apparently in the developmental course of these families the cubital fork (consisting of Cu1b and M4) has shifted still further into the direction of the wing-base (as compared to Bolitophila, Bolitophilella). See Figure 34 above. As a result the pale basal trunk of the Media is shortened, as it was already in Bolitophila , and now still more so in Ectrepesthoneura , and the cross-vein tb1 is pushed into the original position of that basal trunk, figuring now as its extension. So this cross-vein now looks like a basal part of the Media. Also the cross-vein ta (between Radial Sector and Media) has followed this trend (see Figure 34 ). Its point of ending-up in the Media does not lie below or distally from the root of the Radial Sector -- as it still was in, for example, the Ditomyiidae , in Diadocidia and also in Bolitophila -- but has shifted into the direction of the wing-base. This stage has been preserved in Ectrepesthoneura : In this genus the original point of ending-up of tb1 in the anterior branch of the cubital fork (M4) has been preserved. In the rest of the Sciophilidae, the Fungivoridae, and the Lycoriidae, on the other hand, this connection has been severed and the cubital fork is secondarily shortened. See next Figures.
Family Sciophilidae :
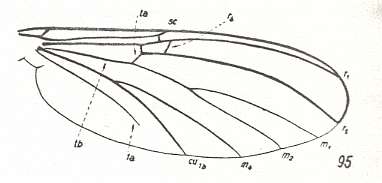
Figure 35 : Wing-venation of Mycomyia limbata WINN. Recent. Family Sciophilidae. For yet another drawing see HERE, number 9 . The anterior branch of the Radial Sector is short, shifted towards the origin of the Radial Sector, and ending up in R1. The cubital fork is secondarily shortened (that is, its stalk has become longer). In the basal part of the wing we see in fact three longitudinal main veins, seemingly Cubitus, Media, and Radius. But we know that the "Media" is in fact the cross-vein tb1. (After HENNIG, 1954)
For the wing-venation of Sciophila, see HERE, number 20 .
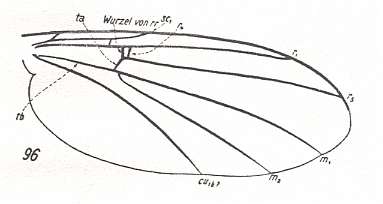
Figure 36 : Wing-venation of Monoclona forcipata STROBL. Recent. Family Sciophilidae. Here the anterior branch of the Radial Sector has shifted still further and is now very close to the point of origin of the Radial Sector. For yet another drawing see HERE, number 21 . (After HENNIG, 1954)
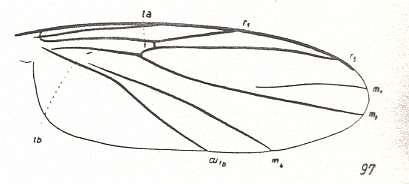
Figure 37 : Wing-venation of Rondaniella dimidiata MEIG. Recent. Family Sciophilidae. The Radial Sector has become unbranched. (After HENNIG, 1954)
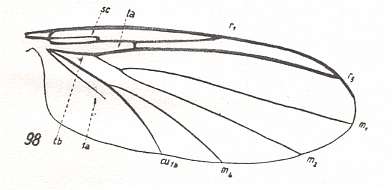
Figure 38 : Wing-venation of Docosia sciarina MEIG. Recent. Family Sciophilidae. (After HENNIG, 1954)
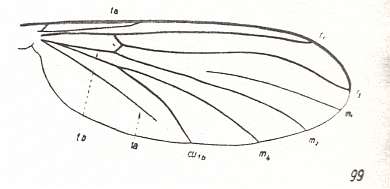
Figure 39 : Wing-venation of Neurotelia nemoralis MEIG. Recent. Family Sciophilidae. For yet another drawing see HERE, number 15 . (After HENNIG, 1954)
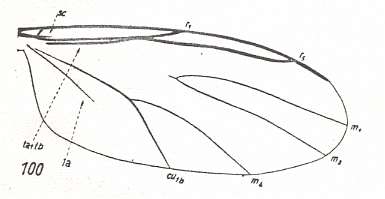
Figure 40 : Wing-venation of Novakia scatopsiformis STROBL. Recent. Family Sciophilidae. (After HENNIG, 1954)
Family Fungivoridae (= Mycetophilidae, fungus gnats) :
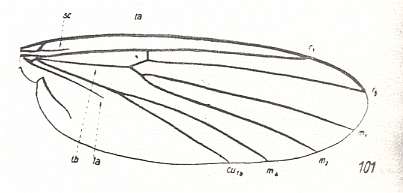
Figure 41 : Wing-venation of Fungivora fungorum DeG. Recent. Family Fungivoridae. (After HENNIG, 1954)
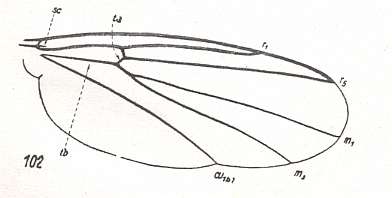
Figure 42 : Wing-venation of Zygomyia notata STANN. Recent. Family Fungivoridae. (After HENNIG, 1954)
Family Lycoriidae (= Sciaridae) :

Figure 43 : Wing-venation of Lycoria bicolor MEIG. Recent. Family Lycoriidae. (After HENNIG, 1954)
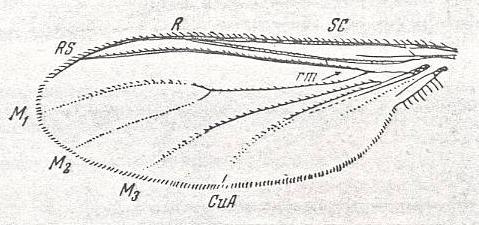
Figure 43a : Wing of Lycoria spec. Recent. Family Lycoriidae. Length 2 mm.
(After HENDEL, form ROHDENDORF, 1951)
As we shall see, analogous processes have occurred in the other series of families of the Fungivoriformia : the Corynoscelidae, Scatopsidae, and the Itonididae (= Cecidomyiidae, gall-midges). Also this group connects -- as regards its wing-venation -- to the family Bolitophilidae. But let us first continue with the series we were discussing : the Bolitophilidae, the Sciophilidae, the Fungivoridae, and the Lycoriidae.
Into the family Bolitophilidae was also placed the genus Arachnocampa, to which the well-known "New Zealand Glow Worm" belongs. See Figure 33, above . See also a Photograph of Arachnocampa . The genus differs from Bolitophila and Bolitophilella by having its Radial Sector unbranched. The drawing of it (Figure 33) shows a m-cu cross-vein (tb1) as well as a well-developed basal piece of the Media (see also the photograph). Nowhere in the Fungivoriformia are these characters present together.
Especially based on larval morphology it was also assumed that Arachnocampa is related to the family Zelmiridae (= Ceroplatidae + Macroceridae). But in all the other species of this family the Radial Sector is 2-branched, and above all, the common stalk of M1 and M2 is, at its base, coalesced with the Radial Sector. Not so in Arachnocampa. So the systematic position of this genus remains uncertain.
The Sciophilidae + Fungivoridae + Lycoriidae form a monophyletic group. The features of their wing-venation has been discussed above. In addition, common to them, is the fact that the anal vein (1a) does not reach the wing-margin. The venation in the genus Ectrepesthoneura is closest to the venational groundplan of the group. Also characteristic of the venation of this group (Sciophilidae + Fungivoridae + Lycoriidae) is the fact that the anterior branch of the Radial Sector never ends up in the Costa. It either ends up, shifted very far towards the wing-base, close or very close to the root of the Radial Sector, in R1 (see Figure 35 and Figure 36 ), or is completely absent (Figure 37 ). The most primitive (plesiomorphous) wing-venation in this group is possessed by the Mycomyini (Sciophilidae) (Figure 35 ). Here the Subcosta is forked at its end, and the anterior branch of the Radial Sector is still relatively far away from the root of the Radial Sector. In the subsequent development of the venational groundplan the second branchlet of the Subcosta (Sc2) has shifted towards the wing-base ( Neurotelia ). Finally, this often already pale branchlet completely disappears ( Rondaniella ). In some cases, on the other hand, the first branchlet of the Subcosta (Sc1) is reduced ( Docosia ), or the Subcosta looses its endings completely and, more or less shortened, ends up freely in the wing membrane ( Novakia , and all Fungivoridae). During subsequent venational development the anterior branch of the Radial Sector comes to lie close to the root of the Radial Sector, often even closer as in Monoclona, and finally very often vanishes totally ( Neurotelia , Rondaniella , Docosia , and all Fungivoridae [for example Fungivora ] ). In Novakia the cross-vein ta is absent. The seemingly longitudinal vein, made up of tb1 and the Radial Sector, is, at the place where ta would be, for some distance coalesced with R1. Further, a reduction of the common stalk of M1 and M2 is seen in Novakia , or a reduction of M1, as in Neurotelia and Rondaniella , and further the reduction of M4 as in Monoclona, and, finally, of 1a, which is completely absent in, for instance, Rondaniella and Monoclona .
Many of these reductions have undoubtedly taken place several times independently in different genealogical groups. They should not without further enquiry lead to the assumption of a close kinship between the forms showing these reductions.
Conspicuous, however, is the fact that all members of the family Fungivoridae possess two clearly apomorphous (= derived) characters : (1) The reduction of the Subcosta, which always ends up freely into the wing-membrane and is shortened, and (2) the unbranched condition of the Radial Sector. And because this family is also uncontestedly a natural genealogic group by the testimony of other characters, one can safely assume that the mentioned venational characters form a synapomorphy and are thus not the result of convergence. Therefore the family Fungivoridae is a genuine monophyletic group.
Not so for the Sciophilidae (as they were conventionally conceived around and before 1954). Their wing-venation suggests that they form a group on the basis of symplesiomorphy (instead of synapomorphy), that is, that possibly some of its subfamilies or tribes are more closely related genealogically with the Fungivoridae. Probably the "Sciophilidae", as being a non-monophyletic group, must be divided up [Of course in a typological perspective they still may form a uniform group].
Close to the "Sciophilidae" and Fungivoridae are placed by some authors also the Lygistorrhinidae. See next Figure.
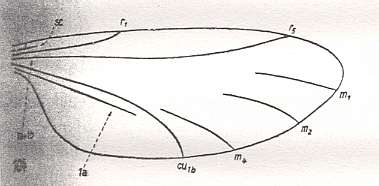
Figure 44 : Wing-venation of Lygistorrhina spec. Recent. Family Lygistorrhinidae. (After HENDEL, 1938, from HENNIG, 1954)
The wing-venation of this small group is so strongly reduced, that it doesn't allow any statements about its relations of kinship (with other groups).
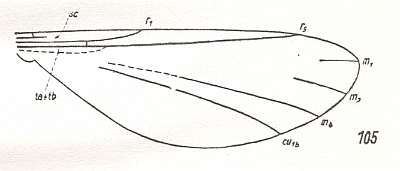
Figure 45 : Wing-venation of Manota spec. Recent. Family Manotidae.
(After HENDEL, 1938, from HENNIG, 1954)
Family Lycoriidae.
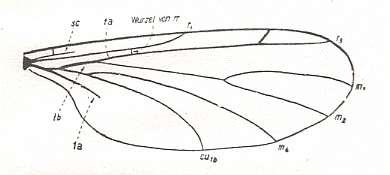
Figure 46 : Wing-venation of Cratyna spec. Recent. Family Lycoriidae. As in Lycoria the cross-vein ta has assumed a horizontal position and now looks like a continuation of the Radial Sector. And it is in turn continued in the same direction by the cross-vein tb1, which also has assumed a horizontal position. The root of the Radial Sector is very short and looks like a cross-vein. (After ENDERLEIN, 1937, from HENNIG, 1954)
If this form really belongs to the Lycoriidae, then the venational groundplan of this family would have the Radial Sector 2-branched, while in all Fungivoridae it is unbranched. So at least one synapomorphy between the two is lost. The phylogenetic line of the Lycoriidae should then go back deeper than merely to the Fungivoridae, that is, it must originate at least there in the ascendency of the Fungivoriformia where the Radial Sector was still 2-branched such as in the Bolitophilidae . However, according to FREY (1942, p.20, footnote) -- so HENNIG reports -- the forking of the Radial Sector in Cratyna is perhaps just a monstrosity. What this should precisely mean is not so clear, because we apparently have not to do with a mere individual variation but with a characteristic of a genus. Nevertheless, because the rest of the venation in Cratyna is virtually identical with that of Lycoria , the extra branch of the Radial Sector must be considered the result of some 'error' or irregularity at the generic level, meaning that a 2-branched Radial Sector does not belong to the venational groundplan of the family Lycoriidae. And now nothing stands in the way to assume a sister-group relation between the Lycoriidae and the Fungivoridae, based on the wing-venation.
We have now finished the exposition of one of the two terminal series of families of the Fungivoriformia, namely the families Bolitophilidae, Sciophilidae, Lycoriidae and Fungivoridae. See Figure 30 above .
Now we will study the last genealogical group in the Fungivoriformia, that is, the other terminal series consisting of the families Corynoscelidae, Scatopsidae, and Itonididae. Among the "Itonididae" (gall-midges s.l.) we must perhaps distinguish the family Lestremiidae which can be thought of as primitive gall-midges.
The venational groundplan of this group is similar to that of the Fungivoriformia. They differ from it by the fact that in their venation the Radial Sector has only two branches [i.e. there are members of the group Corynoscelidae + Scatopsidae + Itonididae that have their Radial Sector still 2-branched, meaning that this character belongs to the venational groundplan of the group, while the venational groundplan of the Fungivoriformia has a 3-branched Radial Sector (see Figure 18 at "to Fungivoriformia") ]. The venational groundplan of this group (Corynoscelidae + Scatopsidae + Itonididae) also differs from that of the Fungivoriformia by the fact that the Subcosta is always shortened and never reaches the wing-margin : While there are Fungivoriformia, other than the Lycoriidae, such as Fungivora , in which this is also the case, there are others, such as Monoclona , in which the Subcosta is fully developed. So a developed Subcosta (which condition is more primitive than that of a reduced Subcosta) belongs to the venational groundplan of the Fungivoriformia.
Decisive for the allocation of the group Corynoscelidae + Scatopsidae + Itonididae to the Fungivoriformia is the weakening and subsequent reduction of the basal trunk of the Media, for this highly characteristic feature does not occur in a comparable form in any other subgroup of the Nematocera. And also the (to us already familiar) tendency in certain Fungivoriformia to replace the reduced basal trunk of the Media by the cross-vein tb1 is clearly present in the groundplan of the group Corynoscelidae + Scatopsidae + Itonodidae and will, in the more derived families, be driven to an extreme.
Closest to the venational groundplan of this group are the members of the family Corynoscelidae :
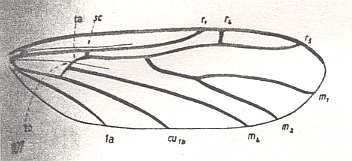
Figure 47 : Wing-venation of Corynoscelis eximia BOH. Recent. Family Corynoscelidae. Subcosta long, but not reaching the wing-margin or R1. The basal trunk of the Media is weak. It is not replaced by the cross-vein tb1 or ta.
(After DUDA, 1928, from HENNIG, 1954)
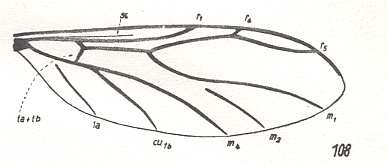
Figure 48 : Wing-venation of Canthyloscelis antennata EDW. Recent. Family Corynoscelidae. Here the basal trunk of the Media has vanished, but it is not replaced. M2 is in a reduced condition. (After DUDA, 1928, from HENNIG, 1954)
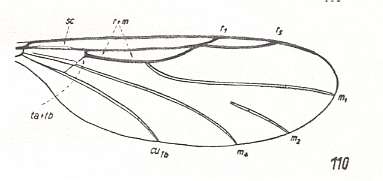
Figure 49 : Wing-venation of Synneuron annulipes LUNDSTR. Recent. Family Corynoscelidae. The Subcosta is short, it ends up in R1. The cubital fork is still present (that is, tb2 is still present [see Figure 4 ] ), and also the basal trunk of the Media, although, it seems, weakened. That is, the trunk has not been replaced.
(After DUDA, 1928, from HENNIG, 1954)
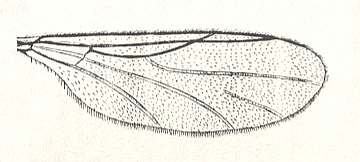
Figure 49a : Wing of Synneuron annulipes LUNDSTR. Recent. Family Corynoscelidae.
(From LINDNER, Die Fliegen der palaearktischen Region)
The Corynoscelidae are, with respect to their wing-venation, the most plesiomorphous forms of the whole group. They have their Radial Sector in a 2-branched condition. The short anterior branch ends up in the Costa (that is, it does not end up in R1). The cross-vein tb1 is only a little oblique, and the cubital fork has not shifted excessively to the wing-base. Also the anal vein (1a) is strongly developed. It reaches the wing-margin.
Family Scatopsidae (see next four Figures).
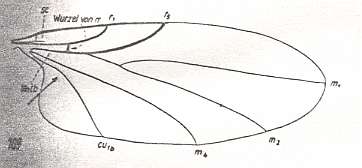
Figure 50 : Wing-venation of Ectaetia clavipes Lw. Recent. Family Scatopsidae. The cross-vein ta has changed its usual orientation : from oblique to horizontal. Its proximal extension is formed by the cross-vein tb1 which is also horizontal as a result of the deepening of the cubital fork. The basal piece of the common stalk of M1 and M2 has coalesced with the Radial Sector. (After HENNIG, 1954)

Figure 51 : Wing-venation of Scatopse fuscipes MEIG. Recent. Family Scatopsidae. The cross-vein ta still has its original position and orientation. But the cross-vein tb1 has assumed a horizontal orientation as a result of the deepening of the cubital fork.
(After HENNIG, 1954)
Figure 52 : Wing of Scatopse flavicollis MEIG. Recent. Family Scatopsidae.
(After LINDNER, Die Fliegen der palaearktischen Region)
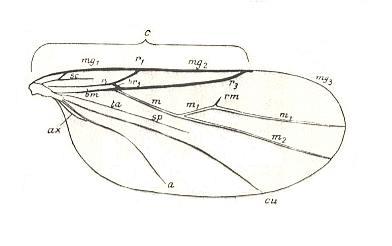
Figure 53 : Wing-venation of Scatopse lapponica DUDA. Recent. Family Scatopsidae.
c = Costa. The interpretation of the veins is of the aurthor of the drawing.
(After DUDA, from LINDNER, Die Fliegen der palaearktischen Region)
The Scatopsidae deviate from the venational groundplan of the whole group in the following points :
Family Itonididae (gall-midges) (or superfamily Itonididea).
The most primitive (most plesiomorphous) genera of the Itonididae may be united into a separate family, the Lestremiidae [and then having Lestremiidae + Itonididae = Itonididea]. See next two Figures.
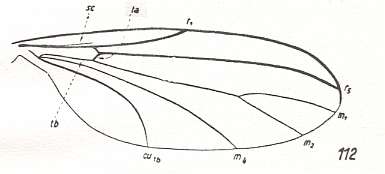
Figure 54 : Wing-venation of Catocha spec. Recent. Family Lestremiidae (Itonididea). The cross-vein ta is still in its original position and orientation, that is, it makes a clear angle with the Radial Sector. The cubital fork is deepened, causing the cross-vein tb1 to assume a horizontal orientation. (After HENNIG, 1954)
The wing of a species of the same genus is depicted in the next Figure :
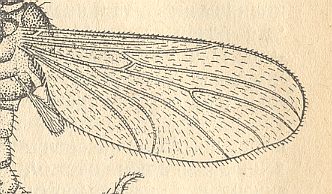
Figure 54a : Wing of Catocha spec., male. Recent. Family Lestremiidae (Itonididea). Here an anal vein (1a) seems to be developed.
(After CURRAN, 1934, from ROHDENDORF, 1964)
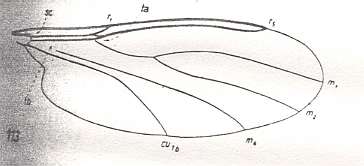
Figure 55 : Wing-venation of Lestremia cinerea MEIG. Recent. Family Lestremiidae (Itonididea). The Subcosta (Sc) is extremely short. The cross-vein ta has assumed an almost horizontal position. It forms the proximal extension of the Radial Sector. The rest of this extension is formed by the cross-vein tb1 which runs horizontally, close to and parallel with, the Radius. The connection between M4 and Cu1b is severed.
(After HENNIG, 1954)
As to their wing-venation, the Lestremiidae represent about the same [developmental] stage as do the Scatopsidae : The Media (M1 + M2) is (still) separated from the Radial Sector and forked at its end. The cubital fork has markedly shifted toward the wing-base. As a result the cross-vein tb1 lies almost horizontally and has come to follow the course of the original basal trunk of the Media (which trunk has now vanished). The anal vein (1a) is absent. In many Lestremiidae, such as Lestremia , M4 has been separated from Cu1b, implying that the cubital fork has been disintegrated. In the true gall-midges (Itonididae) the Media (apart from M4) is unbranched, see next Figure,
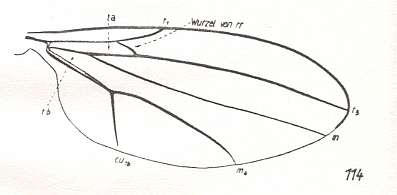
Figure 56 : Wing-venation of Tetraxyphus atra MEIG. Recent. Family Itonididae. The cross-vein ta has assumed a horizontal orientation, and its proximal extension is formed by the cross-vein tb1 which also runs horizontally, probably as a result of an initial deepening of the cubital fork. In the present form this deepening is followed by a secondary shallowing of this fork as a result of partial coalescence of its two branches. Apart from M4, the Media is unbranched. (After HENNIG, 1954)
or has (apart from M4) completely vanished. See next two Figures.
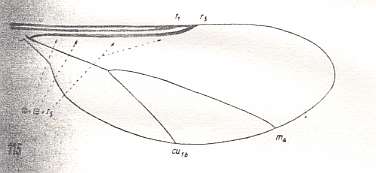
Figure 57 : Wing-venation of Lasioptera ? flexuosa WINN. Recent. Family Itonididae. Below the Radius (R1), and parallel to it, we see a single strong longitudinal vein. This vein consists of (1) the Radial Sector (here, R5), which has lost its connection with R1, (2) the cross-vein ta, and (3) the cross-vein tb1. The vein M1+2 and also its branches M1 and M2, have vanished. (After HENNIG, 1954)
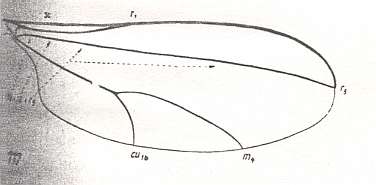
Figure 58 : Wing-venation of Asphondylia sarothamni Lw. Recent. Family Itonididae. The vein, running below the Radius (R1) is composed in the same way as in Lasioptera ? (previous Figure), but it is still much longer and reaches the apex of the wing.
(After HENNIG, 1954)
A wing with similar venation is depicted in the next Figure :
Figure 58a : Wing of Mayetiola destructor SAY., male. Recent. Family Itonididae (Itonididea). (After CURRAN, 1934, from ROHDENDORF, 1964)
In addition to all this, the cubital fork is secondarily shortened in all these forms (that is, the true gall-midges) (compare with Lestremiidae ). Evidently, here its two branches (M4 and Cu1b) coalesced up until the place which also in Scatopsidae is distinguished by a buckle in the course of Cu1b (arrow in Figure 51 ). The formation of this long stalk of the Cu-fork is still clearly recognizable in Tetraxyphus . The shallowness of the cubital fork is therefore in the Itonididae not a primitive, plesiomorphous (as in, for instance, Bibionidae), but a secondary, apomorphous condition.
In the Itonididae the apomorphous development of the wing-venation can still go further : Shortening of the only preserved branch of the Radial Sector (R5), resulting in it not ending up at the wing-apex anymore but close to R1, as in Lasioptera ? , reduction of M4, that is, neutralizing the cubital fork, as in Miastor :
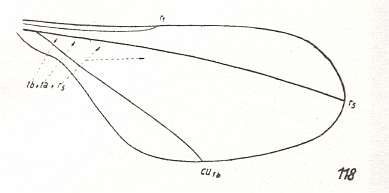
Figure 59 : Wing-venation of Miastor hastatus KIEFF. Recent. Family Itonididae. The vein M4 has vanished, which here means that all veins of the Medial system have disappeared. Only three veins are left : R1, R5, and Cu1b.
(After KIEFFER, 1913, from HENNIG, 1954)
and the reduction of the root of the Radial Sector. In these forms, that is, most of the true gall-midges, the Radius (R1) and the Radial Sector (Rs) run, unconnected, next to each other. In the seeming Radial Sector is not only contained the latter itself but also the cross-vein ta and the horizontally placed cross-vein tb1. See Asphondylia and Miastor .
For determining the phylogenetic relationships between the Corynoscelidae, Scatopsidae, and Itonididea (= Lestremiidae + Itonididae), these autapomorphies of single genera or groups of genera are of course not significant.
Still more important is the question which phylogenetic relationships there are between the group Corynoscelidae + Scatopsidae + Itonididea and the other Fungivoriformia.
With a certain degree of probability one may assume that in the course of the historical development of the fungivoriformian stem the Pachyneuridae first branched off, and then the Mycetobiidae, while all the remaining families, taken together, form a monophyletic group. The latter was later divided into two stems, to one of which do belong the Bolitophilidae + 'Sciophylidae' + Lycoriidae + Fungivoridae, while to the other the Corynoscelidae + Scatopsidae + Itonididea belong.
On the basis of all the foregoing we can draw the phylogenetic tree of the two just mentioned terminal stems of recent Fungivoriformia. For a complete phylogenetic tree of the recent Fungivoriformia, but not yet provided with notes about decisive characters, see Figure 30, above . Before we draw the tree of the two stems we summarize and categorize the venational changes that have ocurred in these stems.
First terminal stem of Fungivoriformia (Bolitophilidae, Sciophilidae, Fungivoridae, Lycoriidae).
The series comes from the Diadocidiidae (from which also the other terminal series comes) and starts with the family Bolitophilidae.
Somewhere during the process of diversification of the family Diadocidiidae a begin was made to replace the vanished basal trunk of the Media by the cross-vein tb1 : The cubital fork becomes deeper which results in a stretching and horizontal orientation of this cross-vein. Although we don't see this yet in the genus Diadocidia , we see it in Heterotricha , and even in Pterogymnus where the Radial Sector is still 2-branched.
In the Bolitophilidae (first member of the first terminal series of Fungivoriformia), all members of which still have a 2-branched Radial Sector, the replacement is well under way it seems, if the interpretation of the veins in the medio-cubital area is correct.
That the vanishing of the basal trunk of the Media and its subsequent replacement by the cross-vein tb1 is not the only development in the Fungivoriformia is shown by Arachnocampa , in which the basal trunk of the Media is still present while the Radial Sector has become already unbranched. But maybe Arachnocampa doesn't belong in the Fungivoriformia at all. Indeed, if we characterize the latter by having lost the basal trunk of the Media, then Arachnocampa would not belong to them. We must admit it to represent some line of fungivoroid midges running independently along their main stem, the Fungivoriformia. A form that is -- as regards wing-venation -- formally identical to Arachnocampa is Rhaetofungivora reticulata ROHD (Figure 14c in next document). from the lower Jurassic of Issyc-Kul (central Asia).
Further, the venation of the Bolitophilidae (perhaps including in them the genus Pterogymnus), cannot (evolutionarily) be derived from that of the Diadocidiidae, say of Heterotricha or Diadocidia , because the Radial Sector of the latter genera is already unbranched (that is, if we would assume such a derivation, then we are forced to assume a reversal in the character transformation Rs 2-branched ==> Rs unbranched). So if the Bolitophilidae really have descended from the Diadocidiidae, then it must be from ancestors of the latter that were still more primitive, that is, from ancestors that still possessed a 2-branched Radial Sector. This fact, which we can safely assume for all other families that are supposed to have been descended one from the other, is expressed by the way we have depicted the phylogenetic line or trajectory between two evolutionarily successive families, a line, not simply going from one family to another (that is from the unchanged proximal ancestors of one family to the recent representatives of another) but from still different (i.e. more primitive) ancestors of one family to the recent representatives of the other ( See, for example, Figure 30, above ), and especially our exposition of specialization-crossing that was given above).
Now we can draw the phylogenetic tree of the first terminal series of the Fungivoriformia. And by clicking on the names of the respective families the reader can check the decive characters :
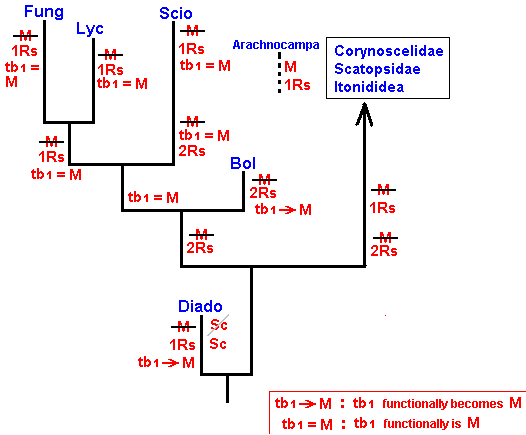
Figure 60 : Phylogenetic tree of the first terminal series of fungivoriformian families.
xRs = Radial Sector with x branches. M = basal trunk of Media present (when scored through, it means : basal trunk of Media absent). Sc = Subcosta present (scored through with a brown score means that it has partly been reduced). For the abbreviations of the family names see Figure 30 .
Of course we could interpret the venation of the Fungivoridae and also of the Lycoriidae differently, because, especially in the Fungivoridae, the bifurcation point of the cubital fork is not close to the wing-base (at least, in the venation of these families, the stalk of the cubital fork is (still) well developed) : Instead of assuming that the cubital fork has secondarily become shallow again (after first having been deepened and pulling the cross-vein tb1 in a horizontal position), we could assume that a shallowing of this fork never took place, implying that the condition of this fork is a plesiomorphous (primitive) character of the wing-venation in these two families. And this means that the mentioned cross-vein (tb1) has not been stretched backwards towards the wing-base, which in turn means that the longitudial vein running between the Radius and the Cubitus is the true Media !. However, in that case the cross-vein tb1 would still be present, and present that is, in its original (oblique) position, as we see it, for example, in Diadocidia (Diadocidiidae) . No such vein can be found in the Lycoriidae or Fungivoridae. Therefore, this alternative interpretation of the wing-venation in these two families will not hold.
Second terminal stem of Fungivoriformia (Corynoscelidae, Scatopsidae, Itonididea [= Lestremiidae + Itonididae] ).
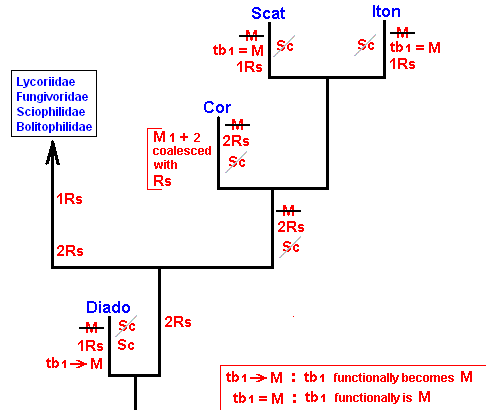
Figure 61 : Phylogenetic tree of the second terminal series of fungivoriformian families (Corynoscelidae, Scatopsidae, Itonididea [= Lestremiidae + Itonididae] ).
xRs = Radial Sector with x branches. M = basal trunk of Media present (when scored through, it means : basal trunk of Media absent). Sc = Subcosta present (scored through with a brown score means that it has partly been reduced). For the abbreviations of the family names see Figure 30 .
The problem of the phylogenetic position of the family Corynoscelidae.
The family Corynoscelidae was -- based on their wing-venation -- placed in the second terminal series of fungivoriformian families as its first member. The next members were the Scatopsidae and the Itonididea (= Lestremiidae + Itonididae).
However, when we again inspect the wing-venation of members of the family Corynoscelidae, Corynoscelis , Canthyloscelis , Synneuron , especially when we do not solely concentrate on a purely formal correspondence of their venation with that of the remaining fungivoriformia, we must admit that their wing-venation does not smoothly fit into the series Diadocidiidae Corynoscelidae Scatopsidae . We can say that, like it is in the previous fungivoriformian families (Diadocidiidae, Zelmiridae, Ditomyiidae, etc.), the basal medial trunk has disappeared or at least weakened, and that its replacement by the cross-vein tb1 has not yet begun (as we see it for example in Diadocidia (Diadocidiidae)). This fact indeed places the family Corynoscelidae in or close to the first series of fungivoriformian families (Pachyneuridae, Mycetobiidae, Ditomyiidae, Zelmiridae, and Diadocidiidae). This is also confirmed by their 2-branched Radial Sector. The family Corynoscelidae would then have -- as a possibility -- evolved from the genus Pterogymnus of the family Diadocidiidae, or, perhaps better (because the Radial Sector in Pterogymnus is a bit more derived as compared with the Radial Sector in Corynoscelidae), from some non-remote ancestor of that genus (Pterogymnus), or from, say, the genus Allactoneura of the family Zelmiridae. But in Pterogymnus the replacement of the medial trunk by the cross-vein tb1 is well under way, while it is not so in Corynoscelidae. On the other hand, in the genus Allactoneura (Zelmiridae) we find not only a Radial Sector that is character-transformationally in line with that in Corynoscelidae, but also the fact that the replacement of the trunk of the Media has not yet begun. Nevertheless, we get the impression that these correspondences, especially of those in the cubito-medial region, are no more than merely formal. And this gives some credibility to the supposition that the venational correspondences between the families Zelmiridae or Diadocidiidae on the one hand, and the family Corynoscelidae on the other, are accidental, and do not prove their phylogenetic kinship. We get the impression that the venation of the Corynoscelidae, in spite of the formal correspondences, has phylogenetically nothing to do with the mentioned two families or with any other family of the first series of fungivoriformian families. The family Corynoscelidae might even have little to do with the 'next' family, the Scatopsidae (according to KRIVOSJEINA, 1969 [there the family Corynoscelidae is called the Hyperoscelididae] ).
Perhaps the family Corynoscelidae very early on branched off from the fungivoriformian stem or even from the bibioniformian stem. In the latter case the weakening or vanishing of the medial trunk occurred independently of that same process in the true fungivoriformians. For the time being it is impossible to allocate the family to its proper place within the phylogenetic tree of the Bibionomorpha. We leave it therefore in the place established above, that is, within the second terminal series of fungivoriformian families.
Problems with other alleged bibionomorphous families or genera as to their proper taxonomic place. Other cases of convergent development of wing-venation.
Comparable problems we have met with the genus Arachnocampa . Also in this case it is not known where to place it in the phylogenetic tree of the bibionomorphs. Its habitus seems to refer it to the Tipulomorpha. See Photograph of Arachnocampa . And about the same goes for the genus Axymyia , and even for the genus Pachyneura .
Status of Pachyneura
The taxonomic place of the genus Pachyneura is uncertain. A part of the authors places it close to the Bibionidae, that is, to the Bibioniformia, but the majority of them (as it at least was so in 1969) places it close to the Rhyphidae [= Anisopodidae], that is, to the Rhyphiformia. HENNIG, 1954, places it (together with Axymyia), at the base of the Fungivoriformia. ROHDENDORF, 1946, placed the genus Pachyneura into the Bibionomorpha, while in 1964 he places it into the Tipulomorpha.
The adult Pachyneura with its long narrow wings, long thin legs, and its nice slender body (KRIVOSJEINA, 1969), let us think rather of the Rhyphidae or of representatives of the families of the Tipulomorpha than of the bibionoids (bibioniformians and fungivoriformians). According to KRIVOSJEINA, 1969, p.236, the trunk of the Media is well-developed. There are two anal veins, the second of which is short ( In many Tipuloids the second anal vein is long, but, for instance, in Idiotipula confluens ALEX. it is short. See next Figure).
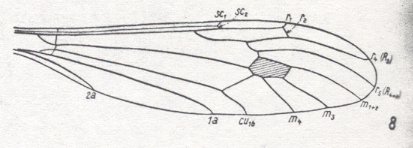
Figure 61a : Wing-venation of Idiotipula confluens ALEX. Family Tipulidae, Tipulomorpha. Second anal vein present, but short. As in Bibioniformia and Fungivoriformia, the media has three branches. The cubital fork is shallow but broad. The cross-vein tb1 is suppressed. Radial Sector 3-branched, with the anterior branch very short, running slightly backwards, and ending up in R1. Subcosta well-developed.
(After ALEXANDER, 1921, from HENNIG, 1954)
If indeed the medial trunk is well-developed in Pachyneura, and if we consider the fact that it is relatively long --- i.e. the Media, measured from its point of origin near the wing-base to the point where it first bifurcates (giving off M4 [= tb1] ), being (at least) a little longer (or much longer) than it is in the primitive Bibioniformia, and also longer than the space where the medial trunk would be were it not vanished (Fungivoriformians) --- to be significant, then we can say that in this respect, together with the shape of the basal half of the wing, the genus Pachyneura is close to the Tipulomorpha.
Status of Axymyia
The genus Axymyia McATEE forms, together with the genera Protaxymyia MAM.etKRIV. and Mesaxymyia MAM., the family Axymyiidae. In this family the abdomen is, according to KRIVOSJEINA, 1969, p.237, massive. And, also according to the latter, p.238 (and her figure), the medial trunk is present. See also Figure 18a , but also Figure 18b . Characteristic of the family Axymyiidae is the 3-branched Radial Sector. The anterior branch is short and ends up either in the Costa close to the endpoint of R1, or directly at that endpoint. The two other branches run parallel, they do not converge proximally. The cross-vein ta (= r-m) lies in the distal half of the wing.
Some of these characters remind us of Tipulomorpha. Of many groups of Tipulomorpha it is, we might say, typical that the anterior branch of the Radial Sector approaches R1, as it can be seen for instance in Hexatoma and Antocha (Limoniidae) (but also in many Tipulidae) :
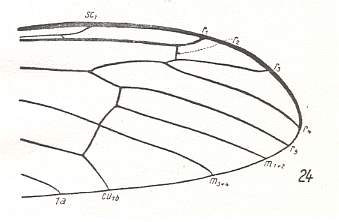
Figure 61b : Wing-venation of Hexatoma bicolor MEIG. Family Limoniidae, Tipulomorpha. The Media has only two branches. The cubital fork is shallow but broad. The cross-vein tb1 is present. Radial Sector 4-branched, with the anterior branch very short and ending up in R1. Subcosta well-developed.
(After HENNIG, 1954)
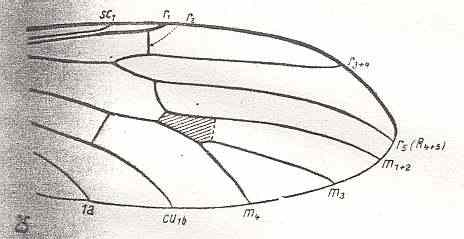
Figure 61c : Wing-venation of Antocha alpigena MIK. Family Limoniidae, Tipulomorpha. As in Bibioniformia and Fungivoriformia, the Media has three branches. The cubital fork is moderately deep. The cross-vein tb1 is suppressed. Radial Sector 3-branched, with the anterior branch very short and ending up in R1. Subcosta well-developed. HENNIG notes that the cross-vein tp , here drawn as an interrupted line segment, is absent in this species, but is present in other species of this genus. He further notes that the anterior branchlet of the Radial Sector is only weakly developed.
(After HENNIG, 1954)
The position of the cross-vein ta (= r-m) distally from the first branching of the Radial Sector is also characteristic of many groups of Tipulomorphs (Tipulidae, Limoniidae, Ptychopteridae). The same can be said of its lying in the distal half of the wing.
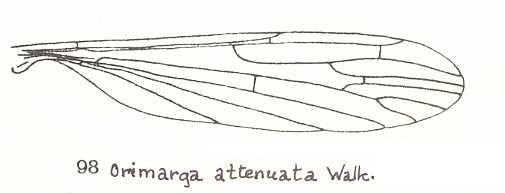
Figure 61d : Wing of Orimarga attenuata WALK. Family Limoniidae (Tipulomorpha). There is no discoidal cell. (From LINDNER, Die Fliegen der palaearktischen Region )
Anyway, the overall structure and topography of the veins are very similar in the compared insects.
Further facts resulting from comparing the wing-venation of Bibionomorpha with that of Tipulomorpha.
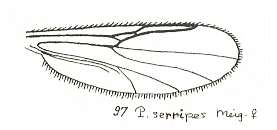
Figure 62 : Wing of Palpomyia serripes MEIG., female. Family Heleidae (= Ceratopogonidae). Radial Sector 2-branched, its anterior branch short and ending up in R1. The cross-vein ta (= r-m) clearly present. Trunk of Media present. M1 and M2 present. M3 absent. Discoidal cell absent (that is, the intermedial cross-vein tp absent). Cubital fork (consisting of M4 and Cu1b) present. The two veins posterior to CuA are respectively CuP and 1A (= 1a). The cross-vein tb1 absent. Wing not elongated.
(After GOETGHEBUER, from LINDNER, Die Fliegen der palaearktischen Region)
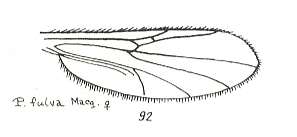
Figure 63 : Wing of Palpomyia fulva MACQ., female. Family Heleidae (= Ceratopogonidae). Wing-venation as in P. serripes (previous Figure).
(After GOETGHEBUER, from LINDNER, Die Fliegen der palaearktischen Region)
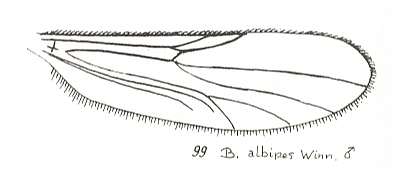
Figure 64 : Wing of Bezzia albipes WINN., male. Family Heleidae (= Ceratopogonidae).
Radial Sector unbranched, short. The cross-vein ta (= r-m) clearly present. Trunk of Media present. M1 and M2 present. M3 absent. Discoidal cell absent. Cubital fork present. The two veins posterior to CuA are respectively CuP and 1A (= 1a). The cross-vein tb1 absent. Wing elongated.
(After GOETGHEBUER, from LINDNER, Die Fliegen der palaearktischen Region)
Apart from lacking the cross-vein tb1 the venation is fairly similar to that in most Bibioniformia, such as Amasia , Hesperinus , or Bibio . Other characters by which the depicted Heleidae differ from bibionoids is the deep fork of M1+2 and the face of the anterior branch (where present) of the Radial Sector which branch is short and ends up in R1 (this latter phenomenon also does occur in Bibionomorpha, such as in certain Sciophilidae ).
All this clearly demonstrates the difficulty inherent in the 'phylogenetic philosophy' (= classifying organisms according to phylogenetic systematics) of tracing the genealogic lineages connecting families and other more or less higher taxonomic units. If many, or indeed almost all, more or less equal character transformations turn out to have taken place independently of each other, then the method of phylogenetic systematics (sensu HENNIG) will not be able to reveal the genealogic lineages, and, moreover, cast doubt on the very existence of them. But, to be more precise, these difficulties cast doubt on the existence of these lineages, with their ongoing bifurcations, in the Explicate Order, because according to our theory they do exist in the Implicate Order in the form of a constantly ramifying noëtic trajectory. But for tracing this trajectory there are no special methods available, that is, we must still use the methods of phylogenetic systematics, but be aware that these methods will reveal parts of this trajectory only in a few 'lucky' cases.
This concludes our discussion about the last terminal series of fungivoriformian families, and at the same time concludes our exposition of the phylogenetic relationships of the recent Bibionomorpha (= Rhyphiformia + Bibioniformia + Fungivoriformia).
In the next document we will discuss the fossil Bibionomorpha and include them into the above established phylogenetic trees. After this we will interpret our phylogenetic findings in terms of the Implicate Order, noëtic trajectory, and projections.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXIX-A.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII