

Example of two totally different groundplans nevertheless suited to be fitted into the same ecological niche.
Each organic family-groundplan intrinsically defines a definite set of potential ecological niches. If a potential ecological niche actually exists in the Explicate Order its noëtic counterpart constitues the existential conditions of this groundplan. And the noëtic content of this groundplan can only be projected into the Explicate Order when it carries with it the noëtic content of those existential conditions. This projection is then in the Explicate Order experienced (by us) as an evolutionary process in which an existing species adapts itself to a given ecological niche. And this colonization of such a new ecological niche often results in the appearance of a new species, that is, the original species splits into two species. In this way a family-groundplan is fanning out over the ecological landscape resulting in its multiplication or differentiation, that is, the appearance of the many species and species groups (genera) of the family.

Figure 1 : Imago of Myrmeleon formicarius, Ant Lion (Order Neuroptera). Wing-span 65-75 mm.
(After ZAHRADNIK, in Thieme's Insektengids, 1977)
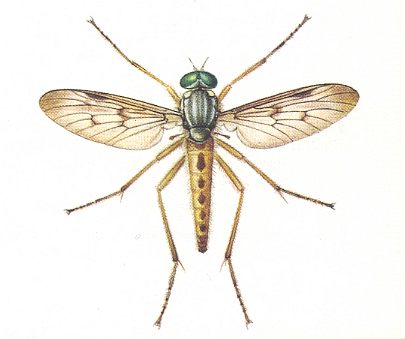
Figure 2 : Imago of Rhagio scolopaceus (Order Diptera). 13-18 mm.
(After ZAHRADNIK, in Thieme's Insektengids, 1977)
Let's now go ahead with the example, taken from WEBER in his Grundriss der Insektenkunde, 1966, p. 324-326 :
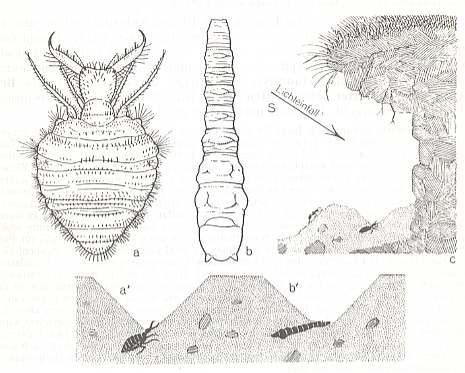
Figure 3 : Constructors of sandpit traps.
a, a' -- Myrmeleon formicarius, larva, dorsal view, and funnel with ambushing animal in profile. (After DOFLEIN)
b, b' -- Vermileo vermileo, larva. (After BUCHNER, redrawn)
c -- Typical habitat, profile, schematic.
(From WEBER, 1966)
The Ant Lion, the larva of the Planipennian Myrmeleon formicarius (See Figure 3, left), living in central and southern Europe, and the Worm Lion, the larva of the Leptid (= Rhagionid) Vermileo vermileo (See Figure 3, center), living in the mediterranean countries, show in their overall structure all prototypic characters of the taxonomic groups to which they belong. The Ant Lion, with a well-differentiated head, with powerful pincers (Mandibles + Maxillae), and possessing true legs, is just as clearly a member of the Planipennia as the legless hemicephalous (= head not clearly differentiated) Worm Lion is a dipterous larva. The only similarity between them consists of the fact that the posterior end of their body is more heavily thickened and bristled, as is otherwise usual in the respective groups (in Figure 3, b, the bristles are not drawn). Both species are markedly stenök (narrowly confined ecologically) and stenotopous (very locally living). Sometimes they (that is, larvae of both species) can be found together at precisely the same type of locality, in places where there is homogeneous fine and dry, and therefore easily mobile, sand, especially under overhanging edges of paths or roadsides (see Figure 3, right), where sandstone disintegrates and where rain seldomly can interfere. Slopes facing south are preferred because of the rich incidence of solar rays.
While in the case of Vermileo the imagines take food from flowers, the imago of Myrmeleon is known to be unable to take food. Its life therefore is short. ( WEBER, 1966).
Before we are going to discuss this example of WEBER's in the context of our theory of organic groundplans, some additional information might be instructive. RICHARDS & DAVIES, 1977, in Imms' General Textbook of Entomology, p. 999, while dealing with the family Rhagionidae, add :
Females of Vermileo and Lampromyia (Hemmingsen, 1963) lay their eggs in sand, and the larvae construct conical pitfalls for the capture of their prey, after the manner of 'ant lions'. The 5th segment of the larva bears a ventral mobile pseudopod which assists in seizing and holding the prey. The 10th and 11th segments each carry a transverse row of long hooklets which serve as organs for boring and fixation (cf. Wheeler, 1930).
So, according to this additional information there exists, in the Rhagionidae, in addition to Vermileo, yet another species the larva of which lives in the same way.
In the example of WEBER we have seen that the ecological niche of at least the l a r v a e of two taxonomically unrelated insects is identical. In describing and defining the ecological niche of a given insect species the habitats of larvae as well as of the adults must be taken into account. In the case of Vermileo and Myrmeleon the adults of the former take nectar (from flowers), and this is only to fuel its flight-muscles. As seems to follow from WEBER's account, no other food is taken. The adult of the latter (Myrmeleon) flies (around), but does not take food at all. So the way of life of the respective adults does not differ significantly. Therefore it is allowed to consider the whole ecological niche of the two species Vermileo (Diptera) and Myrmeleon (Neuroptera) to be identical, or at least virtually identical, while the respective groundplans invoved differ sharply from one another. So it is not a priori impossible that two substantially differing groundplans have instances that occupy precisely the same ecological niche.
Also this example demonstrates that, apart from certain exceptional cases (such as in epizoic diptera), a groundplan's significance is only partially, or sometimes not at all, ecological, that is, its significance is mainly formal.
In the case of the ancestral family (whatever this may be) of the Myrmeleontidae (ant-lion flies) we may suppose that while (this ancestral family) radiating into various ranges of the ecological landscape, and thus successively occupying and adapting to the various potential ecological niches circumscribed by its groundplan, instances of it stumble upon a peculiar member of the set of potential ecological niches : ambushing ants in specially constructed pitfalls. Initial circumscription of this niche, followed by injection into the Implicate Order, noëtic reaction, and projection of the reaction-product back into the Explicate Order (as generally described earlier) result in the definitive adaptation of the larvae to such a way of life and in the establishment of a new neuropterous family, the Myrmeleontidae. But what is interesting here is that the dipterous family Rhagionidae, while radiating into the ranges of the ecological landscape and successively occupying its potential ecological niches, it, while stumbling upon it, finds out that "ambushing ants in specially constructed pitfalls" turns out to be among its potential ecological niches as well. And also here, initial circumscription of this niche, followed by injection into the Implicate Order, noëtic reaction, and projection of the reaction-product back into the Explicate Order result in the definitive adaptation of the larvae to such a way of life and in the establishment of a new dipterous species, Vermileo vermileo ( Here the acquired adaptations are apparently not far-reaching enough to effect a transformation of the original groundplan of the Rhagionidae into a new groundplan).
This concludes our exposition of the fact that it is not as a rule impossible for two different groundplans to have instances that occupy the same ecological niche.
Later, some general insights that still further develop the theory of organic groundplans will be added . . .
In the foregoing four documents (Parts XXVII, XXVI, XXV, and XXIV) we have discussed the idea of groundplan and its relation to ecology and the Implicate Order. The theory of groundplans expounded in these documents is meant to be a general theory, i.e. a theory that is supposed to apply not only to Diptera, and to all insects for that matter, but also to all organisms. Organic evolution is not a process aimed at an ongoing improvement of biological structures, that is, not a process exclusively concerned with intensifying existing adaptations of organisms to their environment. Of course evolution, as we experience it in the Explicate Order (or how it takes place in that Order), is surely driven by ecological factors, that is, by the successive colonization of new ecological niches. And adaptation plays a role therein. But as can already be seen in the Explicate Order, evolution also turns out to be driven by purely formal factors, factors that is, whose nature is not ecological. We have seen this in the morphology of dipterous insects, where we can distinguish a number of different body groundplans that apparently have only little ecological significance, and are mainly of a formal nature. And in order to understand this we have described these groundplans and their (formal) evolution in terms of an analogy based on mineral stability diagrams. The formal derivation (= noëtic evolution) in the Implicate Order of one groundplan (in its noëtic form) from another (also in its noëtic form), where, of course, biological adaptation cannot play a role, is described in terms of unidirectional phase transitions as they take place in certain physical substances when thermodynamic conditions change. Subsequent projections of the noëtic forms of the newly derived groundplans are seen in the Explicate Order as evolutionary transformations of one organic groundplan into another. However, a noëtic form of a groundplan can only project into the Explicate Order if it is provided with the noëtic forms of conditions of existence in this Order. And it is these conditions that contain the relevant adaptations to a certain ecological niche existing in the Explicate Order.
Preceding all these expositions of organic groundplans we were studying the evolution of wing-venation in nematocerous diptera (crane-flies, mosquitoes, midges, etc.). The last family group we have looked into in this respect were the Rhyphidea. And because these have apparently formed the evolutionary point of departure of all the higher Diptera (including rhagionids, horse-flies, robber-flies, hover-flies, and all the muscoid flies [acalyptrates and calyprates] ), much attention was given to the possible evolutionary transition leading from rhyphoid to muscoid flies. And in that exposition the theory of groundplans was born.
In the next document we will pick up again our exposition of the evolution of wing-venation in Diptera (first finishing the Nematocera and then passing on to the rest of the Diptera).
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXVIII.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI