

[In the following enquiry into the wing-venation and its transformations in Diptera-Nematocera we will consider the subcostal vein (Sc) at most only occasionally. This vein, especially its terminal branches (Sc1, Sc2), is -- in Diptera -- often difficult to discern.]
Of all Nematocera the representatives of the family Tanyderidae (superfamily Tipulidea, infraorder Tipulomorpha) have the most primitive (meaning original) wing-venation. There are about 30 species. All of them having Rs and M both 4-branched. All four branches of Rs end up freely in the wing margin. The wings of two species are depicted in the next two Figures.
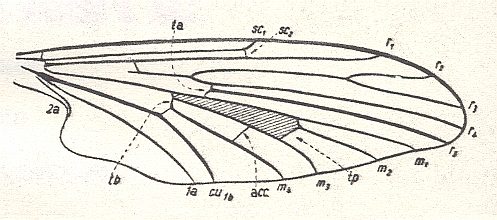
Figure 1 : Wing of Protoplasa fitchii O.S.. Order Diptera, Family Tanyderidae. The shaded area in the wing signifies the Discoidal Cell (in a natural wing this cell is as transparent as is the rest of the wing membrane).
(After HENNIG, 1954)
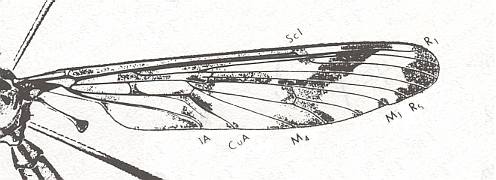
Figure 2 : Wing of Eutanyderus oreonympha. Order Diptera, Family Tanyderidae. Length of wing, ca. 13 mm.
To see this figure better and a little enlarged, click twice on it.
(After COLLESS, 1970, from RICHARDS & DAVIES, 1977)
The wing-venation in the Tanyderidae is so primitive, original, and (thus) complete, that the wing-venation of all other representatives of the Diptera can formally be derived from it. That does, however, not necessarily mean that -- judging things only by the features of wing-venation -- all Diptera have descended from some tanyderid ancestor.
Limoniidae
The venational groundplan of the Limoniidae is represented by the wings of Tricyphona protea ALEX. :
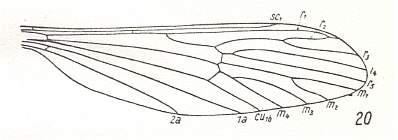
Figure 3 : Wing-venation of Tricyphona protea ALEX.
(After ALEXANDER, 1927, from HENNIG, 1954)
Rs is 4-branched (like in Tanyderidae). The branches are R2, R3, R4, and R5. Rs first branches into R2+3 and R4+5. R2 (unlike in Tanideridae) ends up, not at the wing margin, but in R1, albeit only just so. R2+3 still branches off from the main stem of Rs, that is, not from R4. Discoidal cell absent (as a result of reduction of the cross-vein tp (for the discoidal cell and tp see Figure 1 ). Fork of M1-M2 still present, that is, M still 4-branched. Chief forks of the venation only a little distad of the middle of the wing (i.e. lying just in the apical half of the wing). 2A (second anal vein) long. This feature is apparently connected with the narrowing of the wing-base.
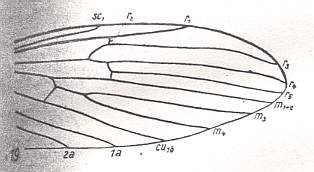
Figure 4 : Distal part of wing-venation of Molophilus flavoscutellatus LACKSCH.
(After HENNIG, 1954)
R2 ends up in R1 more basally. R2+3 still branches off from the main stem of Rs, that is, not from R4. M1-M2 fork absent, leaving only the vein M1+2 (together with M3 and M4). No discoidal cell.
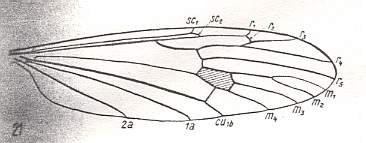
Figure 5 : Wing-venation of Limnophila ferruginea MEIG. (After HENNIG, 1954)
R2 runs into R1. R2+3 branches off, not from the main stem of Rs, but from R4 (this is called "Capture of vein R4 by R2+3"). This feature we will also encounter in other Limoniidae, but also in all Tipulidae (and in Phlebotomus papatasii SCOP., belonging to the Psychodidea, but here R2 ends free, it does not run into R1, see HERE ). Discoidal cell present. M1-M2 fork present. The Media has four branches. 2A long.
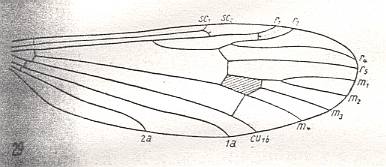
Figure 6 : Wing-venation of Gymnoplistia leucopeza postica ALEX.
(After ALEXANDER, 1929, from HENNIG, 1954)
The venation is the same as in Limnophila (previous Figure).
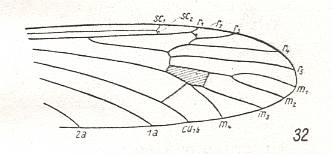
Figure 7 : Wing-venation of Architipula clara HANDL. Upper Lias of Mecklenburg (Germany). (After HANDLIRSCH, 1938, from HENNIG, 1954)
The venation is again the same as in Limnophila (Figure 5) and as in Gymnoplistia (previous Figure).
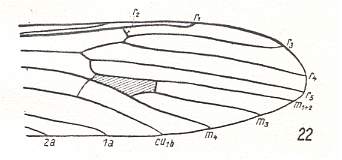
Figure 8 : Distal part of wing-venation of Erioptera trivialis MEIG. (After HENNIG, 1954)
The venation is again the same but now the M1-M2 fork is absent.
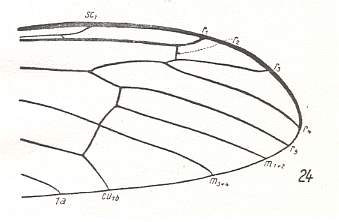
Figure 9 : Distal part of wing-venation of Hexatoma bicolor MEIG. (After HENNIG, 1954)
Radial events take place more distally. Discoidal cell absent. M3 disappeared (or coalesced with M4).
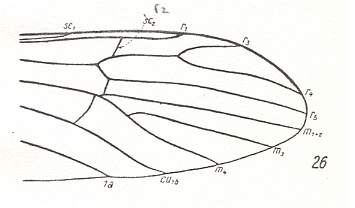
Figure 10 : Distal part of wing-venation of Empeda nubila SCHUMM. (After HENNIG, 1954)
R2 (erroneously indicated by Hennig as sc2) is moving basad (i.e. into the direction of the wing-base). It descends along R3, and ends up on R3+4. No discoidal cell. It is opened as a result of the reduction of the cross-vein between M3 and M1+2. M has 3 branches. This situation, insofar as it comes from M being still 4-branched, is apparently accomplished by the disappearance of the M1-M2 fork.
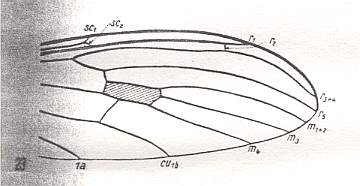
Figure 11 : Distal part of wing-venation of Dicranoptycha livescens LW. (After HENNIG, 1954)
R3 absent. This could be the result of either the coalescence of R3 with R4, or simply by reduction of the vein R3. This situation (absence of R3) can also be seen in Idiotipula confluens ALEX., which belongs to the family Tipulidae, see HERE . Discoidal cell present : It is still closed by the retention of the cross-vein between M3 and M1+2.
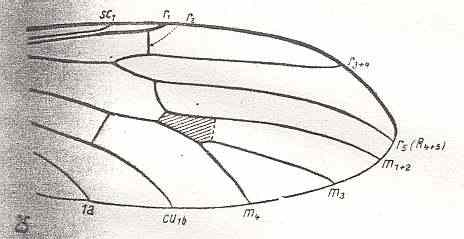
Figure 12 : Distal part of wing-venation of Antocha alpigena MIK. The cross-vein tp, drawn as dotted line, is absent in this species, but is present in other species of the genus. R2 is only faintly present. (After HENNIG, 1954)
R3 also absent, but R4 following a more or less different course. Discoidal cell present (not in this particular species, but in others of the same genus). M still 3-branched : M1+2, M3, M4.
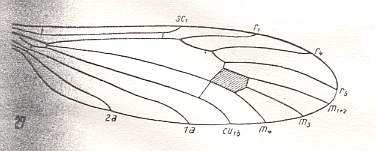
Figure 13 : Wing-venation of Atarba picticornis O.S.
(After ALEXANDER, 1942, from HENNIG, 1954)
R3, and now also R2, absent. So Rs is now 2-branched. Discoidal cel present. M is 3-branched (M1+2, M3, M4). Two long anal veins (1A, 2A) still present.
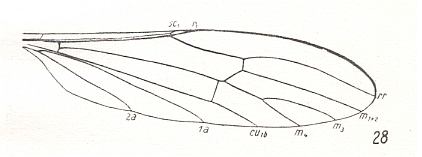
Figure 14 : Wing-venation of Toxorhina muliebris O.S. (After HENNIG, 1954)
In addition to R2 and R3, now also R4 absent. This leaves the Radial Sector consisting of only one vein. Cross-vein between M3 and M1+2 absent, therefore discoidal cell opened and thus absent. 1A and 2A still long. Wing still elongate.
This concludes our discussion of the wing-venation in the family Limoniidae. In this family there is no tendency for R2 to become strongly recurrent as we will see it in the family Cylindrotomidae.
Tipulidae
Especially in the family Tipulidae we see, in the wing-venation, a strong tendency to having all the forks shifted towards the apical part of the wing. As in Limoniidae (but in the present family apparently even more so) the wings are markedly elongate. R2 tends to be a recurrent vein, but, apparently by far not so much as in the family Cylindrotomidae.
The venational groundplan of the family Tipulidae can be represented by Pales dorsalis FABR., except that the origin of Rs must be situated (in this groundplan) a little more proximally as we see it in, for example, Idiotipula confluens ALEX. See for the latter Figure 17 .
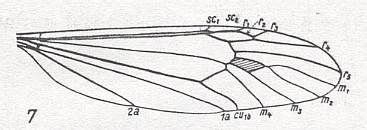
Figure 15 : Wing-venation of Pales dorsalis FABR. (After HENNIG, 1954)
Capture of R4 by the vein R2+3. R2 recurrent, and ending up in R1. R3 short, ending up at wing-margin. M1-M2 fork present (that is, the veins M1 and M2 present). M is 4-branched : M1, M2, M3, M4. 1A and 2A long, and reaching the wing-margin.
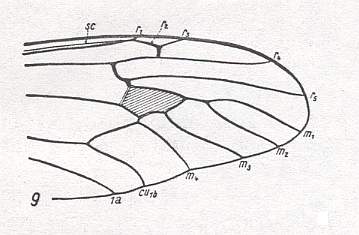
Figure 16 : Distal part of wing-venation of Xiphura atra L. (After HENNIG, 1954)
Same as in previous Figure, but with Rs branching off more proximally. So also this species can (at least as to the part of it that is depicted, its distal half) represent the venational groundplan of the family Tipulidae.
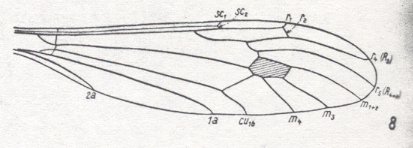
Figure 17 : Wing-venation of Idiotipula confluens ALEX (After ALEXANDER, 1921, from HENNIG, 1954)
Capture of R4 by the vein R2+3 as in the two previous Figures, but R3 absent. Fork of M1-M2 absent, meaning that the media is 3-branched.
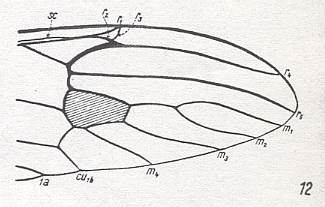
Figure 18 : Distal part of wing-venation of Tanypremna sp. (After HENNIG, 1954)
Still capture of R4 by the vein R2+3 as in the previous Figure, but R2 more clearly recurrent, and R3 still present albeit very short and on the verge of vanishing altogether. Fork of M1-M2 present. The media is 4-branched. So relative to the wing in Figure 17 (Idiotipula) this wing is primitive (plesiomorph) as to R3, and as to M. But it is advanced (apomorph) as to the recurrent course of R2, and, especially, as to the point where Rs branches off from R which point lies very distally (and still more so in Dolichopeza, next Figure).
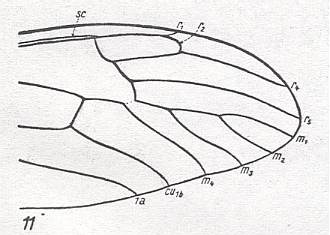
Figure 19 : Distal part of wing-venation of Dolichopeza albipes STRÖM.
(After HENNIG, 1954)
Follows upon Tanypremna (previous Figure) with the complete disappearance of R3. Basal piece of M3 vanished so that the discoidal cell is absent. The fork M1-M2 still present. M still 4-branched. Rs branches off from R still more distally.
Judging from the recent Tipulidae the capture of R4 by R2+3 (that is, shift of R2+3 towards the apical part of the wing along R4) is at least already present in the groundplan of the family. The fact that this situation is already very old is suggested by fossils such as Architipula clara HANDL. (probably belonging to the Limoniidae), see Figure 7 , and also by Architipula radiata ROHD. from the lower Jurassic of central Asia :
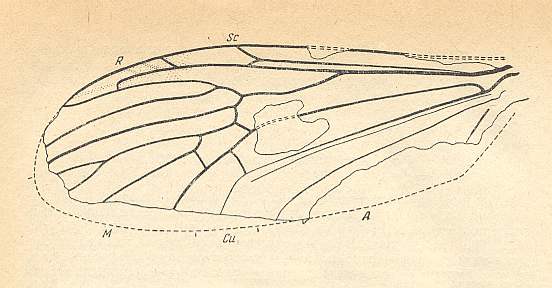
Figure 20 : Wing of Architipula radiata ROHD. from the lower Jurassic of Issic Kul, central Asia. PIN No. 371/403. ( The age of the Issic Kul fauna originally was held as being upper Triassic. Later, however, it was held as being Lower Jurassic). Length of wing 5.9 mm.
(After ROHDENDORF, 1962, from ROHDENDORF, 1964)
We clearly see in this fossil (Architipula radiata) that the Radial Sector first splits into two branches : R5 and R4. Shifted onto R4 we see R2+3, which latter in turn gives two branches : R3, which ends up at the wing-margin, and R2, which folds back and ends up in R1 (Radius). To express this same situation differently : Originally (in more primitive forms) Rs splits into R2+3 and R4+5. Later (in evolution) R2+3 shifts distally along R4, thus resulting in the capture of R4 by vein R2+3. During this shift R2 keeps its connection with R1 and thus becomes recurrent.
M is 4-branched. The cross-vein tp between M4 and M3 is present, implying that the discoidal cell is present. Between R5 and M there is the cross-vein ta (= rm) connecting R5 with the discoidal cell. Between CuA and M there is the cross-vein tb (= mcu), it connects CuA with the discoidal cell. CuP thin, not reaching the wing-margin. 1A long, reaching the wing-margin. 2A probably also long and also reaching the wing-margin.
With R2 strongly recurrent, this fossil leads us structurally to the Family Cylindrotomidae, with which we will deal next.
The tipuloid venational scheme including in it the described "capture of R4 by vein R2+3" can undoubtedly be evolutionarily accomplished in different ways, that is, along different pathways (for instance certain parts of the overall process taking place in a different order). This means that, among other things, in evolutionarily accomplishing the pattern as we see it in Tipulidae as well as in many Limoniidae (and also in the other families of the superfamily) the historical process does not necessarily need to pass through the "tanyderid phase" (see Figure 1 ) in all cases : If the development (origin of the first Tipulidea from a non-dipterous stock) starts from Mecoptera with a reduced venation, such as representatives of the suborder Paratrichoptera (see Figure 1 in previous document ) or of some true Mecoptera such as ancestors of the family Nannochoristidae (see Figure 13 in previous document ), it -- that is, the development -- will probably go its way through the "tanyderid stage" (4 free branches of Rs as well as of M, see Figure 1 ). If, on the other hand, the development (resulting in about the same tipuloid venational pattern just mentioned) starts from Mecoptera having a more complete venation, such as Agetopanorpa (see Figure 12 in Part XIV ) or Platychorista, (see Figure 20 in Part XIV ), then some parts of the process, such as R2 ending up in R1, could already have taken place before the overall reduction of the venation was well under way. And then the "tanyderid phase" is simply skipped. There will be a phase, it is true, that looks like the tanyderid phase. It is, however, not exactly the same.
Two or more different lines of diptera -- in the present case two or more different lines of Tipuloids -- have descended from two or more different mecopterous progenitors.
To underscore the potential of the Mecoptera to give rise to different and separated lines of Diptera (in addition to giving rise to Lepidoptera and probably Hymenoptera) we show a fossil wing that certainly is dipterous, but which cannot be assigned to any known infraorder.
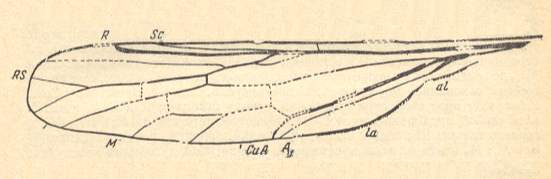
Figure 21 : Wing of Mesophantasma tipuliforme ROHD. from the Middle Jurassic of Karatau (Galkino), southern Kazachstan. PIN No. 2452/560. Length of wing 8.1 mm.
(After ROHDENDORF, 1962, from ROHDENDORF, 1964)
Cylindrotomidae
The groundplan of wing-venation of the family Cylindrotomidae is represented by that in Phalacrocera, but then with the M1-M2 fork present (because it is present in Cylindrotoma (see Figure 23)) [It could be that in Phalacrocera the fork M1-M2 is still present, and that M3 is absent].
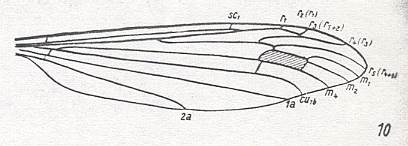
Figure 22 : Wing-venation of Phalacrocera replicata L. (After HENNIG, 1954)
Capture of R4 by the vein R2+3. The Origin of Rs lies before midwing, that is, within the present superfamily, relatively proximally (as we see it also in Trichoceridae, see HERE , and apparently more or less so in all Cylindrotomidae figured in HENNIG, 1954). M3 absent, M1-M2 fork present, or, and probably better : M1-M2 fork absent (only one branch left) and M3 present.
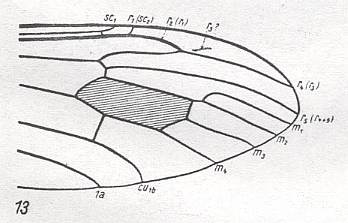
Figure 23 : Distal part of wing-venation of Cylindrotoma distinctissima MEIG.
(After HENNIG, 1954)
M1-M2 fork still present. M3 present. R3 almost vanished. R2 strongly recurrent while ending up in R1.
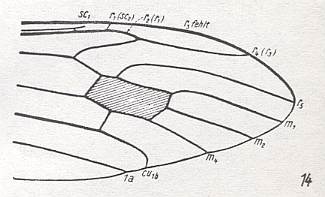
Figure 24 : Distal part of wing-venation of Triogma trisulcata SCHUMM.
(After HENNIG, 1954)
M1-M2 fork absent. M3 present (or -- differently interpreted -- M1-M2 fork present and M3 vanished). R3 completely vanished. R2 strongly recurrent while ending up in R1.
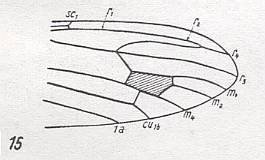
Figure 25 : Distal part of wing-venation of Stibadocerodes tasmaniensis ALEX.
(After ALEXANDER, 1927, from HENNIG, 1954)
M1-M2 fork absent. M3 present, or M1-M2 present and M3 absent. R3 vanished or coalesced with R4 . R2 strongly recurrent while ending up in R1. The latter does not seem to end up in the wing-margin. It continues as R2 and finally as R4.
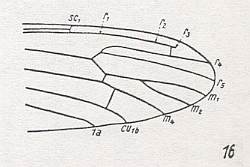
Figure 26 : Distal part of wing-venation of Stibadocerodes australiensis ALEX.
(After ALEXANDER, 1927, from HENNIG, 1954)
Extreme basal piece of R3 seems to be preserved, ending free in the wing-membrane. R2+3 branches off from R4 while it looks like a cross-vein. R2 completely in line with R1 which does not itself reach the wing-margin (as in S. tasmaniensis) but ending blind as R3. M3, including its basal piece, absent, therefore no discoidal cell present.
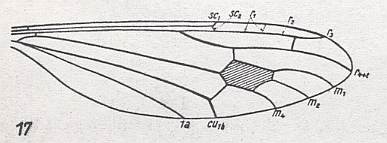
Figure 27 : Wing-venation of Stibadocerella albitarsis de MEIJ.
(After ALEXANDER, 1927, from HENNIG, 1954)
R4 and R5 coalesced. R2+3 present, it originates from R4 (in fact from R4 and R5 coalesced) like a cross-vein. It divides into R2 and R3, where R3 proceeds to the wing-margin while R2 bends back to R1 and ends up in it just before R1 itself reaches the wing-margin. The end-piece of R1 looks like a cross-vein. M is 3-branched. Discoidal cell present.
Trichoceridae
We consider this family as not representing an individual independent line within the complex of Tipulidea. Except for the structure and course of 2A and the very short R2, but long R3, its venation looks much like that of Pales dorsalis FABR. ( Tipulidae), see Figure 15 , which we had established as representing the venational groundplan of the family Tipulidae.

Figure 28 : Wing-venation of Trichocera regelationis L.
(After HENNIG, 1954)
Capture of R4 by the vein R2+3. R2 extremely short, ending up in R1, not recurrent. M is 4-branched. M1-M2 fork present. Discoidal cell present. Rs branches off from R before middle of the wing. Second anal vein (2A) very short and bending toward (and reaching) the hind-margin basally.
Except for the structure and course of 2A, Architipula clara HANDL. from the upper Lias of Mecklenburg, Germany, looks much the same as Trichocera :

Figure 29 : Wing-venation of Architipula clara HANDL. Upper Lias of Mecklenburg (Germany). (After HANDLIRSCH, 1938, from HENNIG, 1954)
Ptychopteridae
The venational groundplan of the family Ptychopteridae ( = Liriopeidae) can be represented by Liriope scutellaris MEIG. :
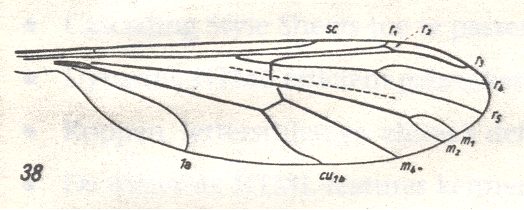
Figure 30 : Wing-venation of Liriope scutellaris MEIG. (After HENNIG, 1954)
[Later on (next document) we could follow the groundplan still further back in history.]
The cross-vein ta (= r-m) ends in R4+5 (instead of in R5), that is, its level is at the proximal side of the fork R4-R5. We see this also in Tricyphona protea ALEX. ( Limoniidae), see Figure 3 . R2 ends up in R1. No capture of R4 by the vein R2+3. The latter branches off from R4+R5, that is, from the stalk of the fork R4-R5. The fork M1-M2 is present, M3 has vanished. No discoidal cell. 1A moderately long, bending towards the wing-margin. 2A absent. Wing elongate.
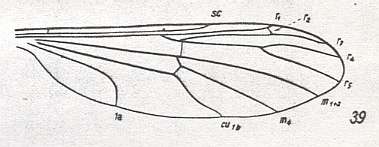
Figure 31 : Wing-venation of Bittacomorphella nipponensis ALEX.
(After ALEXANDER, 1927, from HENNIG, 1954)
This venation is the same as that in the previous Figure, but with the M1-M2 fork disappeared. It leaves the Media only 2-branched. Wing elongate.
The wing-venation of the representatives of the family Ptychopteridae deserves additional attention, because of the features in the basal part of the wing, and because of the uncerainty as regards the taxonomic position of this family.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XVII.