
Organic Evolution in terms of the Implicate and Explicate Orders.
Part XXXVIII
Hymenoptera (wasps, bees, ants) (Sequel)
The evolutionary diversification in the Order Hymenoptera in terms of Strategies (Sequel).
Secondary-phytophagous (phyto-oophagous) phase (Sequel)
Let us now turn to the second major group of secondary-phytophagous Hymenoptera, the terebrant gall-wasps in the broad sense, that is, to the Cynipoidea. With about 1600 species they are widely distributed. The systematists divide them into 130 genera classified across 10 families. Their exotic forms, though, are until now very little known.
Of all this diversity of froms only one family, the Cynipidae, or true gall-wasps, are phytophagous. [We should not confuse these true gall-wasps with gall-producing saw-flies : The true gall-wasps (and their non-phytophagous allies) belong to the Suborder Terebrantia, whereas the gall-producing saw-flies belong to the Suborder Symphyta.]. Here we depict several representatives of the gall-wasps.
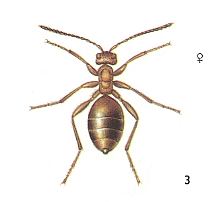
Figure 1 : Biorhiza pallida. Female. Sexual generation 1.7 - 2.8 mm, asexual generation 3.5 - 6 mm. Family Cynipidae.
(After SEVERA, in ZAHRADNIK, 1977, Thieme's insektengids voor West- en Midden-Europa)
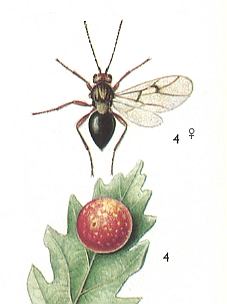
Figure 2 : Oak gall-wasp Cynips quercusfolii. Female., and gall. Sexual generation 2.3 - 2.5 mm, asexual generation 3.4 - 4 mm. Family Cynipidae.
(After SEVERA, in ZAHRADNIK, 1977, Thieme's insektengids voor West- en Midden-Europa)
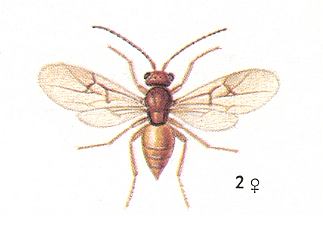
Figure 3 : Andricus lignicola. Female. Asexual generation 4 - 4.5 mm. Family Cynipidae.
(After SEVERA, in ZAHRADNIK, 1977, Thieme's insektengids voor West- en Midden-Europa)
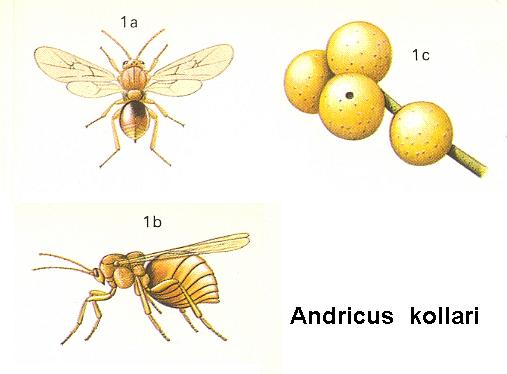
Figure 4 : 1a - Andricus kollari Hrt. about 10 mm. Family Cynipidae.
1b - resting position. 1c - galls on oak.
(After CHINERY, in Elseviers insektengids voor West-Europa, 1983)
The next figure schematically depicts some types of plant galls caused by gall-wasps (in fact, as we will see later, caused by their larvae).
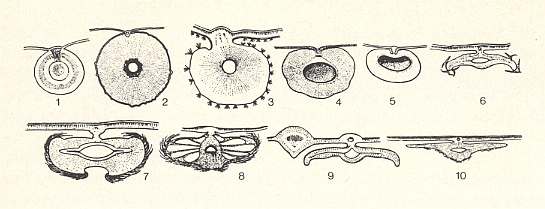
Figure 4a : Cross-sections through galls of gall-wasps (Cynipidae) on oaks.
1 - Gall of Neuroterus quercusbaccarum, bisexual generation. 'grape gall'.
2 - Gall of Cynips quercusfolii, asexual generation. 'common oak gall-apple'.
3 - Gall of Chilaspis nitida, asexual generation.
4 - Gall of Cynips longiventris, asexual generation. 'ornamental gall'.
5 - Gall of Cynips divisa, asexual generation. 'brown shiny gall'.
6 - Gall of Neuroterus fumipennis, asexual generation. 'small lenticular gall'.
7 - Gall of Neuroterus numismalis, asexual generation. 'silk gall'.
8 - Gall of Neuroterus lanuginosus, asexual generation.
9 - Gall of Neuroterus laeviusculus, asexual generation. 'saucer gall'.
10 - Gall of Neuroterus quercusbaccarum, asexual generation. 'great lenticular gall'.
(After ROSS, 1911, in BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)
The next Photograph depicts a typical gall-wasp gall and its structure.

Figure 4b : Cross-section through a gall of the gall-wasp Andricus kollari with larva-chamber and larva. See also Figure 4 .
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)
Some galls contain more than one larva-chamber :
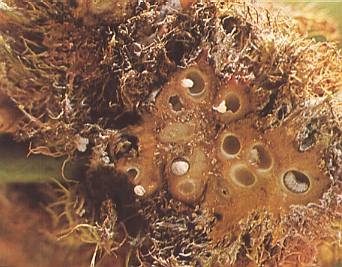
Figure 4c : Cross-section through a gall of the common rose gall-wasp Diplolepis rosae with larva-chambers and larvae.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)
(Speaking about the Cynipidae, true gall-wasps)
To this family belong almost two thirds of all known species of the superfamily (Cynipoidea). The overwhelming majority of the Cynipidae possesses the exclusively developed ability to cause in plants various galls in which the whole individual development of cynipids takes place. Only a few of them are inquilines that develop in galls of their relatives or, more rarely, in galls of other insects, especially of gall-midges (Cecidomyidae).
All the other families of the Cynipoidea belong to the typical terebrants of which the larvae are carnivorous.
The life-activity of the true gall-wasps contains such complex and peculiar features, that, as a result, this, during the larval state phytophagous, group stands out, not only with respect to the other Terebrantia, but also to all remaining Hymenoptera. It is therefore not surprising that an attempt to answer the question about their origin will meet with many difficulties.
Today we can say that most important and at the same time instructive in answering this question is the fact that all gall-wasps (Cynipidae) develop, not at the expense of already formed plant organs, but at the expense of not yet differentiated meristemic tissue, that is of precisely that germ-tissue on which also the larvae of the just discussed seed-eater terebrants feed.
By the term "meristemic (meristematic) tissue", or "meristem" (from the Greek word m e r i s o o = divide) is meant the uniform formative tissue, consisting of polyhedric cells, filled with fine-grained protoplasm and coated with very thin and smooth shells. The cells are in such a degree strongly united with each other that between them no space is left. All of them grow, divide, and, while being transformed, give rise to various definitive tissues. Further, in the for the plants normal formational processes, some parts of secondary tissues are in a state of change and again give rise to tissue that is able to divide [i.e. to tissue of which the cells are able to divide] -- the meristem ("secondary meristem").
With this situation -- the larvae of the cynipids feeding on meristem -- the following series of very important facts is indissolubly connected :
- The fact that in gall-wasps the larvae are helpless, 'sessile', developing at one place within a narrow breeder cell (breeding chamber), similar to the development of the larvae of terebrant seed-eaters inside the shell of the [plant] seed.
- The part of the metabolic meristem guaranteeing proper food for the immobile larva is isolated from its surroundings by a special plant shell. The degree of isolation of this shell usually is closely connected with the location of the breeding chamber : either within a more or less freely developed gall, or, the other way around, concealed under the surface of the plant (inside a shoot, stalk). In the latter case the isolating shell may be developed weakly, but it is always such that proper food for the larva being at one place is guaranteed completely.
The absence of development of a protecting shell, in some cases where the growth of the larvae of cynipids takes place in juicy grassy plants (of which we will speak further down) can be well understood if one takes into account that such a shell may not develop there where it is not needed, that is, when the larva, living inside the plant, moreover is already protected by its [the larva's] integument. Rather, the opposite might take place, namely a more or less complete reduction of this shell, having become unnecessary in the transition from development in free galls to development inside stalks, especially when this transition is connected with a change of food plant.
- Feeding on meristem implicated in the gall-wasp larvae the same feature as is seen in larvae of terebrant seed-eaters : the absence of a connection between middle and hind gut, expressed by the total absence in them of defecation during the whole period of feeding.
- The development at the expense of very small parts of isolated meristem had its influence also in the adult gall-wasps : All of them are very small indeed, having a length of usually 1-2 millimetres. Also this feature connects the gall-wasps with the "seed eaters" and opposes them to the original phytophags which always attain larger sizes.
Such a remarkable versatile similarity of the gall-wasps with the chalcidoid seed-eaters is enhanced still more when we take into account the fact that among the gall-wasps there are in fact quite many of them that develop at the expense of generative organs of plants -- young seeds and fruits. Thus, for instance, the peculiar galls of the medusa gall-wasp, Cynips caput medusae Hart., which are covered with long thread-like outgrowths, projecting to all sides, originate from the marrow [pljuska, I'm not shore whether this means "marrow"] of the acorn. Further, the galls of the acorn gall-wasp Andricus mayri Kieff., developing at the expense of acorns of cork oak and holm oak ( Quercus suber L. and Q. ilex L.). And such are also the galls of the maple-tree gall-wasp, Pediaspis aceris Foest., emerging on fruits of maple. And so many others.
Anyway, in order to clarify the real state of affairs with respect to the gall-wasps as well as to the "seed-eaters", it is very important to know not only the mere place where the larva feeds or where the gall is formed, but also the very point where the egg is laid. Although this question is not yet sufficiently clarified we are, on the basis of a series of observations, probably not in error to maintain first of all that very many gall-wasps, if not the majority of them, lay their eggs into the buds of plants, either into flower buds, shoot buds, or leaf buds. Here we must note that the egg (sometimes several of them) is not placed on the surface but is deeply inserted into the bud. It is remarkable that in infecting buds the females of gall-wasps show a definite inclination to perform this operation as quickly as possible and as it were prematurely, that is, laying the egg before differentiation of the tissue and the formation of organs inside the bud get started. Therefore not a low number of gall-wasps lay their eggs in the buds already from autumn. In middle latitudes one can, even in late autumn or still in December, observe females of some species of gall-wasps, crawling on the snow or climbing up oak sprout in order to lay eggs in the budds of the oak at the right time. Thinking of this, we can easily understand how precisely such relationships could originate in which the females of gall-wasps began to lay their eggs already not necessarily on female flower buds, but also in male flower buds, and also in leaf and offshoot buds. Henceforth also other parts of the plants turned out to be agreeable to them, such as stalks, veins, and edges of leaves, the marrow of young shoots, and even the cambian layer of roots.
The chief connection of gall-wasps with buds, especially the generative ones, is well shown in their heterogony, that is, in the, common to many gall-wasps, individual development with alternation of generations -- sexual (bigamous) and asexual (parthenogenetic) (agamous) generations.
Here some examples that illustrate this.
According to Adlerts the sexual (= bisexual) generation of the oak gall-wasp, today known by the name Neuroterus quercus baccarum L., appears in the first half of June. See next two Figures.
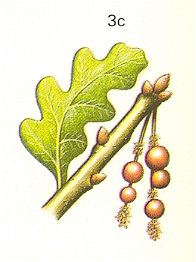
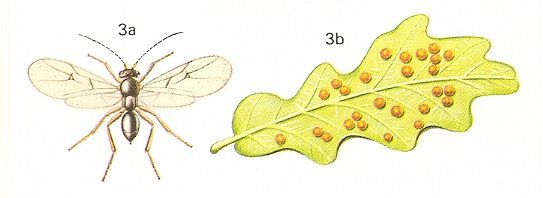
Figure 5 : 3a - Neuroterus quercusbaccarum L. about 4 mm. Family Cynipidae.
3b - galls of asexual generation on oak. 3c - galls of bisexual generation on oak.
(After CHINERY, in Elseviers insektengids voor West-Europa, 1983)
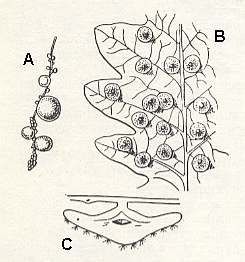
Figure 6 : Galls of Neuroterus quercus baccarum L. Family Cynipidae.
A - Forma baccarum on catkin of oak.
B - Forma lenticularis on leaf of oak.
C - Cross section of the gall of lenticularis.
(After BERLAND, 1951, in MALYSHEV, 1966)
Soon after fertilization the young females begin to lay their eggs on the underside of oak leaves that are still growing. In three weeks on these places appear features of gall-formation, and here, on the underside of the leaves, quickly and usually in great numbers (up to 40-50) circular tablet-like galls develop having a diameter of up to 6 mm. Earlier these galls were attributed to a totally different gall-wasp, but later it turned out that they were caused just by the larvae of the second, that is, the asexual generation of the above mentioned species. Having developed in June, the galls of the asexual forms (lenticularis) ripen in September, and at the end of that month and in October they, together with the leaves, fall on the ground, where they [and their content] hibernate. In the next spring, in April, and partly in May, from these galls emerge the generation of asexual forms (infertile females). These females lay their eggs in the buds of oak plants where their ovipositor, being led under the scales of the bud, proceeds up until the bud's axis and penetrates into the latter, which it can do thanks to its flexibility. At the beginning of the development of the buds one can see on their leaflets the formation of galls, which begins not until the larva emerges from the egg. These spring galls not only appear on the underside of very young leaflets of the oak, but also on the stalks of its male flowers -- catkins. They have a spherical shape and reach a diameter of 3-5 mm. From them, in June, emerges the bisexual generation -- forma baccarum. The fact that the spring galls are formed on leaflets as well as on the flowers of the oak is caused by, as was already indicated, laying eggs in the buds of oaks too soon, at which time the (infertile) females (lenticularis) were evidently not able to distinguish, in the bud, between the not yet differentiated germs of a future sprout, the germs of flowers, or those of a leaf, and therefore their eggs ended up in all these germs (that is, now in this, then in the other).
Similar relationships are observed in the gall-wasp Andricus noduli Htg. in the interaction with its parthenogenetic form Aphilothrix radicis F. The parthenogenetic females radicis, that fly off at the end of April or in the beginning of May, climb up the stems of [young] oaks and lay their eggs in the buds, namely into the tissue from which will begin the apical growth of the future sprout. As a result, among the annual sprouts galls develop from which in the first half of August flies off the bisexual generation noduli. After mating the females noduli go to the roots [of the tree] where they lay eggs in the cambium layer. The galls on the roots begin to develop in that same year, but have their final development only in May of the next year : However, the gall-wasps of these galls do not leave them in that summer but hibernate again, and then emerge as the form radicis. So the developmental cycle of this gall-wasp is drawn out over two years. We must note that in the period in which the parthenogenetic generation develops in the layer of root cambium, the bisexual generation develops in the buds of apical sprouts. Because the bisexual mode of reproduction in gall-wasps is undoubtedly primary, and the asexual [mode] obtained later, we have in this fact a direct indication of the greater ancientry of the habit of the gall-wasps to lay their eggs precisely into buds and not into the root cambium or into already developed leaves.
Also the widely known wingless parthenogenetic females of another root-dwelling gall-wasp, Biorhiza aptera Bosc., in late autumn or even in winter thaw climb up the stems of [young] oaks in order to lay, in the upper buds (more rarely in the axillary ones), some tens of eggs in every one of them. See next Figure.
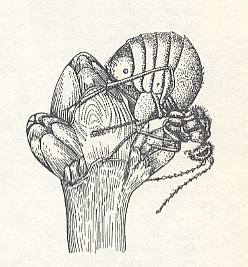
Figure 7 : Wingless gall-wasp Biorhiza aptera laying its eggs in a bud of an oak. In the Figure this bud is opened laterally to show the position of the ovipositor and of the eggs (some dozens), placed in the center of the bud.
(After BERLAND, 1951, in MALYSHEV, 1966)
In the next spring and summer on the apices of the sprouts from the in this way infected buds large yellow-with-red multi-chambered galls develop that look like small apples. From these galls in June emerges the bisexual generation consisting of winged males, and females that are wingless or with reduced wings -- Biorhiza pallida Oliv. (See Figure 1, above ). The fertilized females of this generation lay eggs in the bark of the roots of the oak, on which also develop, in groups, small reddish galls giving, as in the previous case, the parthenogenetic generation. See next Figure.
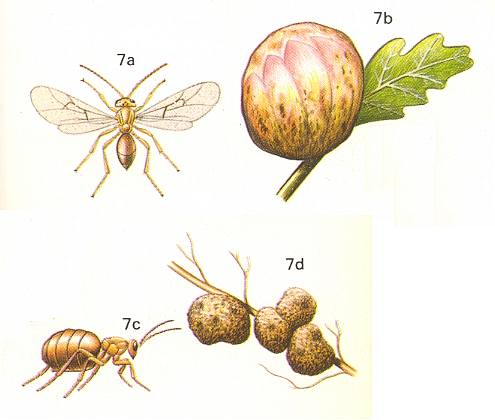
Figure 8 : Biorhiza pallida Oliv. Family Cynipidae. 7a - sexual form, about 4 mm.
7b - gall of sexual form on oak. 7c - Asexual form. 7d - root galls of asexual generation.
(After CHINERY, in Elseviers insektengids voor West-Europa, 1983)
[Regarding the life-cycle of Biorhiza pallida we can add the following taken from BEIDERBECK and KOEVOET, Plantegallen, 1979, Dutch edition, 1981, p.29-30] :
On the roots of oaks we can find, often at a depth of 1 m, the spherical galls, having the size of a pea, with one single cavity. Later, several galls may coalesce, resulting in irregular-shaped galls with several 'chambers'. These are formed by the asexual generation of this gall-wasp. [We must realize that the galls of gall-wasps (Cynipidae) are not formed by the egg-laying female, but by the developing larva, and thus by the generation next to the one to which that egg-laying female belongs]. From these, in December - beginning of January, emerge the always wingless parthenogenetic females which are, as compared with the sexual forms, fairly big. They crawl up along the stem of the tree, laying their unfertilized eggs in buds. This results in the formation of many-chambered bud galls, from which a great many wasps appear in July. These are males as well as females (bisexual generation). The females are polymorphic as to their wings. In addition to long-winged individuals and short-winged ones that cannot fly, one also encounters wingless females. The degeneration of the wings has advantages, because the fertilized females must enter the ground in order to lay their eggs on the oak roots in which act the wings would be impeding. In the ground the mentioned root galls are formed again from which not before December of the next year again females appear. So the complete cycle with alternation of generations lasts two years. See also next Figure.

Figure 8a : Sponge gall at the end of an oak branch of the gall-wasp Biorhiza pallida, oak sponge gall-wasp. Size of these galls 2-4 cm. Formed by the bisexual generation.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)
Also in the gall-wasps Andricus pillosus Adl. the bisexual generation emerges in May from small hairy galls that develop on catkins of the oak. Its parthenogenetic generation, known as Andricus (Aphilothrix) fecundatrix Htg. develops from large scaly galls, looking like the fruits of hopplants, and sitting on the axils of oak leaves. See next two Figures.
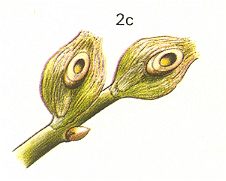
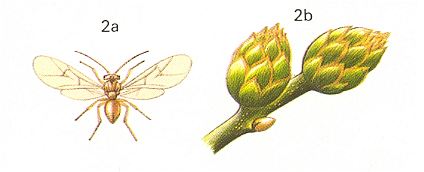
Figure 9 : 2a - Andricus fecundator Hrt. about 5 mm. Family Cynipidae.
2b - pineapple-galls on oak. 2c - cross section through the galls, showing the interior chamber.
(After CHINERY, in Elseviers insektengids voor West-Europa, 1983)

Figure 9a : Cross-section through a gall of the gall-wasp Andricus foecundatrix (= acc. to me [JB] fecundator). In the center we see the hard in-gall.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)
In August from this scaly gall a hard nutlet falls off that lies on the ground for 2-3 years, after which in April the adult insect emerges. Here, accordingly, the galls of this, as well as of the other generation, originate from buds, but here, at least the bisexual generation, undoubtedly emerges from flower buds.
Here we must also mention the "keel" gall-wasp, Cynips quercus-calicis Burgst, in which the different generations are adapted to different species of oak. Its parthenogenetic generation develops on the summer oak (Quercus pedunculata), causing in its fruits one-chambered galls having the form of a truncated conus that envelop the "pljuska" of the acorn. At the top they are open, but laterally they carry a great many keel-like projections. The bisexual generation, called Andricus cerri Beyer., develops on Quercus cerri L., forming galls that look like amphoras (ceramic bottles with a narrowed neck) on male flowers. Here, accordingly, the galls develop on the generative organs of the plant : On the female ones the asexual generation, on the male ones the bisexual generation.
That it is typical of the gall-wasps to develop their bisexual generation from the generative organs of plants is clear from the fact that this phenomenon is not only observed with respect to the oak, but also to maple. Thus, the maple gall-wasp Pediaspis aceris Foerst. causes, in place of seeds of the maple [say, by pollination], galls (partly also on neighboring leaves), in which the bisexual generation develops, while the asexual generation forms galls on the roots of the maple-tree. See next Figures.
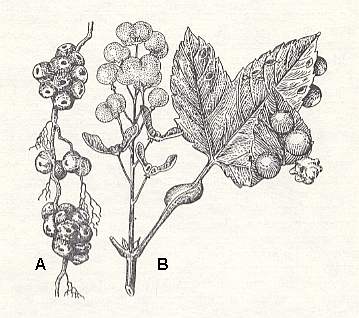
Figure 10 : Galls of Pediaspis aceris on maple.
A - Galls on the roots : one can see the openings from which the gall-wasps came out. B - Galls on fruits, on a stalk, and on leaves. One gall (right), heavily modified, is occupied by a gallwasp-inquiline
(After BERLAND, 1951, in MALYSHEV, 1966)

Figure 10a : Galls of the gall-wasp Pediaspis aceris on maple leaves. The small warts on the normally spherical galls are caused by 'subtenants'
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)

Figure 10b : Galls of the gall-wasp Pediaspis aceris at the underside of a maple-leaf (Acer pseudoplatanus (host-plant)). Diameter of galls 6-8 (10) mm. Bisexual generation.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)

Figure 10c : Galls of the gall-wasp Pediaspis aceris in the inflorescence of maple. Diameter of galls 6-8 (10) mm. Bisexual generation.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)
Such a widely distributed connection of gall-wasps with reproductive [or at least generative] organs of the food plant is, of course, not accidental : It has very deep [historical] roots. Earlier it was indicated that in gall-wasps, of whom it is typical to have alternation of generations, the reproduction in the bisexual way was the original way. Today we see that precisely these bisexual generations usually develop on the generative parts of plants. All this points to a single conclusion : The original gall-wasps developed in the generative organs of plants, that is, buds or flowers, in the same way as did also the terebrants seed-eaters.
Among gall-wasps that form galls on oaks a number of species of the genus Aphilothrix was discovered that are represented by a parthenogenetic generation only. In them, evidently, the bisexual generation completely disappeared. Having emerged in springtime from hibernated galls these gall-wasps lay their eggs in the buds of oak. The galls, having resulted from this, are formed directly from buds or develop on leaves or on catkins. Hartman, 1905, also obtained from galls of Diplolepis disticha Htg. many thousands of females, but among them no single male was found.
The common rose gall-wasp Rhodites rosae L., forming large rough galls on wild rose and on escaped sorts of rose, also develops without alternation of generations. The females coming out in the spring lay their eggs in the buds of the mentioned plants. In three weeks after oviposition galls appear on developing leaves. They ripen in September, and in the next year from them, from the end of April, the fully grown generation begins to emerge, represented chiefly by females. Males in this gall-wasp occur very rarely, and, as is supposed, do not have any essential significance for the reproduction of the species, and find themselves on the way to ultimate extinction. Meanwhile, in North America from some galls of this species of gall-wasp, as observations of Kinsey, 1920, indicated, males and females nevertheless sometimes emerge and in roughly the same numbers. Such divergence of observations points, according to Kinsey, "to a great variability of the eggs laid by these or those individuals". This statement, however, hardly says more than a simple recording of a fact.
Before us the question arises whether the appearance of males is connected with the laying of eggs in already formed buds or directly into the young ovary of the wild rose? [that is, is the appearance of (also) males related to the laying of eggs in the generative organs, i.e. in tissues not yet differentiated, or in the already differentiated reproductive organs?]. Corresponding experiments might throw light on this interesting question.
Anyway, according to data of Kuznetsov-Ugamsky (1930), in another species, namely in the Turkestanian rose gall-wasp Rhodites mayri Schlech., the number of males corresponds to that of the females, answering to a more primitive state than is the tendency for the males to disappear, as in the previous case. But this gall-wasp shows also other very important biological features. The period of its activity, connected with the laying of eggs, starts from the middle of April and coincides with the blooming period of the wild rose. The formation of galls on the wild rose the mentioned author describes as follows :
"After the blooming is concluded the shell of the fruit starts to swell, and the whole fruit becomes more and more asymmetric. Subsequently, through the opening of the calyx (that is, from the receptacle -- C.M.) (small) particles of the developing gall start pushing outward. These particles are nothing else than individual seeds of the wild rose, which grow rapidly and ultimately form the very gall. The swelling of the shell of the fruit proceeds only up to a certain moment, and then this shell bursts (the crack can start anywhere) and the particles of the gall, in cases united with each other only by their narrow bases, in other cases consolidated into one common mass, obtain the possibility for further growth . . . . Not rarely in one and the same gall one can find, on the one hand, particles with diameter of up to 1.5 cm and even a bit larger, and, on the other hand, almost unchanged seeds of the wild rose. It is clear that in these cases not all seeds of the fruit were infected by the insects.".
Here, in the Turkestanian gall-wasp one can distinguish two categories of galls :
"Primary galls (as were just described -- C.M.), in their formation connected with hypertrophy and deformation of the developing fruit. . . . , and galls that are secondary [i.e. derived], which are formed later at the expense of vegetal [i.e. not participating in reproduction] parts of the plant. . . . Numerically they lag behind the galls of the first category which are markedly more numerous and typically. It is clear that their formation represents a more or less accidental episode in the life of the insect."
Unfortunately the investigator did not breed the gall-wasps from galls of either category.
Anyway, it is remarkable that also in gall-wasps causing galls in rose plants [Rosaceae] we again have to do with laying the eggs in the generative organs of the plants as indeed does the Turkestanian gall-wasp, Rhodites mayri Schlech., laying its eggs in the ovaries of the wild rose. In this respect one should note that the widened, invaginated, urn-like receptacle of [the infloresecence -- here one flower -- of] the wild rose [ receptacle = base of flower] looks surprisingly much like [that of] the inflorescence of the fig. In fact the difference is only that in the wild rose we have not to do with an integration of many small flowers into one, the inflorescence, as it is in the fig, but with only one flower at a time, each one carrying many ovaries. And it is here in these ovaries, concealed inside the urn-like receptacle and each one of them having only one seed-bud (ovule), that the gall-wasp Rhodites lays its eggs. The infected ovaries of the wild rose, as it is in the fig, do not yield seed, but only here (wild rose) they grow excessively, resulting in a complex, multi-chambered gall, of which the individual chambers are separated by firm smooth walls such as the shell of nuts. From this it is clear that, essentially, also the rose gall-wasp can be called a "seed-eater" with the same right as we do it with the fig blastophags [fig wasps] [The word seed-eater is here placed between quotation marks to express the fact that, as a result of egg-laying, the seed does not develop. So in fact we have to do here with "(plant) egg-eaters", phyto-oophags. And this is the reason why the gall-wasps (Cynipidae) are included in the Secondary-phytophagous (phyto-oophagous) Phase of evolution]. All this leads to a single conclusion : The [larvae of the] gall-wasps also originally [already] developed at the expense of the generative organs of plants [that is, it is an original, primary, not a derived, habit within the gall-wasps as a group], as they still do today.
It is interesting to note that even in grassy plants galls are encountered that are closely connected with the flower-carrying part of the plant. Thus, according to the description of Kinsey, galls of the wasp Aulacidea annulata Kins. are apical excessive growths of the plant-carrying stalk of Latuca, or, possibly Prenanthes because dry specimens could not be determined precisely (as to their identity). See next Figure (concerning a related species of gall-wasp).
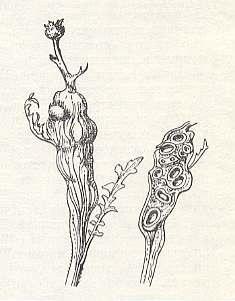
Figure 11 : Galls of the gall-wasp Aulax scabiosae.
(After BERLAND, 1951, in MALYSHEV, 1966)
The galls (of Aulacidea annulata) are club-like with their greatest diameter apically (up to 18 mm). Many leaf-stalks or stalks of the flower(-carrying)-basket are grouped at the apex of the gall, such that their bases seem to be drawn into its uncontrolled growth.
With respect to the question of the origin of the gall-waps let us now anwer it from a different angle. Some of the aulacidines, those such as Aulacidea abdita Kins. and its relatives, infecting the stalks of Latuca or of other composites [Compositae, plants of which the many small flowers are strongly integrated resulting in fact in one single flower as we see it for instance in daisies] do not cause on them gall-like excessive growths that can be seen from the outside. From such a state of affairs, especially when we have to do with dry dead [plant] material, as in the mentioned cases, we could easily come to the conclusion that these insects simply "live in the marrow of the stalks of Latuca". On closer inspection it turns out, however, that the larvae of these Aulacidini, which Kinsey considers to be the most primitive of all known gall-wasps, develop all the same in "larval cells", albeit with a hardly expressed or even not visible (in dry material) distinction of their walls. In this way, the larvae of these gall-wasps developing in the marrow of grassy stalks, also in these conditions remain sedentary [i.e. not mobile], that is, living and feeding -- not gnawing channels -- at one and the same place inside a very narrow space. In Aulacidea abdita Kins., for example, the larval chamber is only some 2 mm long, and is 1.2 mm wide. In A. annulata Kins. its size is 2.5-3.5 mm. But we must realize that in such very small chambers also develop the larvae of those gall-wasps which cause well-formed galls that rise above the surface of the plants. Such is the case, for example, in the larvae of Andricus pellucidus Kins., that form spherical galls, on the underside of oak leaves, and develop in central "cells" having a size of only 2-3 mm, whereas the gall itself has a diameter of 8-15 mm. (see also Figure 9-2c, above ).
From here it is clear that the larvae of the Aulacidini, as in the other gall-wasps, get, at their place with its extremely narrow space, all they need for their growth and development. This is only possible in such conditions in which at the larva's place all the time the necessary nutritive substances are coming in, in line with what is required. But this can only be guaranteed by the plant's formational tissue, that is, by the meristem, and certainly not by already formed parenchym of the marrow. Assuming that the Aulacidini are in many respects the most primitive group of gall-wasps, Kinsey, together with this asserts that the biology of the species of the genus Aulacidea is hardly distinguishable from the biology of "normal" (i.e. primitive, original) phytophagous Hymenoptera [That is, that, supposedly, the Aulacidini just feed on the marrow, as do the original phytophags, whereas Malyshev points to the fact that they feed on meristem and therefore not being distinct from the true gall-making wasps and thus not being primitive phytophags (such as the Symphyta --saw-flies and allies)].
On closer inspection one can easily see, however, that this position is fundamentally wrong. In fact, even those "normal" phytophags, as are the endophytic saw-flies, which develop in the soft and juicy marrow of sprouts (for instance the spring-time sprouts of the rose-plants), must, for their feeding, make a channel in the sprout, and must move in it, and leave behind undigested particles. Moreover, even larvae of those saw-flies that cause well-formed galls on the leaves of willow, similar to the galls of the gall-wasps, do not stay at one and the same place but move around in the gall's cavity while feeding on the juicy spongy parenchym of its walls, giving off excrements from already the first hours of the beginning of feeding. We must add that on concluding their feeding, the larvae of saw-flies make cocoons, in contrast to the larvae of the gall-wasps which develop in their narrow cradles always without cocoons.
Thus, contrary to the position of Kinsey, between gall-wasps and primary phytophags not only there does not exist a close similarity with hardly visible differences, but, on the contrary, there exists a very deep-seated difference with a weak superficial similarity, which bears witness to the absence of a direct phyletic connection between them. This was already partly expounded above but will find its confirmation also later on. Anyway, when we take into account the fact that the Aulacidini, by Kinsey taken as the most primitive of the living gall-producing Cynipoidea, develop on composite plants, that is, on plants that have reached the highest evolutionary stage, then the secondary character [within the Cynipoids] of the biological features of the Aulacidini will be without doubt. Evidently, for answering the question of the origin of the gall-wasps, it is necessary to not proceed from that viewpoint to which Kinsey points.
The best conditions for life the gall-producing Cynipoids found in the oak (Quercus). This is visible already by the fact that on it 86 percent of all known species of gall-wasps live. It is possible that the preference of the gall-wasps for living on the oak has its reason in the fact that while slowly developing and for a long time retaining the freshness of its sprouts, it is in the position to offer accommodation for even not just one, but for two successive generations of insects during a single season. But the oak, as is known, is not widely distributed, thus the Cynipidae, apparently, began their way of life in other plants [This might mean either that they originally (= early in their evolution) lived on oak, but when it became less abundant passed to other plants, or (it might mean) that the oak, never having been abundant, was not their original food plant but was added to the family's circle of food plants later on.]. At the second place of preference of the gall-wasps stands the rose (Rosa), or more precisely, the wild species and escaped sorts of rose (i.e. forms in which not all male parts are transformed into flower-leaves (petals)). With the roses are associated 7 percent of the gall-wasp species, and the remaining 7 percent live for a part on other Rosaceae, for another part on maple and on grassy plants especially compositae. In answering the question about those plants on which originated and remained developing gall-producing Cynipoids, that indication by botanists is interesting that is about the phylogenetic close connection of the rose plants (Rosales) and the catkin-carrying trees (Hamamelidales -- Fagales), to which belongs also the oak. The common ancestors of both were closely related to the Magnoliales. They undoubtedly stood (phylogenetically) far from the Compositae. Therefore we must suppose that on about them [= the mentioned common ancestors of Rosales and Fagales] it was that the first gall-wasps caused the formation of galls, from which first gall-wasps the cynipids then evolutionarily dispersed into different directions (Malyshev, 1964).
Here it is interesting to note that the gall-wasps are by their structural features clearly related to the Callimomidae -- a family of chalcidoid terebrants, itself closely related to the Eurytomidae and Agaonidae. Meanwhile, among these callimomids there are "seed-eaters" which develop chiefly at the expense of seeds of pine trees and rose plants (Rosaceae). Thus, from the 18 palaearctic species of the genus Megastigmus, which is, by a series of features, the most primitive genus, 14 are "seed-eaters". Half of them develop in young seeds of pine trees (silver fir, spruce, cypress, juniper), and the remaining develop on Rosaceae (trees and bushes - rowan tree, apple tree, cornel tree, wild rose, and others), and one species develops on the turpentine tree. These traits of similarity between the gall-wasps and the primitive group of chalcidoid seed-eaters, with some difference (with respect to pine trees), are very indicative. They certainly have deep roots.
To the origin of the gall-wasps independent of the primary phytophags also points the very way that galls are formed. Indeed, while the galls of [gall-producing] saw-flies are produced by the action of enzymes given off by the female during oviposition, around the eggs laid by gall-wasps no excessive growth of plant tissues does take place before their larvae start to develop. Therefore it is easy to find a gall of a saw-fly in which lies merely an egg [i.e. not yet a larva] or even one without any egg, while one cannot find a gall of a gall-wasp in which there was no egg, or in which there is an egg containing a not yet developed larva [in the sense of a not yet developed embryo]. Usually, in a young gall of a gall-wasp we can find an already emerged larva. In addition, direct experiments point to the fact that when the egg, laid by a gall-wasp, is removed from the plant, the gall will not develop. The cause of the formation of galls of gall-wasps is, consequently, the life activity of their larvae, and not the adult individuals, which merely lay their eggs at proper places. Therefore galls of saw-flies and galls of gall-wasps are different products. The galls of saw-flies can, in virtue of the mentioned reason, be called "imaginal galls", while the galls of gall-wasps can be called "larval galls". Also in the very structure of the one and the other there is an essential difference, with which we will deal below.
Thus we have evidence that clearly speaks against a direct phylogenetic connection of gall-wasps with the original phytophags. We now will deal with this problem from yet another angle. As was shown above, the terebrants evolved from gall-producing saw-flies that went through an inquilinoid phase, from which several different lines of evolution of terebrants came off, including the line of chalcidoid seed-eaters. In addition a whole series of basic features was found that emphasizes a fundamental connection of the gall-wasps with the terebrant seed-eaters : feeding on germ tissue, absence of defecation, development in one place -- with brood chamber, the small size of larvae and adults. When we in addition realize that not a few gall-wasps develop at the expense of seeds, the immediate connection of them with the terebrant seed-eaters will be clear -- the gall-wasps themselves are, in many cases, in fact seed-eaters.
Earlier it was already noted that also from a morphological viewpoint there exists an indication of a certain connection of the gall-wasps (in virtue of the structure and position of the ovipositor) with the chalcidoid terebrants to which also belong the above considered phyto-oophags -- seed-eaters. More so, among the latter exists, as we know, the small aberrant group Harmolita (= Isosoma) of which the larvae, as in certain Eurytomidae, are able to cause larval galls on the place of their growth and development. Thus also from this viewpoint the chalcidoid phytophags are similar to the gall-wasps.
When we now, on behalf of a more complete analysis, turn to the group of parasitic cynipoids, we will see that they too did not go away far from the egg-eaters. Thus, the relict Ibalia (from the Ibaliidae) lays its eggs into the eggs or into the just emerged larvae of horn-tail wasps, and the Eucoilidae [lay their eggs] into first-instar larvae of certain flies that lay their eggs on cabbage, mushrooms, and others. However, the lines of evolution of the gall-producing and the parasitic cynipoids diverged, evidently, since the very beginning of the origin in them of oophagy [egg-eating], and we have no basis to assume, for instance, that the gall-producing cynipoids first were parasites, or, the other way around, that the parasitic cynipoids once upon a time developed in galls. The absence of defecation in developing larvae might have originated independently or already before the separation of these groups.
Basing ourselves on what has been found out above, we must, accordingly, assume that the ancestors of the gall-producing cynipoids, going through the archaic inquilinoid phase [of evolution], stood on the same path of [evolutionary] development as stood the "vegetable egg-eaters" - the seed-eaters. On this path they, however, did not stay, they went into another direction, working out their characteristic features. We will now further expound the latter.
The first characteristic feature of the gall-wasps, as noted earlier, was their striving for laying their eggs at the right time [with respect to the developmental stage of the food plant]. Under the influence of perhaps the competition with the "seed-eaters" or by some other reasons, they left the young ovaries of flowers to the chalcidoid seed-eaters, whereas they themselves aimed at the same organs, but that were still in a germ state, that is, in a not yet differentiated state, in the form of hardly woken-up or still dormant buds. Although in this as well as in the other case the emerged larvae found themselves amidst germ tissue suitable for feeding, the conditions for further development of them became already different [by reason of the eggs being laid at different times in the two groups]. Finding themselves inside the developing ovary [of the flower], the larvae of the "seed-eaters" received from the plant all what they needed as a result of a natural flow of saps to the place where the seeds grow. On the other hand, the larva of the gall-wasp, finding itself amidst non-organized germ tissue not yet showing definite formational processes, fell into other conditions in which a regular supply of food to it was not acquired in the normal way. This was to become that particular thrust that produced the most characteristic feature of the gall-wasps -- the ability to cause an increased excessive growth of the plant tissue around their larvae. In this way, the first -- seemingly little significant -- deviation of the gall-wasps as to laying their eggs earlier, led to an unusual result -- the formation of characteristic galls.
In working out their remarkable ability to cause galls the larvae of the gall-wasps widely made use of the fundamental property of plants to transform already formed living tissues again into their embryonic condition -- into meristem of second, third, or higher order. Thanks to this the larvae of gall-wasps, finding themselves not only amidst formed meristematic tissue but also amidst already formed parenchym or even amidst vascular-fibrillar knots of the oak, are always surrounded by a layer of young and juicy dividing cells which provide the larva of the gall-wasp with all it needs. See next Figures.
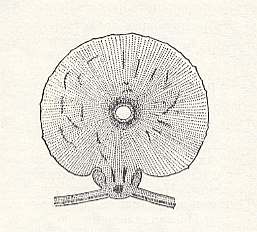
Figure 12 : Young gall of the oak gall-wasp Cynips (Diplolepis) follii L. Cross-section.
In the center the larval chamber [larva-chamber] is visible, walled by nutritive tissue.
(After ROSS, in BERLAND, 1951, in MALYSHEV, 1966)
Assuming that "Cynips follii" and "Cynips quercusfolii" point to the same insect, we here have a photograph of the gall and the adult insect :

Figure 12a : Cross-section through a gall of the gall-wasp Cynips quercusfolii with adult insect. The beginning of the feeding-channel of the larva leading to the exit opening can be clearly seen. (After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)

Figure 13 : Galls of the gall-wasp Cynips (Diplolepis) follii L., on oak leaves.
(After MALYSHEV, 1966)
The action of the meristem here is twofold : in the centripetal direction it gives, as it is in the case of the development of the "seed-eaters, nutritive elements, and in the centrifugal direction coating elements, originating normally in the case of the seed-eaters [as a regular structure in plants], but in the case of gall-wasps specially caused by them. In this regard the action of the larvae of gall-producing cynipoids on the plant tissue is very effective. There is an indication that the action of the larval secretion on the plant tissues expresses itself even in organs of plants apparently having definitively stopped growing. Thus, the gall-wasp Trigonaspis renum Mayr causes the development of galls on oak leaves that have already become yellow.
Here we may point to the gall-wasp-inquiline Synergus melanopus Htg., which lays its eggs into ready-made galls of the gall-wasp Cynips kollari Htg., but not into the larva-chamber of the host-gall, but into the layer of spongy coating parenchym of the gall, that is richly soaked through with tanine worthless for the feeding of the inquiline. However, in this case around the emerged larvae of the inquiline there develops a protective shell within which the larva of the inquiline independently feeds on meristem caused by it, and in no way impeding the host-larva.
The forceful action of the secretion of larvae of gall-wasps on plant tissues is especially strongly expressed there where a number of larvae of gall-wasps develop in each other's neigborhood, each one of them in its own chamber together forming one single many-chambered gall. Such are, for instance, the galls of a series of rose gall-wasps, and of many oak gall-wasps, reaching a size of sometimes up to 5 cm and more. See Figure 4c, above .
If we now take the history of the origin of the gall-wasps as plant egg-eaters, and also take into account that all gall-wasps each develop in a special chamber, then the formation of tightly grown-together galls must be seen as a secondary (derived) phenomenon, as an adaptation to optimally use their ability to cause gall formation. As regards the protecting layers and the exterior shells of the galls we must, with respect to the widely distributed infection of galls by various parasites and inquilines, see this as a weak measure being taken, or better, a measure that comes too late, because the infection of galls takes place, evidently, at an early stage of their development. On the other hand, at a later stage, to already ripened galls, they [protecting layers and shells] offer a wonderful protection against other insects as well as against dehydration and other detrimental influences.
Thus we see that the ancestors of the gall-producing cynipids, in search of places to lay their eggs gave up, essentially, their original development at the expense of more or less formed generative organs of plants, and a mass of species strove to infect these same organs, it is true, but now these organs as they are still in a germ-condition, and later also wholly [strove to infect] vegetative organs. [The germ-state generative organs of the plants, and also the vegetative organs, do not by themselves offer some protecting shell]. In these new conditions their larvae, having originally fed on formed seeds, worked out a remarkable ability -- to generate galls. As a result galls were obtained that have amazed investigators : On vegetative parts of plants began to grow structures that were similar to generative ones. The similarity indeed turned out to be remarkable : Within the fruit-like body lies a concealed hard shell (a "kernel" or "seed") -- a fruit stone or nut (hence the original name "nut-former" (Russian, orjechotvorka)), isolated to a certain degree from the exterior surface of the gall. The similarity is further increased by the fact that the mentioned interior structures are, when they are still young, filled with juicy and delicate meristemic tissue similar to the kernel of a seed, that is eaten by the larvae of the gall-wasps. In this way the insect itself began to create on vegetative parts of plants a grain of milk fruition which is eaten by it.
Concluding his description of galls, Frost, 1942, writes : "It will always remain mysterious in what way such remarkable structures have originated, such as the rough galls on the oak with the seed-like grains included in them". In the light of what has been found out above, I think, it is clear that this problem is not "eternal" and that there is a definite path leading to its solution.
Having now dealt with the gall-wasps, mainly following MALYSHEV, 1966, we conclude our exposition of MALYSHEV's account of the Secondary-phytophagous (phyto-oophagous) Phase of the evolution of Hymenoptera. But before we do so we add some interesting photographs concerning the cynipids (gall-wasps).

Figure 14 : Green marble gall of the gall-wasp Andricus inflator in an oak bud. Size of such galls 3-4.5 mm. Asexual generation.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)

Figure 15 : Sponge galls of the gall-wasp Andricus kollari on lateral buds of an oak. Size of such galls 10-25 (30) mm. Asexual generation.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)

Figure 16 : Satin button galls of the gall-wasp Neuroterus numismalis on the underside of an oak leaf. Width of such galls 2-3 mm. Height 1.5-2.5 mm Asexual generation.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)

Figure 17 : Lenticular galls of the gall-wasp Neuroterus quercusbaccarum at the underside of an oak leaf. Width of such galls 4-6 mm. Height 2 mm Asexual generation.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)
Galls of saw-flies
In the three Figures that follow (Figure 18, 19, 20) we also show plant galls, but this time not caused by true gall-wasps (Hymenoptera-Terebrantia-Cynipidae) but by certain saw-flies, namely the saw-flies of the genus Pontania (Hymenoptera-Symphyta-Tenthredinidae).

Figure 18 : Double-sided galls on willow leaves, caused by Pontania proxima (Symphyta-Tenthredinidae). Width of such galls 4-5 mm. Length 7-10 mm.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)

Figure 19 : Two galls on the upperside of a willow leaf, caused by Pontania vesicator (Symphyta-Tenthredinidae). Width of such galls 15 mm. Length up to 20 mm.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)

Figure 20 : Haired marble gall at the underside of a willow leaf, caused by Pontania pedunculi (Symphyta-Tenthredinidae). Size of such galls up to 6 mm.
(After BEIDERBECK and KOEVOET, Plantegallen, 1979/1981)
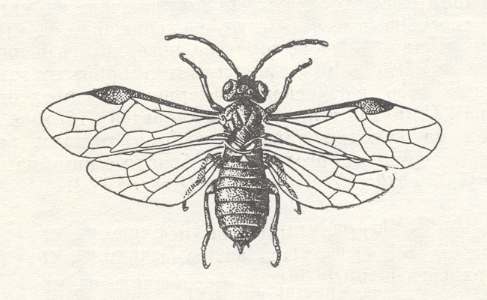
Figure 21 : Willow berry saw-fly Pontania viminalis (Symphyta-Tenthredinidae),
ca. 8 mm. (From BERLAND, 1951, in MALYSHEV, 1966)
* * *
In the next document we will describe the next phase of the evolution of the Hymenoptera, the Delayed-parasitic (metaparasitic) Phase.
e-mail :  ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXXIX.
Back to Homepage
Back to Contents
Back to Evolutionary Part XIV
Back to Evolutionary Part XV
Back to Evolutionary Part XVI
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XIX
Back to Evolutionary Part XX
Back to Evolutionary Part XXI
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII


































 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )