

In the evolutionary history of the terebrants (parasitic wasps and allies) we see the remarkable and also unexpected fact of a return to feeding on plant tissue : The terebrants, as it were, again changed into phytophags [like they were when they were still inquilines]. This secondary phytophagia appeared only in two lines of terebrant evolution : (1) in only some Chalcidoideans (in the families Eurytomidae, Callimomidae, Agaonidae [such as the fig wasps], in a few Eulophidae, and, as an exception, in Encyrtidae and Perilampidae, and (2) also in the majority of the gall-wasps, Cynipidae. So the vast majority of terebrants preserved the inherited (of their ancestors) carnivorous way of life.
From a biological standpoint all phytophagous terebrants (not considering, of course, the archaic inquilines considered above) fall into two groups : "seed-eaters" and "gall-producers". The meanings of these divisions demand, however, essential qualifications.
Although the terebrant-seedeaters indeed emerge as adults from seeds, which lets them therefore be true pests of seeds, it is for us important that their larvae develop not at the expense of ripe seeds, but at the expense of very young seeds. They feed on not yet differentiated meristemous cells of the plant-germ. Of still more significance for understanding the essence of the process [= return to phytophagy] is the very manner of laying the egg by the female "seed-eater". Unfortunately this moment is still studied insufficiently. But in some cases it is nevertheless clarified that the egg of the terebrant is laid into the endosperm of the germ, that is, in its egg-cells, which essentially means in the egg of the plant. These data directly point to the fact that the so-called terebrant-seedeaters are really vegetable egg-eaters, phyto-oophags.
If our views reflect the real course of evolution, then we can indeed expect that among mutually closely related primitive forms there exist species that [nevertheless] live a different life : Some [of these closely related species] would live as inquilines or as typical [animal-]egg-eaters (oophags), and others would live as [plant-]seed-eating terebrants, that is, feeding on plant-eggs. And this is indeed observed. Thus, among the family Eurytomidae which is the most primitive family of the extensive group of chalcidoid terebrants (Chalcidoidea), and even in that family's dominating genus Eurytoma, we can see examples of this. As was reported earlier, Eurytoma inquilinum R.- Kor. lays eggs in the galls of the eurytomid Isosoma rossicum R.- Kor., where it first destroys the egg or young larva of the latter, and after that feeds on the gall-walls. Similarly, the larva of the American Eurytoma parva Gir., emerging [from its egg] in the gall of the wheat-Isosoma first destroys the young host-larva, and after that feeds on juices and tissues of the gall. In experimental conditions E. parva can develop entirely normally on a vegetarian diet, as well as only at the expense of the Isosoma-larva, but it is remarkable that in natural conditions the instinct of E. parva to lay the egg is only put into action when the Isosoma-larva is present. [So in the terebrant family Eurytomidae Eurytoma inquilinum and Eurytoma parva both are true inquilines, and they are inquilines of an eurytomid, Isosoma].
On the other hand, a series of Eurytoma (Eurytomidae) develops at the expense of host-egg clutches, and these eurytomae are therefore already genuine [animal-] egg-eaters (oophags) (zoo-oophags). Thus for instance, the larvae of the cricket-eurytoma, Eurytoma oophaga Silv., feeds on eggs of the wood-cricket (Oecanthus), laid in the integument of stems or branches. [And, generally, egg-eaters can have been evolved from inquilines].
Finally, of the Eurytomidae-seed-eaters [and thus true phytophags] we can mention the following ones :
The almond seed-eater, Eurytoma amygdali End., which lays its eggs in the young ovaries of almond, cherry, blackthorn, and of some sorts of plum, and
the acacia seed-eater, E. caraganae Nik., stinging with its ovipositor the young beans of the yellow acacia above the place where in them are tied up the seeds, and which lays its egg under the seed box.
Among the other genera of the Eurytomidae we also find a whole series of "seed-eaters" : for instance, Bruchophagus develops at the expense of seeds of clover and alfalfa (lucerne), and Systole at the expense of coriander and anise and others.
To the other chief group of chalcidoid seed-eating terebrants belong fairly many representatives of the closely related family Callimomidae. Thus for instance, the apple seed-eater Callimome (Syntomaspis) druparum (Boh.), the spruce seed-eater Megastigmus abietus Seith., the wild-rose seed-eater M. aculeatus Swed., and others.
Only in the form of an exception is phytophagy encountered among other families of the Chalcidoidea. Thus, representatives of Tanaostigama haematoxyli Doz., belonging to the family Encyrtidae, damage seeds of logwood, whereas the single species of the tropical genus Mimaspis forms galls on the branches of Scutia. Further, of the family Tetractichidae a case of phytophagy is known in one species, the larvae of which bore the pulp of the berries of juniper. And still two genera, for the time being placed in the family Eulophidae, are also considered to be gall-producers in Australian eucalyptus'. Moreover, to the family Perilampidae belong five phytophagous exotic genera which form galls from buds on young branches of the south African asparagus, on flower buds of the Australian acacias and on leaves of eucalyptus'. Such are the multi-cavity galls of Asparagobius braunsi Mayr, looking like proliferantly-grown fruits of asparagus, and others.
The fact that among [genealogically] related forms and even among representatives of one and the same genus one encounters, on the one hand, inquilines and typical egg-eaters, as well as vegetable egg-eaters (seed-eaters), on the other, not only confirms the very fact of the transition of some of them into the others, but also points to a certain ease to realize this transition. This ease came from -- one should suppose -- the unstoppable striving of the egg-eaters for finding new sites to lay their eggs, and, in no less degree, also by the fact that the egg-eaters have evolved from archaic inquilines, that is, from forms that chiefly lived at the expense of vegetable food.
To clarify the concrete circumstances that have accompanied the process under discussion (return to phytophagy), it will be most illustrative when we turn to the so-called "fig pollinators" (fructificators) or blastophags -- Blastophaga [and other genera], that is, to the Chalcidoids of the family Agaonidae, that develop in inflorescences ("wine berries") of fig-trees (Ficus). Let us, to begin with, note that the family Agaonidae counts 20 genera and more than 100 species. The majority of them feeds, in the larval state, in inflorescences of fig-trees [of which there are many -- mainly tropical -- species] [and feeds on, i.e. eats, ovaries], but many tropical species, as for instance species of the genus Agaonella, are -- apparently -- parasites of their own relatives. [The flowers of the fig-tree are very small and appear in groups (inflorescences), each such a group consisting of many flowers. In most fig species the common substrate of the flowers of such a group is curled up, resulting in a flask-like 'pseudo-fruit' -- the fig -- carrying its many flowers -- together forming the inflorescence -- on its interior walls.
The widened and inwardly bent inflorescence of the fig-tree looks, as is known, like a pear, or, maybe better, like a large gall, the interior of which connecting with the exterior world by a narrow opening [the 'eye'] at its distal end. The walls of the cavity of this gall-like inflorescence are plastered with a mass of very small flowers of which the ovaries contain only one seed-bud -- the germ of the seed. Having discovered such an inflorescence the female blastophag (Blastophaga) penetrates in it through the closed scales blocking the opening, and usually in all this looses her wings. After this she begins egg-laying. For this the blastophag directs its ovipositor to the seed-bud through the seed-channel and places its egg under the interior integument of the seed-bud -- to its nucleus (see next Figure). Each female (somtimes 2-4 penetrate into the inflorescence [syconium], rarely more) can lay up to 400 eggs, and after this she dies inside the syconium.
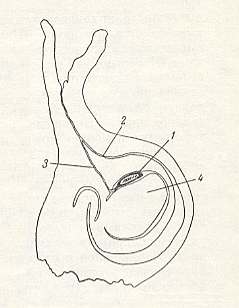
Figure 1 : Schematic cross-section through the ovary of [a flower of] the fig-plant, in which (ovary) Blastophaga has laid an egg. (After GRANDI, 1929, in MALYSHEV, 1966, simplified).
1 - egg. 2 - winding pollen-channel leading to the seed-bud. 3 - straight channel made by the ovipositor of the blastophag for placing the egg directly on the nucleus (4) of the seed-bud.
In 5-6 days from the egg, laid by the blastophag, the larva emerges and begins feeding on the primary white (endosperm) of the seed-bud. In exceptional cases inside the white of the seed-bud two larvae are present, but their fate has not been observed. The germ of the seed-bud does not develop, and the seed is not formed, but an increased division of the second nucleus of the macrospore (germ vesicle) takes place. As a result, in line with the growth of the larva of the blastophag, the ovary turns red, slightly increases its size, and forms something that resembles a small spherical gall, from which later emerges the blastophag (Grandi 1929, Nikolskaja, 1954).
Extract from "Figs and fig wasps" by WIEBES, J.T., 1970
The ecological relationships between fig wasps and figs is so interesting, complex, and evolutionarily significant, that it is certainly instructive to dwell on these matters a little longer. For this we will use one of the texts of lectures held at the symposium Biosystematics, organized by the Koninklijke Nederlandse Botanische Vereniging and the Nederlandse Dierkundige Vereniging in cooperation with the Biological Counsil of the Koninklijke Nederlandse Akademie van Wetenschappen at the 14th and 15th of April 1969 in the Koninklijk Instituut voor de Tropen in Amsterdam, the Netherlands.
The text with which we will deal here -- "Vijgen en vijgewespen" (Figs and fig wasps) by WIEBES, J. T. -- was published (in Dutch) in 1970 in the volume dealing with the mentioned symposium, p.180-206. We will (not necessarily technically) translate (and add comments where necessary) precisely that part of the text dealing explicitly with the ecological relationships between figs and fig wasps (while we will not consider its systematic and taxonomic conclusions).
Of course we expect that since 1970 still more knowledge has been acquired about these ecological relationships and also about the systematics (taxonomy) of figs and wasps. For the time being, though, the present text will suffice (for building up a knowledge of the g e n e r a l w a y s of organic evolution).
Introduction
Few animal and plant species ultimately would manage to exist and persist without the presence of other organisms. When one speaks about "symbiosis" (in the broad sense : a living-together of two or more species of organisms) one does, however, not mean this general connection, but a more direct mutual dependency. For example that between parasite and host, where the benefit lies clearly on the side of the parasite, or the living-together of organisms, mutualists, where the benefit is mutual.
What will be told here about figs and fig wasps is an interim report of an investigation that is collectively carried out by a number of biologists from different countries. Each one of them approaches the problem in his/her own way, or rather : derives from the subject his/her own formulation of the problem.
The symbiosis of figs and fig wasps is a remarkable one, even among the extraordinary close ties that are known to exist between plants and their pollinators. Also in the well-known pollination relation between Yucca's and yucca-moths the symbionts do not influence each other in such a high degree as is the case in figs and fig wasps.
CORNER (1960, 1961, 1962, 1965) has completed a systematic treatment of figs from Asia and Australia. The genus
Of earlier overviews that of MAYER (1882) is historically important. The results of GRANDI, who has, in the first part of the 20th century, published much about fig wasps, are summarized by him a number of times, for the last time in an article (GRANDI, 1961), and in the sixth edition of his catalogue of the Agaonidae (GRANDI, 1963).
The symbiosis of figs and fig wasps
Figs and their pollination. The fig is a pseudo-fruit. Inside the fig (further designated by the name "syconium", as distinguished from "fig" when a species of the genus Ficus is meant) one finds flowers, either male and female flowers together in one single syconium (monoecious), or the male flowers and those in which, after pollination, seed is developed, in syconia on different plants (diecious). Pollination takes place by the action of small wasps of the family Agaonidae (Hymenoptera, Chalcidoidea). In what way this happens can best be expounded with a diagram of the cycle in the monoecious Ficus sycomorus from Eastern Africa. See next Figure (compare with Figure 5 giving a diagram of the cycle in a diecious fig).
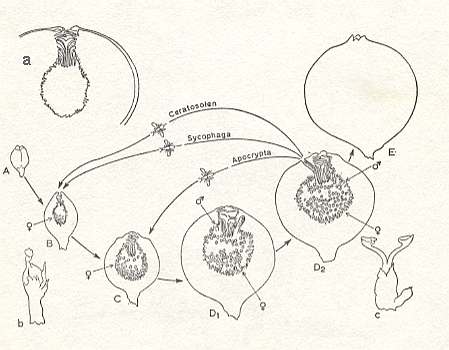
Figure 2 : Ficus sycomorus. The developmental cycle of the syconium.
A-E : Different phases of the syconium.
a : detail of syconium of phase B.
b : female flower of phase B.
c : male flower of phase D.
The pollinator wasps of the genus Ceratosolen (Agaonidae) , born in an advanced syconium, fly to a younger syconium to pollinate it and to lay eggs in it.
The wasps of Sycophaga (Torymidae) and Apocrypta (Torymidae), being true parasites of the fig, also fly to a younger-stage syconium to lay their eggs.
(After GALIL and EISIKOWITCH, 1968, changed by WIEBES, 1970)
The flowers of Ficus sycomorus are pollinated by the fig wasp Ceratosolen arabicus. See next Figure.
In a young stage of the syconium the female flowers are fully-grown (Figure 2b ) and their stigmata are susceptible to pollen. The scales in the eye (= opening) of the syconium (see Figure 2a ) become more loose than they are in a very young syconium, and now the female wasps attempt to crawl inside between the scales. Some of them are unsuccessful. Others are, but in the act they loose their wings and parts of the antennae. In the cavity of the young syconium one eventually finds one to ten living but mutilated wasps. See next Figure.
There they walk around on a layer of stigmata. Although the stigmata of all flowers are about at an equal distance from the syconium-wall, the styles have significant different lengths, often much longer than the ovipositor of Ceratosolen arabicus. The wasp tries to lay an egg through the style into the ovary of the flower. When they succeed (usually in about 85 percent of the short-styled flowers), from such an egg a larva emerges that feeds on the gall-tissue that has resulted from the oviposition. From such larvae will develop (in phase D, see Figure 2 ) the adult wasps of the next generation. See next Figure.
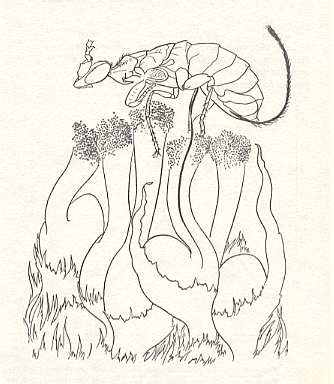
After oviposition something remarkable happens, discovered by Galil and Eisikowich (1969). A few seconds before the female wasp withdraws her ovipositor out of the flower, she takes with the tarsal (= foot-) segments of the forelegs pollen from pollen-baskets (that is, morphological structures) which are situated at the ventral side of the thorax, and rubs (the pollen) with the tarsal segments onto the stigmata of the flowers. By this act she pollinates the fig flowers : In the flowers of which the ovaries are uninfected (by an egg of the wasp), that is, most of the long-styled flowers, now seeds will develop.
In a diecious fig, on the other hand, one finds in the one plant syconia with short-styled female flowers ('gall flowers') and male flowers, and on the other plant only long-styled female flowers. See next Figure.
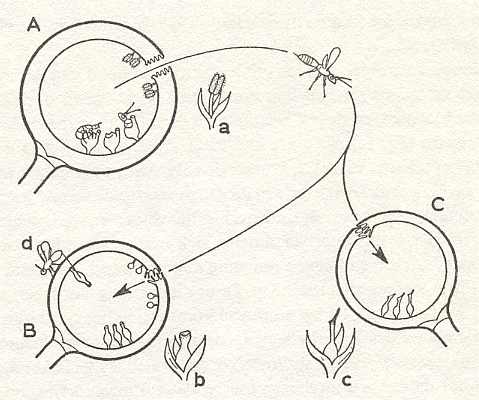
Figure 6 : Individual developmental history of the syconia of a diecious fig.
In the ripe fig (A) the wasps emerge from the gall flowers. The females take with them the pollen of the male flowers (a) to a young gall fig (B) where they lay eggs in the short-styled flowers (b), or they fly to a young seed fig (C) where they pollinate long-styled flowers (c). At d an egg-laying parasite (of which we shall speak later).
(After WIEBES, 1965, changed by him (1970))
In the syconium with the long-styled flowers the female wasps, therefore, can only pollinate, and not lay eggs because the styles are so much longer than the ovipositor. In what way the pollination precisely takes place here is not known. Galil and Eisikowitch (1969) expressly write that 'trial pricks' with the ovipositor into long-styled flowers, and thus not followed by oviposition, are in Ceratosolen arabicus never followed [still in the case of diecious figs] by pollination movements. In wasps that pollinate [and not only parasitize] flowers of a diecious fig this must take place. In the other syconium (that with gall flowers and male flowers) eggs are being laid and from these the wasps develop that can, while flying off out of the syconium, take with them the pollen of the male flowers.
The parasites. In addition to pollinators in the syconia also develop other insects. Of these we can mention only some wasp groups that are in a special way adapted to living together with the fig. They are enumerated here in the usual systematic order, while as much as possible the symbionts of Ficus sycomorus will be taken as an example.
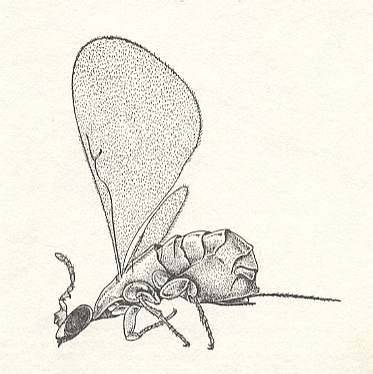
[So in addition to the pollinators, the Agaonidae, in the next table we list the parasites (of the fig) belonging to the family Torymidae. A representative of the family Agaonidae was already depicted in Figure 3, while a representative of the family Torymidae is depicted here : ]
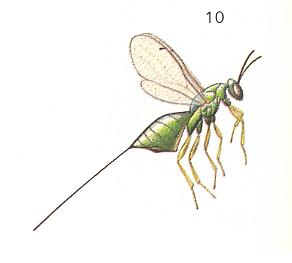
Figure 6a : Torymus nigricornis Boh., about 3.5 mm., Torymidae, Chalcidoidea.
(After CHINERY, 1983, Elseviers Insektengids voor West-Europa)
| GROUP | EAST AFRICA | ISRAEL |
| Agaonidae |
Ceratosolen arabicus Ceratosolen galili |
-- -- |
|
Torymidae-Sycophaginae Sycophagini |
Eukoebelea sycomori Idarnes gracilis Parakoebelea gigas Sycophaga sycomori |
-- -- -- Sycophaga sycomori |
|
Torymidae-Sycophaginae Apocryptini |
Apocrypta longitarsus |
Apocrypta longitarsus |
|
Torymidae-Sycophaginae Sycoryctini |
Sycoscapter spec. Sycoscapteridea spec. |
-- -- |
|
Torymidae-Sycophaginae Philotrypesini |
-- |
-- |
|
Torymidae-Sycophaginae Otitesellini |
-- |
-- |
|
Torymidae-Sycophaginae Sycoecini |
-- |
-- |
It is remarkable that, as far as known, in the syconia of Ficus sycomorus, in addition to Ceratosolen arabicus, a second species of Agaonidae develops, namely Ceratosolen galili. The life-cylcle of this wasp is almost identical to that of C. arabicus, but the females do not perform pollination movements after oviposition (in flowers with short styles). The pollen-baskets, that these wasps do possess, are always empty for that matter. The males cooperate in gnawing the exit channel.
In the above table it is also indicated in what way the symbiosis of
Ficus sycomorus is constituted in Isreal. There this species had been imported by man centuries ago, with only two symbionts [in fact parasites of the plant or of other inhabitants of the plant] : Sycophaga sycomori and Apocrypta longitarsus. Sycophaga can make galls, and the males gnaw a channel in order that the females can leave the syconium. The fig flowers are, however, not pollinated, resulting in the fact that in Isreal Ficus sycomorus does not form seeds.The relationships between the symbionts (including parasites).
Origin of the symbiosis. One supposes that the symbiosis between figs and fig wasps is old. Corner (a.o. 1958) takes the time of origin of Ficus to be the Cretacious or earlier. Croizat (1968) speaks about the distribution from a certain region in "Triassic-Jurassic" and "Jurassic-early Cretacious". No matter how indistinct all this might be, one can savely assume that the symbiosis has a history of 100-150 million years. Paleontological data concerning the symbiosis are not available, and we shall have to base our speculations on the recent situation as compared to what is known in related groups of plants and wasps.
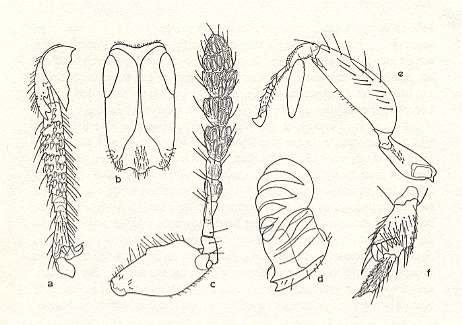
Figure 7 : Parts of female fig wasps, in which some structures are visible that are used in entering the syconium.
a-d - Agaonidae : a - tibia and tarsal segments of the foreleg, with teeths and spines. b - anterior view of head, with unchitinized median groove, rendering the head easily deformable. c - antenna, with large shaft and strongly elongated third segment. d - mandible with ribs, and appendage with ribs.
e-f - Sycophaginae : e - foreleg with platy appendage on the tibia. f - tibia and first tarsal segment of the hindleg, with teeths and spines.
(Largely after GRANDI, 1955, and WIEBES, 1968)
In suppositions about the origin of the symbiosis and the form of the original symbionts one can only conjecture. There is still not much to say about the place of the Agaonidae in the system of the Chalcidoidea. They are most similar, also in characters not related to the phytophagous way of life, to some Torymidae (see table above ).
Ficus is partly characterized by Corner (1962) by the symbiosis with the Agaonidae. The genealogical relationship of Ficus with other Moraceae (for example Sparattosyce, next Figure) is clear.
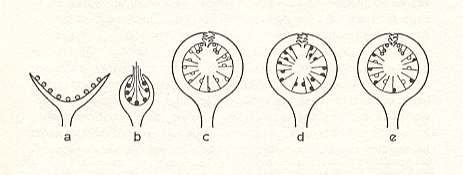
Figure 8 : Inflorescences of Sparattosyce (a - male, b - female),
A diecious Ficus (c - male, with gall-flowers. d - female), and
Sicomorus [subgenus of Ficus] (e - monoecious : male and female flowers, and gall-flowers, in one single syconium), diagrammatic.
Male flowers are indicated as shaded, female flowers black, and the gall-flowers white.
[In c and e the male flowers are clustered around the syconium's eye]. (After CORNER, 1962, modified by WIEBES, 1970)
Moreover, one might suppose that some proto-Moraceae from which Ficus as well as Sparattosyce have originated possessed such closed inflorescences that when blooming either bursted open (as is today still the case in the male inflorescences of Sparattosyce, and, less regularly, in some species of Ficus), or in which the styles of the female flowers projected outwardly through the 'eye'. In that sense Croizat (1968) might be right in holding that the Ficus-form of the syconium is an early appearance in the evolution of the Moraceae. Here his arguments are not directly relevant : It is not the shape as such of the syconium that limits further differentiation, but rather the narrow mutualistic symbiosis with the Agaonidae, which, once having originated, limits both participants to a differentiation at a [merely] low taxonomic level.
In Sycophaga-females one finds adaptations that are comparable to those of the Agaonidae (see Figure7 ). The head is flat, with a narrow longitudinal groove. The fore-tibia possess a series of teeths, and the hind-tibia have a comb with flat spines. Also in other Torymidae (see table above ) one finds special structures : appendages on the mandibles or the fore-tibia (some Sycoecini) that make us think of the jaw-appendages of the Agaonidae but apparently not being homologous with them, or spinelets laterally on the thorax, or a comb on the hind-tibia as in Sycophaga (some Otitesellini). It is clear that in different groups different adaptations have originated all making possible the penetration into the syconium through its eye. Later we will see that this also holds for the adaptations of the oviposition through the thick wall of the syconium.
The transformations in the Agaonidae (with respect to other Chalcidoidea) that directly have to do with the pollination-syndrome are the pollen-baskets and the pollination movements, that is, the behavior dealt with earlier. "Syndrome" in this sense is a concept in flower-biology (pollination-ecology) that names the total of features having to do with a certain type of pollination. The behavior of the males of Agaonidae also belongs to this syndrome insofar as it causes the liberation of pollen out of the male flowers (as a result of the gnawing activities of those males making an exit opening). If one compares the result of the gnawing activity of the Agaonidae-males with that of the males of Sycophaga, it is clear that in the former case the behavior belongs to the pollination-syndrome, while in the latter case precisely the same behavior has nothing to do with pollination : Each part of a given syndrome makes as such only sense when the other parts are also present. In that way one can imagine how a given feature, originated in some other context (for example : leaving the syconium), can come together with other features (that also may have been originated individually, that is, under different circumstances) to form a complete pattern of a very complex pollination-type.
In this way one can globally imagine the origin of the symbiosis of Ficus and Agaonidae as being a development from a living-together of proto-Agaonidae with the proto-Moraceae, in the inflorescences of which the larvae live, to the mutual dependency in which now also the Ficus depends, with respect to its reproduction, on the other symbiont : The agaonid wasp changes from 'parasite', and Ficus from host, to mutualistic symbiont.
The interspecific relationship must have been originated fairly early on in development, perhaps already during the parasitic stage (as for example also the phytophagous Megastigmus-species are fairly limited in their choice of hosts [which might finally lead to a single-species <==> single-species relationship] ). This supposition seems to be implied by the systematic parallel that we find in the classifications of the Agaonidae and Ficus.
The parasites, already mentioned in passing or in comparison with Agaonidae, presumably come from very different groups. Here we can point to the wasps still placed into the subfamily Sycophaginae of the family Torymidae. The division into smaller groups (tribus) is indicated in the table above . Van der Vecht (1960) has pointed to the fact that the elongation of the abdomen in the 'tail wasps', necessary to guide the ovipositor during oviposition through the shell of the syconium, might have originated in different ways. One can suppose that in the figs, in the course of evolution, natural selection took place in the direction of a thickening of the wall (shell) of the syconium. The way that, in their turn, the parasites keep up with this development by increasing the length of their ovipositor and undergoing morphological transformations related to it, might give a clue to their genealogical relationships.
The Sycophagini are, with respect to their ovipositor-mechanism as well as to other features, clearly genealogically related with Torymus-species also living in moderate climates as parasites in various galls. In the Sycophagini the ovipositor is driven into the syconium by walking backwards. This is not only the case in the tail-wasps that prick the syconium from the outside, but also in Sycophaga sycomori which lays its eggs while being inside the syconium. Galil and Eisikowitch (1969) describe the behavior of this species as follows : " The wasp stands up high on its legs and directs the ovipositor straightly backwards. When the proper oviposition site has been found the wasp walks backwards while pricking its ovipositor in the ovary ". This behavior differs from, for example, that of Ceratosolen arabicus, which pricks rightly beneath it into the pistil (see Figure 5, above ).
In the Apocryptini (see table above) the rings [segments] of the abdomen can be slided apart from each other as in a telescope, resulting in the ability to lift the tip of the abdomen high above the ground (= wall of the syconium) on order to drive the ovipositor straight into the syconium.
In the Philotrypesini the last two abdominal rings are elongated.
In the Sycoryctini only the ninth (= the last) abdominal ring is very long and tube-shaped. The movements during oviposition are similar to those of the Sycophagini.
Such habits point to the fact that we here have to do with at least two genealogic groups of parasites, which is supported by many other, morphological features. One group, the Sycophagini, consists of Torymidae that must have specialized themselves already fairly early on (that is, in a rather early developmental state) (the group lives in all continents) in living with Ficus. The other groups (the Apocryptini, only in Africa, Asia, and Australia, the Sycoryctini, up to now also known only from these continents, and the Philotrypesini, known only from the Old World) might have been evolved from one or more other groups of short-tailed Torymidae, at a later stage than have the Sycophagini.
There exist more such groups of parasites.
Until now in our considerations the male fig wasps are almost not mentioned. They so much differ from the females that they are hardly recognizable as being wasps at all. See next Figure.
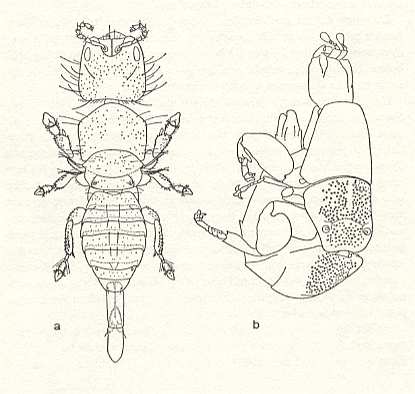
Comparative morphological investigation of these males is indispensable for setting up the genealogical groups. So, the close relationship between Sycophaga (of which the females enter the syconium through its eye, and possessing for this organs making them similar to Agaonidae) and other Sycophagini (and thus Torymidae) (of which the females with their long ovipositor bore through the wall of the syconium, and therefore being more similar to other Torymidae) is evident from the morphology of the males, which until now are not distinguishable even generically.
[We now continue with the considerations of MALYSHEV, 1966, concerning -- still in the context of the Secondary-phytophagous (phyto-oophagous) Phase of the evolution of Hymenoptera -- the relationships between, and the biology of, several families such as the Eurytomidae, Callimomidae and Agaonidae. In MALYSHEV's discussion we encountered the genus Philotrypesis, belonging, according to him, to the family Callimomidae. This genus was also encountered in the just finished extract from WIEBES on figs and fig wasps. And here the genus is placed in the family Torymidae. On page 210 Malyshev states that the family Callimomidae is the same as the family Torymidae of earlier authors.]
In searching proper sites to lay their eggs, some chalcidoid terebrants belonging to the family Eurytomidae began to exploit other organs of plants, but organs also rich in metabolic meristemic tissue, functionally and morphologically similar to embryonic tissue. Such first of all were the growth-points at the base of the internodes from where growth proceeds and where, as a consequence, takes place an increased growth of meristemic cells. In precisely these places on the stems of grasses (and somtimes also on the apical growth-sites), places, that is, protected by envelopes of leaves emerging from them, the Eurytomidae of the genus Isosoma (= Harmolita) lay their eggs. Here their larvae feed on the delicate plant tissue, where at the place of injury usually longitudinally-oval galls appear.
Thus, the wheat isosoma (Harmolita tritici Fitch.), according to data from Phillips and Dicke, 1935, lays its eggs in young stems of wheat, "infecting the meristemic zone of the internode abover the node in the upper part of the young stalk. The layed egg does not cause changes of the tissues. Their hypertrophy (excess of growth) only starts when the larva emerges. In the course of the first larval instar a strong division takes place of the cells of the phloem and parenchym (secondary meristem). When the larvae reach the second instar this cell-division stops, and the hypertrophic growth of the cells begins, which loose polarity in their spatial ordering. The central cavity of the stalk gradually disappears. It is being filled with grown cells of the gall, and as the larva grows the vascular system of the gall is destroyed and lignified, causing the fragility of the stalk (Nikolskaya, 1952).
If we take into account the fact that the chalcidoid phytophags originally are, as was explained above, plant-egg-eaters, then the secondary status of the behavior of the representatives of Isosoma, -- that develop, in contrast to genuine "seed eaters", already not anymore in the generative, but in the vegetative parts of the plants -- naturally follows. This also fits with the data of Bugbee, 1936, according to which the group isosoma originated from an eurytomid kernel as a side- and aberrant group.
The second large grouping of secondary-phytophagous Hymenoptera -- and thus, like the first grouping [just discussed], also representing the Secondary-phytophagous (phyto-oophagous) Phase of hymenopterous evolution -- consists of the gall-wasps and allies. We shall deal with them in the next document.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXXVIII.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI