

Predatory Hemi-Familial Phase
This document continues to expound -- following MALYSHEV, 1966 -- the successive evolutionary Phases (or main strategies) of the insect Order Hymenoptera. In earlier documents we have dealt with the Phases in the hymenoptera symphyta and hymenoptera terebrantia. Of the hymenoptera aculeata we have dealt with the solitary wasps, and the social wasps. And now we have arrived at the second major group of aculeates, the ants (Formicoidea) [the third (and last) group is formed by the bees (Apoidea)].
Together with the diverse wasps and bees the ants make up the large suborder of aculeate Hymenoptera, counting more than 50000 species. The superfamily of ants (Formicoidea) belongs to the smaller part thereof, that is, counting about 6000 species and varieties, distributed in all inhabitable parts of the world. It is remarkable that all ants are social insects, living more or less in an organized way. Their colonies chiefly consist of closely related female individuals, usually being descendants of a single mother. From this it is clear that the colonies of ants, as indeed also of the other social insects, are, essentially, with respect to their contents and origin, families.
Just noted was the basic fact that all recent ants are social insects. To this we should add the fact that also among the fossil ants, in great numbers known from Eocene [= early Tertiary] times, we do not encounter some or other primitive forms living solitarily, but only representatives of the same families of social ants which also live today. But most remarkable, apparently, is the fact that the social way of life of the ants appears in them in all developmental stages [of the individual]. Even the young fertilized female, beginning to independently found a colony, directly [that is, without transition] lays her eggs not isolated one from the other but in small heaps or packets, for which, just as for the emerged larvae, and later also the pupae, she simultaneously cares. Therefore, in an ant's nest there are absolutely no "cells" -- individual chambers for the separate nursing of the young [as we have such cells in wasps and bees].
All this sharply distinguishes the ants from all wasps (and equally from bees), which, in contrast, in their great majority of species live a solitary life, and with it in such a degree solitarily that the mother usually even does not see her descendants which, moreover, develop isolated from one another, which is also oberved in Myzine, Tiphiidae, and Mutillidae. It is very indicative that even in those relatively very few species of wasps, which live socially, the eggs are nevertheless laid isolated from each other, and the young fed individually, each one in a special chamber-cell.
A few exceptions from this are observed only in higher [evolutionary] stages of development of bees, but also here these phenomena are, undoubtedly, of a secondary nature.
This difference undoubtedly has deep roots, and therefore for us there is absolutely no reason to turn to wasps or bees, and, with respect to their evolutionary development, look for solitarily living forms also among ants or their ancestors : such, evidently, do not exist.
Accordingly, we conclude that the social form, or, more precisely, the familial form, is the original form of the life of ants, and thus also the ancestors of the ants, which had differed from them morphologically, already in one way or another lived a familial life. From this viewpoint for answering the question of the origin of ants we must, consequently, proceed not from the highly-developed aculeate hymenoptera, but from the lower ones -- the parasitic ones, that is, from the hymenoptera terebrantia. Meanwhile the question of the birth of familial life among terebrants, of its development in them, and of the various complexifications connected with it, has not yet been answered in one way or another completely. On the contrary, in the literature concerning terebrants in such cases not seldom is used the term "multiple", or "gregarious", parasitism. Here, one usually has in mind the development at the expense of a single host of several parasitic individuals which might be descendants of several mothers and which might have different relationships to each other. Such a term, consequently, points to different relationships and masks those which are here of interest to us. The latter should therefore be indicated in an exclusive way, namely as [indicating] familial parasitism, underlining the development of several descendants of a single mother at the same place at the expense of a common food source or of a single host.
In it being our task to demonstrate elements of familial life in [some] "parasitic" (carnivorous) forms, and to evaluate them with respect to the origin of the formicine structure [= structure as ants have it] of life, we must now turn to those terebrants in which several descendants of a single mother live together. In this it is very important to us, independently [i.e. not being influenced by] even from the systematic position of the given groups, to clear up under precisely what circumstances and in what way originated and developed in [certain] terebrants elements of familial life. Of course, the cases of internal parasitism -- excluding the possibility of contact of the parasitic larvae with their mother, let alone the peculiarity of the living-conditions of these larvae inside the body of the host -- do not, essentially, belong here. Nevertheless, it is sometimes not without interest for the completeness of the explanation to consider also them [i.e. internal parasites] during the course of the investigation.
As a point from which to begin in answering the question which interests us we must take the origin of carnivorous forms -- terebrants in the broader sense (Terebrantia) -- from lower originally phytophagous forms (Symphyta). This central moment of the transformation of in their larval stage phytophagous forms into carnivorous, parasitic forms, took place, as was already expounded in earlier documents, in the Inquilinoid Phase of the evolution of the Hymenoptera in the conditions of gall formation.
At first it was the egg stage, or the very young just emerged larva -- the host of the gall -- that became subjected to attack by the larvae of the [evolutionarily] originated terebrant displaying predatory habits. After having destroyed the egg or this larva, the original terebrant's larva turned back to its originally vegetable food, that is, feeding on the walls of the gall. By this the original proper inquilinoid line of evolution of the Terebrantia was determined, clear traces of which are preserved still today in the habits and development of certain primitive terebrants (from the evaniids, ichneumonids, and chalcidids). This first line of evolution does not contain any elements of familial life, but in these or similar conditions we will soon see several other lines of diversification of the terebrants.
Predatory Hemi-Familial Phase
From the Inquilinoid Phase, not far from its beginnings, originated also that particular line of evolution in which the egg of the terebrant became laid there where before it were placed not one, but several eggs of the host. In this case the larva of the terebrant obtained the possibility to pass over into feeding on just the egg clutches of the host, and to remain, consequently, on the stage of being a predatory larva.
When the size of the parasite's body was in this case more or less equal to a single or a few eggs of the host, laid in a common clutch, there appeared a new possibility for the terebrant, namely to lay several eggs on one clutch to be infected, where then began to develop its predatory larvae together at the same place.
Such relationships are observed among Chalcidoidea which is a large superfamily of terebrants, counting more than 30 000 species, almost always being small or minute (from 0.1 mm to 9 mm). From the whole of this mass of terebrants, generally very diverse in outer appearance, divided into 27 families, a series of most primitive forms, especially Eurytomidae, Callimomidae, Eupelmidae, and Perilampidae, stand out. Primitive and outstanding they are on the basis of structural features. Their representatives are, for chalcidoids, medium-sized (2-6 mm), and possess normal larvae, similar to the usual larvae of aculeate hymenoptera. It is interesting that precisely among these morphologically primitive chalcidoids we find examples of the most primitive habits. Here are some examples concerning our presently considered [evolutionary] moment -- the moment of development of the predatory oophagous [egg-eating] line.
The cricket-eurytoma (Eurytoma oophaga SILV.) and relatives oviposit into egg-clutches of wood crickets (Oecanthus), placed in longitudinal cracks in branches of shrubs and trees. Each egg of the eurytoma is here laid close to the anterior end of the host's egg. First the larvae of the eurytoma only consume those eggs of the cricket onto which they had emerged [i.e. eggs of the cricket, next or onto which they had emerged from their eggs], but then the larvae become very motile and destroy all eggs of the cricket's clutch. After this they may gnaw channels through the marrow of the stalk having a length of 4 cm and more, in order to attack the second egg-clutch.
The cicada-eupelmus (Eupelmus cicadae GIR.) places its eggs in the egg-chambers of cicadas, distributed in groups in the tissues of plants, and also not into the eggs themselves, but next to them. Going from one chamber to the next, the larvae of the eupelmus destroy their content.
In the same way the larvae of the chalcidoid Cirrospilus ovisugosus C. and M. (family Eulophidae) devour the eggs of the grass bug Poecilocapsus lineatus F., placed in stalks of living plants. See next Figure.
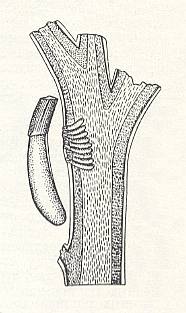
Figure 1 : Egg-clutch of the bug (Hemiptera) Poecilocapsus lineatus F. inserted into the marrow of a twig. Left - individual egg, strongly magnified.
(From SANDERSEN, 1921, in MALYSHEV, 1966)
Having hatched from eggs laid nearby the egg-clutch of the bug, the chalcidoid larvae make their way through the marrow of the plant to the egg-clutch. In order to reach maturity each chalcidoid larva consumes three or four eggs of the bug.
Sometimes reported is a fully-grown larva and pupa of P. pachymerum WLK. lying inside an egg of a mantid. Insofar as the egg of the chalcidoid is [always] laid outside the egg of the mantid, one, also in this case, reckons the chalcidoid larva as being predatory.
To the third group of chalcidoids, having predatory larvae, we must reckon those that develop outside plants directly under insect-hosts which lay their eggs unconcealed. Such habits we encounter among higher organized chalcidoids.
Thus it is observed that Microterys titiani GIR. (Encyrtidae) lays its eggs -- which are colorless or bleak-pink, provided with a stalk -- amidst the spherical eggs of the coccid Lecanium corni BUCHÉ [Hemiptera-Coccidae, scale insects, fam. Lecaniidae] where the eggs of the former look very much like those of the latter. When the female of the scale insect finishes oviposition, and [when thus] beneath her on the surface of leaves or stems of the food plant (grapevine, citrus, plum, white acacia) a small heap of freshly-laid eggs is formed, then, next to it we can see a group of minute larvae (up to 10) of the mentioned chalcidoid devouring from below the female scale insect the eggs laid by it. Also the larvae of Scutellista cyanea MOTCH. and other chalcidoids of the small family Microgasteridae devour eggs of lecaniid scale insects. When the eggs turn out to be unsuited for this, the chalcidoid larvae develop as ectoparasites on females of the scale insect.
In the given diverse examples one can see how, among chalcidoids, appeared in one or another degree essential traits of familial life of [such] terebrants : jointly living larvae at the place of their birth from one single mother, and, in addition, next to a store of food shared by them. However, in this case no conditions are created for a direct, and in one way or another prolonged, contact of the larvae with their mother. This did not happen already only by the fact that during the development of the predatory larvae inside plants, and also in egg-cocoons and oothecas for the chalcidoid-mother such a contact was impossible. In the last case, on the other hand -- of the development of larvae in open places, no remote place was obtained where the mother might spend a long time with her descendants. Moreover, the very temporary nature of the food (developing eggs) and the insufficient stores of it at one place, also may significantly interfere with the development of living-together-in-one-place of the mother-chalcidoid with her descendants in the present Phase.
This was, as we might say, still a very primitive family of orphans, consisting of young developing individuals -- predatory larvae, accidentally taken together only as a result of the common descent from one mother, and the presence of food next to them, having been left on their own, without any care for them of their mother. But this "semi-family" was already an important point -- the prerequisite for the birth of a new, higher stage of life in terebrants in the considered direction.
With all this we have expounded the Predatory Hemi-Familial Phase of hymenopterous evolution. This Phase, and also some other (next to be considered) Phases, tentatively scout the opportunities of familial life, in the end resulting in the ways in which ants live.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part LVII.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII