

Introduction
The question of the origin of the carnivorous, or so-called parasitic Hymenoptera, from their originally phytophagous ancestors undoubtedly has decisive significance to the understanding of the whole course of the historical development of the order Hymenoptera. It is very important theoretically as well as practically. From this it is clear that this or that result obtained from its solution will influence phylogenetic constructions [in the sense of typologic systematics] determining their essence and directions.
When looking to the lower groups of Hymenoptera, saw-flies and horntails, we do not find direct indications of in what way the transition from phytophagous forms to zoophagous forms took place : Larvae of the one and the other -- phytophags and zoophags -- are not encountered [that is, we cannot find there one group, or species, that is in the larval stage phytophagous, and another group, or species, that is in the larval stage zoophagous], if we do not consider the above mentioned enigmatic Orussidae, with which we will deal later on. Also morphologically we cannot with conviction indicate any concrete transitional forms that would clearly connect these two large, standing far apart from each other, groups of Hymenoptera. All this has given rise to the different, and from time to time contradictory, views of investigators regarding the origin of the zoophagous Hymenoptera. [Without discussing all the suggestions given by various authors, we will directly move over to Malyshev's hypothesis about the origin of zoophagy in Hymenoptera and with it the origin of the Terebrantia (parasitic Hymenoptera).
Transition from phytophagy to zoophagy, and the origin of the parasitic Hymenoptera
In the evolutionary history of the hymenopterous phytophags a very decisive junction took place from which several lines of development began. This was some sort of phytophagous complex that had originated already in Permian times. Its base was formed by the most primitive forms of the known phytophagous Hymenoptera, the Xyeloidea and Megalodontoidea. In the same way as their descendants (the few that have managed to survive up into recent times) they, in their larval stage, developed on the surface of plants, feeding on pollen of pine-trees ( Xyelidae ), or needles and leaves of shrubs and trees ( Pamphiliidae and Megalodontidae ).
Let us reproduce some members of the descendants of these first Hymenoptera ( Figure 1, 2, and 3) :

From this central core the great majority of true saw-flies originated, that were also ectophytic, and that during their egg-phase developed under the skin of the plant, but as larvae on its surface. From this core also those true saw-flies originated that were endophytic, which had already the whole of their development inside the plant, in leaves as well as in green stalks and shoots. With these latter, totally in line with their habits, also connect the Cephoidea, having later given rise to the trunk horntails (Siricoidea).
It is interesting to note that in some cases in saw-flies there are "weak gall formations" that are soon left by the emerged larvae. Such weakly developed galls, or so-called procecicia, are found on a series of plants -- on Rubus, Clematis, Heleborus, Pteridium, and others. With respect to such phenomena we must see an indication not only of the existence of gradations, but also of the very method of development of gall-formation in saw-flies -- not from "miners" gnawing channels through the plants, but directly from "ectophytic" species.
In such a situation it is natural to assume that also among ancient saw-flies, in the period of the origin and development in them of the ability to cause galls, also were created relationships of inquilinism. The first thrust leading to it was, we might think, a physiological factor : Complete or partly loss in some of them of the secretion from the appendant genital glands indispensable for generating a gall. Possibly, as a result [of this loss] newly originated biological species had to lay their eggs in alien galls in which also their larvae would develop. To realize this, no great changes of instincts were needed in the just originated sawflies-inquilines. The insect continues to lay its eggs in the same species of plant and even in the same part of it and in the same way as it had layed them [evolutionarily] before. Only now it begins to lay its egg not until an egg of a related species had been laid there and, as a result of this, the characteristic uncontrolled growth of the plant cells-gall appears. In such conditions the guest-larva having emerged from the egg unavoidably runs into contact with the egg or young host-larva. Finding themselves in close vicinity of each other in the gall-cavity with a limiting amount of food, they necessarily become competitors for one and the same stock of food, and sooner or later become enemies of each other.
So, no great structural, physiological, and instinctive adaptations were needed for the guest-larva -- emerged from its egg placed in an alien gall, having until this moment [of its emergence from the egg] lived of the protein content of its own egg -- to gnaw with its mandibles the very delicate integument of the egg or host-larva, destroy it and to take over the gall.
In this way the first small, but utterly significant, step was taken on the path of the transformation of originally phytophagous Hymenoptera, the S y m p h y t a, to the (first) zoophagous Hymenoptera, the T e r e b r a n t i a.
The absence of carnivorous larvae in recent saw-flies is noted in the literature more than once. However, among saw-flies one will look in vain for such a sort of carnivorous habit where the larva during the whole of its life develops at the expense of the prey : in them it never existed, neither does it today. But this sort of entomophagia [preying on insects], which, according to our views, had to appear at the [evolutionary] starting point of the origin of the Terebrantia at their (still) inquilinoid phase, might be observable in today's fauna, or at least in conditions of experiments.
In the case under discussion, in the development inside galls we clearly see the disappearance of contact between larvae emerged from eggs of one and the same female. After having developed inside the gall at the expense of the content of its egg, the larva of the saw-fly feeds exclusively on the walls of the gall. From this moment onwards, having tasted vegetable food, the larva of the saw-fly will always remain phytophagous. As was already noted, around young larvae of Euura amerinae L. together living in shoots of willow, at first also form individual galls that only later on can coalesce together, forming one complex gall with a common room in which the larvae of Euura peacefully live together.
When things are indeed such, we might think of artificially creating conditions which might lead to a contact between young larvae of a saw-fly inside a gall, in view of the possibility that precisely in such conditions cannibalism among them may occur. As an object for such experiments was taken the willow saw-fly Pontania proxima Lep. Its small humble female lays her very small eggs individually under the upper skin of willow leaves, especially of Salix viminalis L., and of Salix fragilis L. She thereby gives off an enzymatic substance causing an accelerated growth of the plant-tissue at the place of the sting. See next Figure.
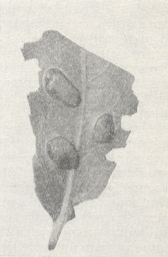
Figure 4 : Ripe galls caused by the female saw-fly Pontania proxima Lep., on the leaves of the willow tree Salix fragilis L. (After MALYSHEV, 1966)
As a result, around the laid egg in the first days an elongated shield-shaped gall develops, projecting from both sides of the leave, especially its underside. The thick and very juicy green walls of the gall enclose a wide, but not high cavity in which, on the spongy lower wall, lies one oval, not reaching a length of 1 mm, egg of the saw-fly. See next Figure.
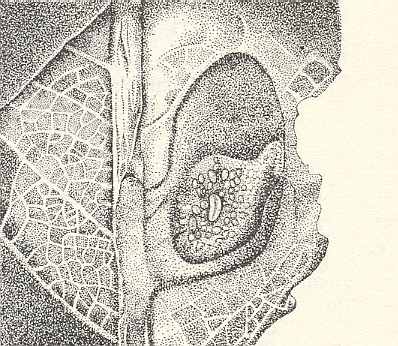
Figure 5 : Opened gall of Pontania proxima. On the spongy lower wall of its cavity lies an egg of the saw-fly. (After MALYSHEV, 1966)
This egg can be found in young green galls on the delicate leaves, still covered as it were with varnish, of the mentioned willows, especially on their watery shoots, emerging in the beginning of May and until the first days of June. The gall develops at the place of the sting of the Pontania, even in the case when, for some reason, no egg has been laid here.
Chopersky nature reserve :
These experiments remove the veil that covered the, seemingly anomalous phenomena that are somtimes observed in inspected galls of P. proxima collected in nature. Thus in one large elongated gall, with constrictions in the middle pointing to the coalescence of two galls, was found one common cavity containing two eggs of Pontania lying at a certain distance from each other. Further in this gall there was one very small larva, and next to it lay a head of a second one. In another such gall a small larva of Pontania was found holding with its mouth the remains of the anterior end of the body of another larva, evidently having killed it.
These observations with sufficient definiteness indicate that young larvae of P. proxima, when finding themseves near to each other, and although not showing definite aggression, nevertheless do not remain indifferent with respect to each other and soon attack each other : One larva bites through the integument of the other, sucks up the haemolymph and may destroy the defeated almost completely, leaving only its empty shell.
Consequently, in the described experiments and observations the young larva of the gall-making saw-fly shows predatory habits and presents itself as an inquiline.
In evaluating individual findings one should be careful not to confuse the casted-off skin of the original larva with the remains of its prey. The case may become still more puzzling when other inquilines and parasites are present. Thus, in one experiment at a young larva of Pontania, which had already begun feeding on the walls of the gall, was placed an egg, qua size and shape similar to the egg of Pontania proxima, but taken not from the cavity of a [the] gall, but from a small cell in the gall's wall. The next day from this egg a legless larva with a tiny head emerged, possibly that of some beetle, and bit to death the Pontania larva.
Archaic inquilinoid phase
As was expounded above, the [evolutionary] first preys of the first Terebrantia [parasitic wasps] having evolved from endophytic saw-flies, were also saw-flies, that is, representatives not only of the same Order Hymenoptera, but also of the same family and genus and even of a closely related species. They belonged to precisely those saw-flies in which the ability was developed to form galls. The first state of the host-sawfly that was subjected to attack by the guest-sawfly (inquiline) was the egg or the young larva that had just emerged. After having destroyed the egg or this host-larva, the larva of the [evolutionarily] original parasitic wasp proceeds to feed on the gall-walls, as it was until now its usual way of feeding. There was no other choice to it. In this way the archaic inquilinoid phase of the evolution of the Terebrantia was determined.
The following task now lies before us :
To enquire, namely, about the conditions during which, from the archaic inquilinoid phase, could have been originated and developed the various lines of evolution of the Terebrantia. Based on morphological investigations, it is assumed that the Terebrantia evolved from phytophags. But precisely the way along which one group was transformed into another is not determined [i.e. is not yet known] and it remains unclear precisely what transitional forms do connect the two hymenopterous suborders (Symphyta - Terebrantia). Concerning this, very valuable morphological and partly paleontological data are obtained that point to the great ancientry and primitiveness of a whole series of systematically different recent Terebrantia, such as (seem to be), for example, the Gasteruptiidae, the Trigonalidae, Orussidae, Heloridae, Ibaliidae, and some others. The next three Figures show some of these.

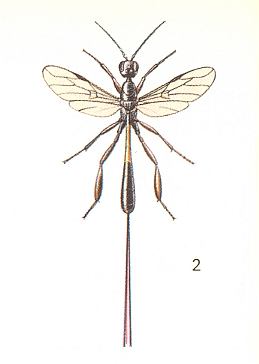
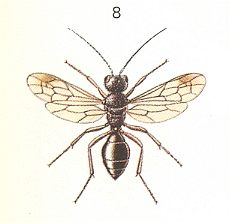
For us it is very important to know how precisely these different, taxonomically primitive groups of Terebrantia are related to each other. The until now (1966) available relevant morphological indications are scarse indeed and not definite. From them we can only see how far these most primitive terebrantia have diverged from each other and from their common ancestors -- the cephoid saw-flies.
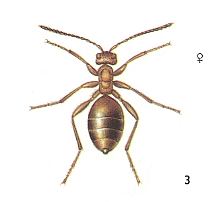
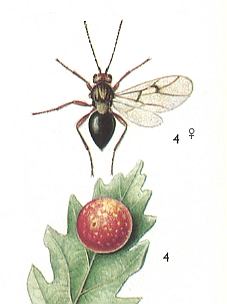
If we, from the remaining 44 hymenopterous gall-making species, do not consider certain Eurytomidae -- which are terebrantia that produce galls -- then in the Nearctic (= North America) there is only a very small number of species of s a w - f l i e s that produce true galls, and which belong to only three genera -- Euura, Lycaotella and partly Nematus. In the same way, in Central Europe the number of species of saw-flies that form true galls is very limited and they chiefly belong to two genera -- Pontania and Euura.
Consequently, it is clear that the gall-producing [members of the] family Cynipidae -- being evolutionarily a relatively young branch of the Terebrantia -- is much richer in species than the ancient group of gall-making saw-flies living today. This relationship is very significant. It clearly expresses the fact that the chief mass of endophytic gall-prodicing saw-flies became extinct a long time ago. Today we see only their relicts. It is natural to think that the extinction of these saw-flies coincided with the replacement of vegetation that took place in the geological past. The grandiose transformation of the plant world, about which we spoke earlier, could not fail to express itself in the life and preservation of forms so narrowly specialized to definite plants as gall-producing saw-flies. The majority of these disappeared, and only a small number of them, having adapted to new plants, could persist and now represents a sort of relict.
Above it was already noted that the larvae of many recent ectophytic and some of the endophytic saw-flies still continued to feed on such primitive plants as ferns, horsetails, pine-trees, sedges, grasses, willows, etc. The saw-fly Selandra coronata Klg., for example, causes gall-like swellings on the fern Nephrodium (Aspidium) filix mas.
It is natural that, following the gall-producing saw-flies, their inquilines -- the archaic terebrantia -- found themselves in similar difficult conditions. The majority of these latter, having lost their original gall-producing hosts, also became extinct. Others, on the other hand, having in one or another degree changed their habits went over to new hosts and together with them fell into different conditions of life.
In this way the fundamental divergence of the chief lines of evolution of the Terebrantia took place. Therefore, the forms representing them today seem to stand so far away from each other that the genealogical relations between them and with their common ancestral form seem to have been disappeared [that is, because of (1) the many gaps -- as a result of extinctions -- and (2) the high degree of divergence, the genealogical relations are hardly traceable].
Attempts to clarify this connection with morphological means did not, as we saw, give any firm and definite result. But archaic terebrantia generally must be archaic not only in their structural features, but also in their inherited behavioral features and instincts. From this it follows that clearly archaic terebrantia must in one way or another express fundamental traits of the live of their ancestral carnivorous forebears closely related to phytophags. Working according to the "principle of functionality" can be very productive, especially in those cases where a pure morphological method fails to provide indications.
Following this biological approach it is clear that the foremost condition for a successful development of the archaic terebrant-inquiline was for it to lay an egg into a normal gall of the saw-flie host. In the opposite case of the absence in the gall of an egg of the host, and, connected with this, the weak development of the gall, the inquiline larva would have been forced to leave it in order not to perish by hunger. In such conditions the decisive moment in the egg-laying of the inquiline soon turned out to be precisely the presence in the gall of an egg of the host, and not the gall itself. Subsequently the destruction of the egg of the host by the young larva of the inquiline became, at least in certain cases, for it even a life necessity : Not having sucked out the egg of the host, it looses the ability to further develop.
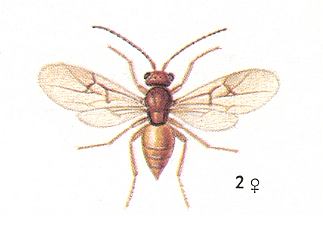
Having analyzed the conditions in which the [evolutionary] origin and development of the archaic inquilines took place, let us now turn to those phenomena that are observed today in the most primitive of terebrantia, and first of all in the E v a n o i d e a. The structural differences among them are so significant that, according to the investigations of Crosskey, 1951, the evanoids are considered to include three families, the Gasteruptiidae, the Evaniidae, and the Aulacidae. Let us insert three Figures to illustrate some of them.
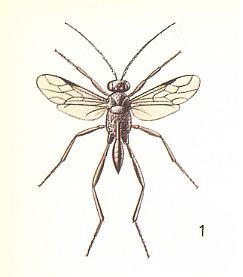
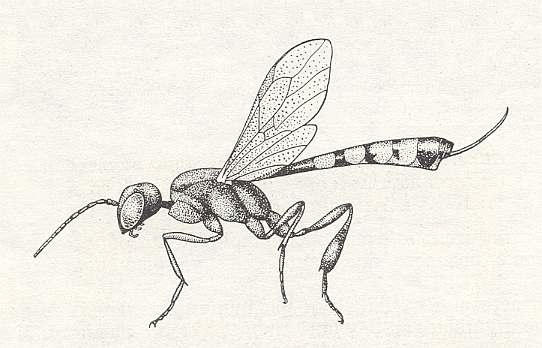
Unfortunately, knowledge of the individual development and the behavior of the Evanoids is still insufficient, but what one does know about them is, with respect to our views, very interesting.
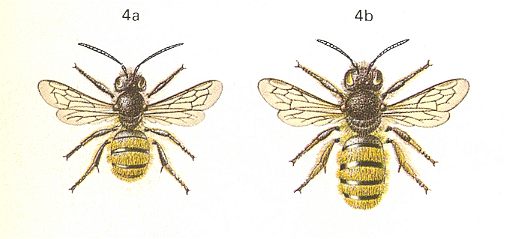

Figure 15 (top and bottom) : Osmia rufa L. 8-12 mm. Superfamily Apoidea, bees.
Top : Left : Male. Right : Female.
After CHINERY, in Elseviers insektengids voor West-Europa, 1983.
Bottom : After SEVERA, in ZAHRADNIK, Thieme's insektengids voor West- en Midden-Europa, 1977.
A statement of this finding [host-nest of Gasteruption caudatum : that of the solitary bee Osmia parvula], at the time sealed by the successful photograph -- see next Figure -- was published by the author (Malyshev) later, together with other materials, in Russian as well as in foreign editions (Malyshev, 1937, 1949). It remained, however, unnoticed in the literature, perhaps because it did not go with the current interests. But now, as a result of the discovery of the inquilinoid phase of evolution of the Terebrantia as an evolutionary point of departure of the parasitoid Hymenoptera, the question of the life and [individual] development of the archaic terebrants has become decisively significant, and therefore demands a systematic approach to its solution.

With the help of placing bait, widely used in the Chopersky nature reserve, the author succeeded in obtaining substantial information about the behavior and development of 8 species of Gasteruptiidae. They all were found in nests (cells) of solitary bees. Together with this, also a larger number of cells of solitary wasps were inspected, wasps that lived in the same ecological conditions. However, no features were found indicating infection of the wasp nests by the gasteruptiids. Many, and repeated observations, later published by the author (Malyshev, 1959, 1964) indicated that Gasteruptiidae show a predilection for a strict circle of hosts and with it only for bees.

Trichofoenus pyrenaicus lays its egg on the honey provisions [prepared by the bee] a bit in front of the egg of the bee Ceratina. For a representative of the latter genus, see next Figure.

Further, Gasteruption thomsoni lays its egg on the cell wall in front of the provisions of the bee Colletes daviesanus Gm., and, finally, Gasteruption rugulosum (see Figure 13, above ) places its egg even outside the cavity of the cell [which itself is made in the stem of one or another plant] of the bee Hylaeus, outside its membranous plastering. See next Figure.
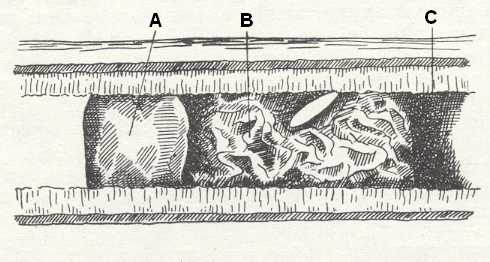
Figure 19 : Egg of Gasteruption rugulosum Ab. laid outside the membranous plastering of the cell of the bee Hylaeus sp.
A - Honey provisions. B - Plastering of the cell. C - Anterior division wall.
(After MALYSHEV, 1966)
The newly-born larva [and thus of the first instar] of the representatives of the Gasteruptiidae belongs to the eruciform (erucoid) type. See next Figure.
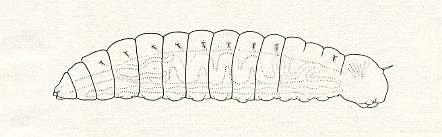
[ E r u c i f o r m larvae are polypod [[sometimes these legs in a rudimentary state]] caterpillar-like larvae as, for instance, in Mecoptera (Scorpion-flies) :

They contrast with worm- or maggot-like larvae.]
(Continuing with the newly-born larva of gasteruptiids) It has a large, almost square, head with well-expressed antennae. Its body is nude [i.e. without hairs, spines, etc.], tapering posteriorly, and ending with a short forklet which is only visible when the larva moves. On the ventral side of the larva, when seen in profile, "micropods" can be distinguished -- paired projections similar to the abdominal limbs of young larvae of saw-flies (Pontania).
After emerging from the egg-shell the larva of the gasteruptiid (Gasteruption caudatum, T. pyrenaicus) usually quickly finds the egg of the host-bee. Having nestled itself longitudinally on it, it cuts through the very delicate egg-shell with its sharp piercer-like mandibles. After twelve hours, or around that, it devours the complete content of the egg of the bee, and its emptied shell remains, hardly visible under the gasteruptiid larva. As a result, the outer appearance of the gasteruptiid larva -- initially erucoid (eruciform) -- now changes dramatically : Its body bulges, the segmentation almost completely disappears, and, apparently, the head is relatively very small. In this way, the larva, still in its first instar, becomes vesicle-like.
Corresponding with the difference of species the further development and feeding of the larvae of the Gasteruptiidae proceed differently. The larvae of the second instar, G. caudatum and T. pyrenaicus, now feeding on the honey provisions in the same place where normally also the bee-larva eats, are very similar to the latter also with respect to their outer appearance, such that a quick look might confuse them with these bee-larvae. But this cannot be said of the second instar larvae of G. rugulosum and G. thomsoni, because they do not lie on their side on the surface of the here semi-liquid honey, bent in a ring, as do the larvae of the bees Hylaeus, and Colletes, but in contrast to them, float on their ventral side in a straight posture.
Still not having reached half of their complete development, the larvae of the Gasteruptiidae enter the third instar. Now their behavior and outer appearance significantly change. First of all, from this moment onwards in the larvae of Gasteruptiidae the connection between the middle- and hind-intestine opens and defecation begins. The little lumps of excrements produced by them are similar to those of their hosts, the bee-larvae. With their anterior end (head-end) the half-grown gasteruptiid larvae now direct themselves toward the posterior wall of the cell and engulf themselves into the provisions. On their middle segments -- laterally as well as dorsally -- appear small projections, (which are) in cases sucker-like [If my rendering of "sostsevidnije" is correct (the word is absent in my dictionaries)], in other cases column-like. See next Figure.
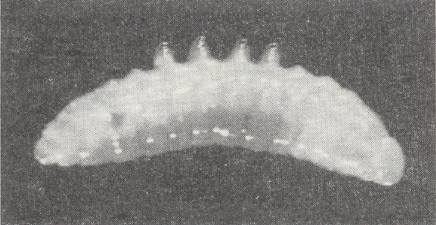
Further on in development, corresponding to the attained degree of larval growth, the larva's integument extends (especially laterally), and the shape and size of the mentioned projections change significantly. Eventually, the latter become lower and wider, and sometimes completely vanish.
Relationships, similar to those described above, are observed also in Ichneumonoid terebrants of the genera Grotea, Macrogrotea, and Ectropsis. Also in this case the young larva of the terebrant begins to devour the egg or newly-born larva of the solitary bee Ceratina (see Figure 18 ), and then feeds on the vegetable material (honey dough) prepared in the cell [So it is a genuine inquiline]. Thus, according to a series of observations of Graenicher, 1905, the small egg of Grotea anguina Cress. (of the subfamily Pimplinae of the family Ichneumonidae (Terebrantia)), as a rule, is laid longitudinally on the end of the egg of the solitary bee Ceratina dupla Say. The larva of the terebrant may emerge earlier or a little later than the larva of the bee, but in both cases it quickly starts to suck out the contents of the egg or of the young larva of the Ceratina, (the terebrant larva) largely clung to the anterior end of the prey. Soon, a shriveling-up of the egg or the host-larva is seen, and after 24 hours the anterior end of its body becomes empty. After this the Grotea larva turns around and empties the rest of the prey such that during the course of two days in the cell one can see only the dry remains of the egg or larva of the bee. After this, the larva of Grotea in the course of 3-4 days so greedily devours the bee-bun as if it were the bee-larva itself. As a result of this it significantly increases in size and then gnaws itself a way to the next cell and sooner or later devours in it the host-larva. Usually it penetrates into a third cell, and even into a fourth one, with the same result. The complete feeding period of the Grotea larva lasts 13-14 days. After this it prepares a firm cocoon at the place of the destroyed cells, and in it it hibernates.
Concerning the question whether defecation takes place while the Grotea larva is feeding (as in Gasteruption), the investigator (Graenicher) doesn't say anything, but only tells us about it dropping its excrements in the lower part of the cocoon [in which the larva, of course, does not feed].
This information is compatible with the interesting, but old data of Giraud and Cameron, telling us that in the genus Pimpla (see next Figure) there exist species that can live in galls and feed on their tissue or saps.
Interesting cases of inquilinism are also observed in Chalcidoid terebrants belonging to the primitive family Eurytomidae. [Having no picture of a represesentative of particularly this chalcidoid family, we can show the general appearance of C h a l c i d o i d e a in the following Figure] :

Thus, according to observations of Ivanova-Kazas, the larva of Eurytoma aciculata Ratz. begins with the destruction of the egg or young larva of the saw-fly Pontania viminalis L. -- for this saw-fly see next Figure
an then starts feeding on the walls of the gall of the Pontania. The death of the egg or larva of the saw-fly is caused here not by the life activities of the larva itself of the Eurytoma, but apparently takes place already at the moment of egg-laying by the adult Eurytoma.
If we take stock of all these data, we see that already the original phytophagous Symphyta (saw-flies and allies) possessed the very important necessary preconditions creating the possibility of the transition from them (all having their abdomen broadly attached to the thorax [i.e. without a stalk or petiole] ) to the "stalked-abdomen hymenoptera", the Apocrita. Such a precondition was first of all the formation of a thin membrane -- the homologue of the funiculus -- at the place where the abdomen is attached to the thorax. In subsequent development this became the stalk (petiolus) of the higher Hymenoptera (Apocrita) -- Terebrantia and Aculeata. This device also guaranteed proper movement of the abdomen of the female, at first merely to be able to lay eggs into the tissue of plants, but later [in evolution] also connected with other tasks.
Therefore the assertion ( Viktorov, 1959 ) that the Hymenoptera with a stalked abdomen could not have originated from Hymenoptera with a broadly attached (sessile) abdomen, that is, from Hymenoptera supposedly lacking a proper mobility of the abdomen at the place where it is attached to the thorax, is not convincing.
Another no less important precondition (for the origin of the Apocrita from Symphyta) was the establishment in certain endophytic saw-flies of the ability to inject in the green tissue of plants a special secretion -- a product of the appendant genital glands -- causing at the place of the sting by the ovipositor a pathological growth -- a gall, which is rich in nutritive substances.
There is no doubt also that the first terebrants ( Terebrantia ) could not be sharply distinguished [i.e. were not very different from] as to their feeding habit in the larval stage from their originally phytophagous ancestors. As it was already explained above, the ancestors of the terebrants, developing in galls of cephoid saw-flies as their original inquilines, could, it is true, begin with destroying [eating] the egg or young larva of the host-sawfly, but later, nevertheless feeded on the juicy walls of the gall (and thus being phytophagous after all, like their ancestors), a gall, that is, now not produced by them.
A very important point in the understanding of the, presently under discussion, central moment of the evolution of the Hymenoptera is the fact that in recent representatives of the Terebrantia (and even generally in the Apocrita !), as was shown above, primitive forms are still present today, forms, that is, showing the behavior of typical inquilines, which are found in three isolated primitive groups of Terebrantia. As we will see later on, typical vestiges of primitive inquilinism are even farther distributed and in other directions.
In all primitive abdomen-stalked Gasteruptiidae, apparently the largest number of archaic features is preserved. This is not only the case in their behavior and development, but also in the structure of their fully-grown larvae, which can, in virtue of their morphological features, be compared with larvae of [primitive] Aculeata, and partly also in the structure of the adults. The transition of the primitive Gasteruptiidae from infecting galls to the laying of eggs in the nests of ancestral bees must be dated at the beginning or middle of the Cretaceous period in which took place the replacement of the ancient gymnosperms by new plants, the angiosperms, and in which (period), connected with this, took place the development of vespoids and of sphecoid apoids (bees).
To the real possibility and even a certain ease of such a transition points the presence among the terebrants of such genera as Eurytoma, where some representatives, such as Eurytoma aciculata Ratz., develop as inquilines in galls of saw-flies, while others, such as E. robusta Mayr., develop in cells of solitary bees. Similar phenomena are observed also in ichneumonid terebrants of the genus Pimpla (see for a representative of this genus Figure 23 ).
We must assume that a favorable circumstance for such a transition was the inclination of many solitary bees to nest in already prepared spaces, including the cavity of galls left by their original possessors. A decisive significance for the transition from infecting galls to infecting cells of bees undoubtedly had the presence for the larvae of the inquilines of enough and proper food, in both cases [that is, no new adaptations were required].
In connection with this it is interesting to note that most species of Gasteruptiidae infect nests of precisely primitive bees of the genus Hylaeus and relatives, inhabiting [as larva] prepared cavities. Here, apart from the already mentioned 8 species of Gasteruptiidae, infecting nests of Hylaeus (and relatives), we might point to Gasteruption diversipes Ab., obtained from a nest of Hylaeus deceptoria Peréz, built on a branch of bramble. It is remarkable that also in Australia one not further determined Gasteruptied, also infects nests of hylaeoids, namely that of Pachyprosopis linettae.
As a primitive feature of the Gasteruptiidae we should mention also their use of the ovipositor. By them it is exclusively used for leading the egg to where it has to be placed, and not for paralyzing the prey or for executing some other function, as is generally the case in Terebrantia and Aculeata.
The newly-born larva being of the eruciform type -- albeit with this or that differences -- is seen also in some terebrants belonging to the Ichneumonidae and Braconidae, and sometimes also in primitive Aculeata (Sapygidae, Chrysididae). To interpret such larvae as secondary, evolved from the usual "worm-like" larva of Terebrantia (similar to what is taken to be the case in some specially adapted larvae of the bee-cuckoos), has no basis.
The presence in larvae of Gasteruptiidae of well-developed chewing mouthparts, that is, not adapted to sucking, as they are in other terebrants, also demonstrates the archaic nature of this family.
If our ideas with respect to inquilinism as a starting form of behavior and (individual) development of the Terebrantia are correct, then we naturally will expect the discovery of remnants of incipient inquilinism also in other groups of Terebrantia.
The next evolutionary phase of the Hymenoptera, the ecto-oophagous phase, will be expounded in the next document.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XXXV.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII