

[Also in these enquiries into the wing-venation and its transformations in Diptera-Nematocera we will consider the subcostal vein (Sc) at most only occasionally. This vein, especially its terminal branches (Sc1, Sc2), is -- in Diptera -- often difficult to discern.]
Sequel to wing-venation in the superfamily Tipulidea.
Having considered the last family -- the Ptychopteridae -- of the superfamily, we must dwell upon the venational relationships existing between this family (Ptychopteridae) on the one hand, and some other families in the Tipulidea, Culicidea and Bibionomorpha on the other, because especially the structure and shape of the basal part of the wings in Ptychopteridae (= Liriopeidae) seem to indicate a different historical development of their wing-venation than that what has taken place in Limoniidae, Tipulidae, Trichoceridae (= Petauristidae), and Cylindrotomidae.
For the sake of clarity we here again produce the two Figures of the previous document that depicted the wing-venation of representatives of the family Ptychopteridae :
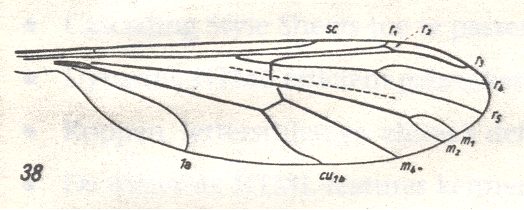
Figure 1 : Wing-venation of Liriope scutellaris MEIG. ( Ptychopteridae).
(After HENNIG, 1954)
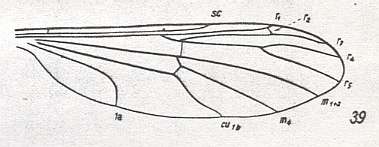
Figure 2 : Wing-venation of Bittacomorphella nipponensis ALEX. ( Ptychopteridae).
(After ALEXANDER, 1927, from HENNIG, 1954)
In the groundplan of the flight-apparatus of the Order Diptera only the forewings have been preserved, while the place of the hindwings have been taken by the so-called halteres -- small petiolate organs provided with a terminal knob -- which probably fulfill a stabilizing function in flight. In connection with an increase of wing-beat frequency the transfer of muscular force has also in the now only pair of wings been concentrated on the anterior part of them. The posterior part of these wings retained -- as a result of the mutual approach of the veins CuA and CuP -- the ability to passively give way to air resistance. A certain narrowing of the wingbase, having taken place in its hind part, have led to the loss of the third anal vein (3A) which is still present in Mecoptera. In this stage of development of the wings the Tipuloid families remained, that is, they did not further develop in this respect. See next Figure.
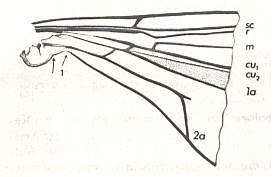
Figure 3 : Basal part of a wing of Trichocera sp. ( Trichoceridae). The left arrow indicates the distal boundary of the Neala ('wing-scale'). The venational stump attached to 2a is an anomaly. But it indicates that the sharp curvature of the second anal vein in Trichocera is a secondary feature. The with 1 marked arrow indicates the distal boundary between the neala and the wing proper.
(After HENNIG, 1968)
In other respects the tipuloids did develop further, however, namely in the progressive elongation of the wings carrying with it the shift of branching-points of the longitudinal veins toward the wing-apex. Connected with this is probably also the course of R2, having it to end in R1, and maybe also the "capture of R4 by the vein R2+3". See next Figure.
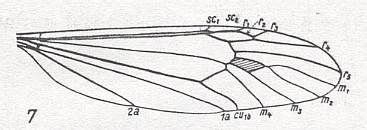
Figure 4 : Wing-venation of Pales dorsalis FABR. ( Tipulidae).
(After HENNIG, 1954)
In all other Diptera a distinction was developed between the wing-base (basiala) and the wing-blade as a result of an incision at the basal part of the wing's hind-margin. As a result of this the second anal vein (2A), still present in the Tipuloid families, was reduced. See next three Figures.
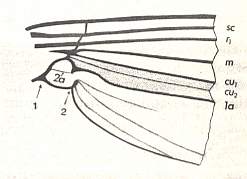
Figure 5 : Basal part of a wing of Culiseta annulata SCHRANK. ( Culicidae). The mentioned incision is indicated by the arrow marked with "2". Also the reduced second anal vein (2a) is indicated.
(After HENNIG, 1968)
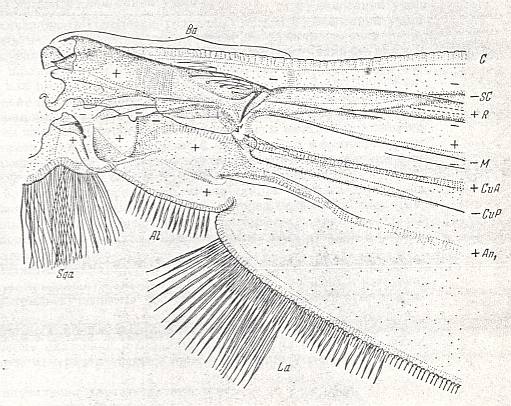
Figure 6 : Theobaldia alaskaensis LUND. ( Culicidae).
Basiala and base of wing-blade of right wing of male. Top view.
Specimen nr. 2570. Length of whole wing 6.5 mm., of basiala 0.75 mm. Macrotrichia and scales not drawn.
For abbreviations see Figure 1a in Part II (of Evolution of Insects).
(After ROHDENDORF, 1951)
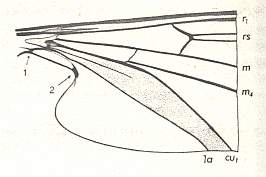
Figure 7 : Basal part of a wing of Liponeura bilobata LOEW. ( Blephariceridae). Here, especially, we see the clear distinction between the wing-stalk and the wing-blade, and, of course, also here the second anal vein is reduced.
(After HENNIG, 1968)
Although in all tipuloid wings the second anal vein is still present, in Trichoceridae (which is a tipuloid family) this vein is rather short :

Figure 8 : Wing-venation of Trichocera regelationis L. ( Tipulidea-Trichoceridae). Compare, with respect to 2a, Figure 4 . (After HENNIG, 1954)
Now we turn to the wing-venation in the family Ptychopteridae (= Liriopeidae) ( Tipulidea).
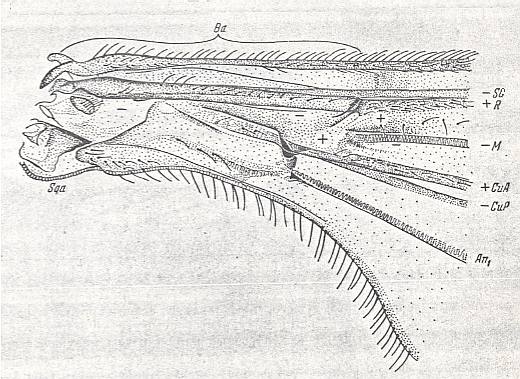
Figure 9 : Liriope contaminata MEIG. ( Liriopeidae [ = Ptychopteridae] ).
Base of right wing of male. Topview.
Specimen Nr.3684. Length of wing 7.50 mm. Length of part starting from the level of the base of the costal vein to that of the humeral cross-vein [the cross-vein between Costa and Subcosta], supposedly delineating the basiala, 1.35 mm.
For the abbreviations, see Figure 1a in Part II (of Evolution of Insects). The brace (in upper part of figure) with the sign Ba indicates the extent of the supposed basiala.
(After ROHDENDORF, 1951)
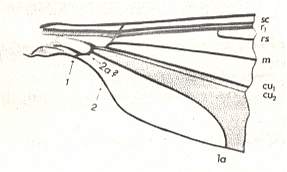
Figure 10 : Basal part of a wing of Ptychoptera sp. ( Ptychopteridae [ = Liriopeidae] ). The arrows with the respective marks "1" and "2" in following five Figures indicate the boundaries of the wing-stalk (basiala). At 1 lies the distal boundary between the neala and the wing proper.
(After HENNIG, 1968)
I now characterize (that is, not literally produce) HENNIG's (1968, p. 12-13) discussion of the venational relationship of the Ptychopteridae with other nematocerous families.
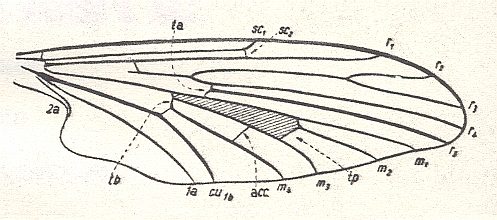
Figure 11 : Wing of Protoplasa fitchii O.S.. Order Diptera, Family Tanyderidae. The shaded area in the wing signifies the Discoidal Cell (in a natural wing this cell is as transparent as is the rest of the wing membrane). The second anal vein is reduced, but still present. There is a distinction between wing-stalk and wing-blade, however, not as the result of a sharp incision into the wing-margin. There is no shift of the main bifurcation points toward the wing-apex. (After HENNIG, 1954)
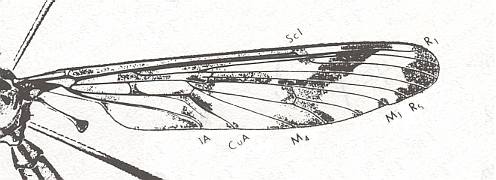
Figure 12 : Wing of Eutanyderus oreonympha. Order Diptera, Family Tanyderidae. Length of wing, ca. 13 mm.
To see this figure better and a little enlarged, click twice on it. Also here the second anal vein is reduced, but apparently still present as a short vein. The wing here is markedly petiolate, and elongate. Also here there is no shift of the main bifurcation points toward the wing-apex.
(After COLLESS, 1970, from RICHARDS & DAVIES, 1977)
The wing-venation in Tanyderidae is, it is true, more primitive than in any other Dipteron (including the tipuloids). [This means that the mentioned differentiation into wing-base and wing-blade might be only seemingly so.]
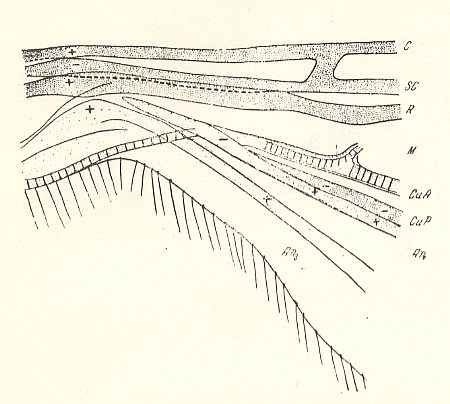
Figure 13 : Pachyneura fasciata ZETT. ( Pachyneuridae). Wing-base, basiala. Schematic.
Abbreviations as usual.
Here we have an example of a weakly developed basiala, that is, lots of elements are missing.
(After ROHDENDORF, 1946)
[and with Petaurista (Tipulidea-Trichoceridae) :]
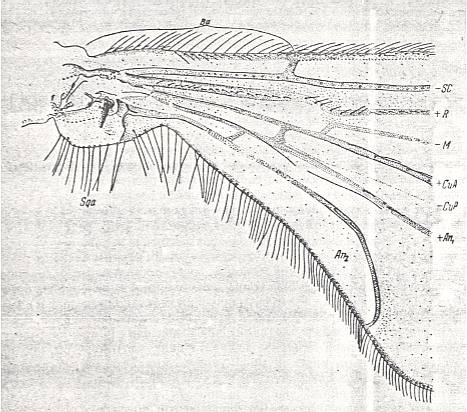
Figure 14 : Petaurista maculipennis MEIG. ( Petauristidae [ = Trichoceridae] ).
Base of right wing of male. Topview.
Specimen Nr.2534. Length of wing 7.35 mm. Length of part starting from the level of the base of the costal vein to that of the humeral cross-vein [the cross-vein between Costa and Subcosta], supposedly delineating the basiala, 0.94 mm.
For the abbreviations, see Figure 1a in Part II (of Evolution of Insects). The brace (in upper part of figure) with the sign Ba indicates the extent of the supposed basiala.
(After ROHDENDORF, 1951)
Apparently the wing-structure in Ptychopteridae has secondarily changed markedly. The development here has been partially resulted in a re-appearance of some original features of the tipuloids (pseudoplesiomorphy of the Ptychopterids : undoing of the differentiation between wing-stalk and wing-blade at least with respect to the wing's outline). As to other features, the narrowing of the wing has resulted both in the tipuloids and in Ptychopteridae in a correspondence of derived (apomorphous) characters : the ending up of R2 in R1.
So it indeed seems that the groundplan of the wing and its venation (or at least the starting condition) in the (evolution of the) Ptychopteridae must be different from what we originally set out it to be, that is, different from the wing and its venation of Liriope as depicted in Figure 1, above . And certainly, it seems, the venational starting condition is different from those of the (other) tipuloids.
But, although such non-tipuloids which later gave rise to the tipuloid-like Ptychopteridae might have existed in Jurassic or Triassic times (fossils of them not found), we cannot find descendants of them among recent Diptera : Because the Radial Sector (Rs) in Ptychopteridae is 4-branched (see Figure 1 ), we cannot find a non-tipuloid having at least 4 branches of its Rs among the Bibionomorpha (they all have a Radial Sector that is at most 3-branched). So from types like these the Ptychopteridae cannot have evolved (unless we allow a turning back having taken place in the evolution of the Radial Sector). They cannot be found even among the Rhyphidea (which are also Bibionomorphs with primitive venation, but with Rs having at most 3 branches), neither in Blephariceridae.
The only non-tipuloids (by which we here mean not-belonging-to-the-superfamily-Tipulidea) having a venation from which that of the Ptychopteridae can formally be derived -- which here means that Rs must have at least 4 branches, and, in addition, these forms possessing no characters that are in a derived (apomorphous) state with respect to those (characters) in Ptychopteridae -- are representatives of the family Nemopalpidae of the superfamily Psychodidea, such as Nemopalpus zelandiae ALEX. :
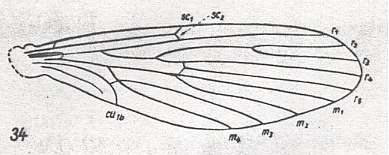
Figure 15 : Wing of Nemopalpus zelandiae ALEX. Order Diptera, Family Nemopalpidae, Superfamily Psychodidea.
(After ALEXANDER, 1927, from HENNIG, 1954)
In Nemopalpus Rs has four branches, all of them still ending up in the wing-margin. The media has 4 branches (M1, M2, M3, M4), and the second anal vein is lacking. Indeed, from such a venation that of the Ptychopteridae can, at least formally, be derived, by letting R2 end up in R1, by losing M3. The problem seems to be that we then have to assume that the specialized condition in the cubito-anal area of the wing in Nemopalpus has, as it were, when changing so to say into Ptychopteridae, evolutionarily turned back to the more original condition : Cubitus and first anal vein long and strong as we see it, for example in Tipulidae, see Figure 4 (but here the second anal vein is still present). However, we have no right to a priori call the cubito-anal area as it is in Nemopalpus a "specialized condition", although it probably is. The only real specialization is the loss of the second anal vein, and this vein is also absent in Ptychopteridae. Anyway, the derivation of the venation of the latter from that of Nemopalpus is not without problems.

Figure 8a : Wing-venation of Trichocera regelationis L. ( Tipulidea-Trichoceridae). Compare, with respect to 2a, Figure 4 . (After HENNIG, 1954)

Figure 2a : Wing-venation of Bittacomorphella nipponensis ALEX. ( Ptychopteridae).
(After ALEXANDER, 1927, from HENNIG, 1954)
By reducing the second anal vein (2a), M3, and the intermedial cross-vein, in Trichocera , in addition to undoing the capture of R4 by R2+3 (by shortening the R4-R5 fork), we get, first, a venation resembling that of Liriope, and after reduction of the M1-M2 fork we get a venation resembling that of Bittacomorphella.
This possibility to formally derive the venation of the ptychopterids from genuine tipuloids, allows us to determine the venational groundplan of the Ptychopteridae as to be a venation very close to that of Trichocera. And this adds to the evidence that the family Ptychopteridae really belongs to the superfamily Tipulidea and not to the Psychodidea as was assumed by HENNIG.
Having looked into the wing-venation of the representatives of the superfamily Tipulidea, we will now take a closer look to the established venational groundplans of the different groupings within this superfamily, and speculate about the possible independent origin of these groups from different mecopterous ancestors.
1. Venational groundplan of the family Limoniidae ( Figure 3, previous document ) :
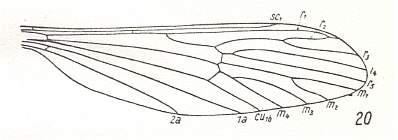
Figure 16 : Wing-venation of Tricyphona protea ALEX.
(After ALEXANDER, 1927, from HENNIG, 1954)
2. Venational groundplan of the family Tipulidae + Trichoceridae ( Figure 15, previous document ) (except that the origin of Rs must be situated (in this groundplan) a little more proximally) :

Figure 17 : Wing-venation of Pales dorsalis FABR. (After HENNIG, 1954)
3. Venational groundplan of the family Cylindrotomidae ( Figure 22, previous document ) (but then with the M1-M2 fork present) :
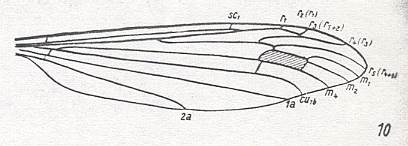
Figure 18 : Wing-venation of Phalacrocera replicata L. (After HENNIG, 1954)
4. Venational groundplan of the family Ptychopteridae ( Figure 8a, present document ) :

Figure 19 : Wing-venation of Trichocera regelationis L. ( Tipulidea-Trichoceridae). (After HENNIG, 1954)
Let us place these venational groundplans still closer together in order to facilitate comparison :




So we see that although these wings are pretty much alike, they nevertheless differ in certain respects. It is certainly remarkable that even within the confines of a single dipterous superfamily -- Tipulidea -- the venational points of departure are not the same. This does not necessarily prove the polyphyletic origin of the Tipulidea, but doesn't disprove it either.
The various brands of tipuloid wing-venation have originated from several mecopterous groups having -- we theorize -- a relatively rich venation such as we see in Permochorista :
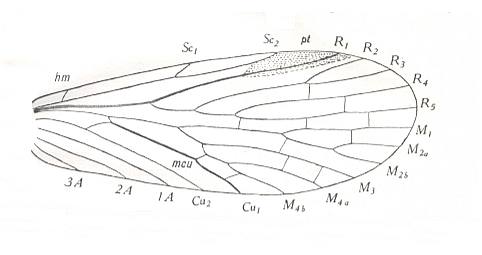
Figure 20 : Forewing of Permochorista sinuata TILL. Order Mecoptera, Suborder Eumecoptera, Family Permochoristidae. Upper Permian of New South Wales, Australia.
(After TILLYARD, 1935)
In this (mecopterous) form we see Rs having four branches. There is no capture of R4 by R2+3, and R2 does not end up in R1 but simply at the wing-margin. There are still fairly many cross-veins present in the wing. M has four or more branches. Discoidal cell present (which is the one at the 'primary' fork of M, namely where M4 originates). CuA and CuP (= Cu1 and Cu2) still relatively far apart from each other, but (already) running parallel to each other [ In at least all Nematocerous Diptera CuP (= Cu2) runs very close alongside CuA (= Cu2). Most of it, however, consists of only a fold or groove in the wing-membrane. It is not drawn in most of the figures in HENNIG 1954.]. There are (in Permochorista) still three anal veins present (1A, 2A, 3A). The wing is moderately elongated.
We can imagine that from such a mecopterous wing the typical tipuloid dipterous wing can be produced (after having lost the hindwings) : Continued elongation of the wing, which process mainly concerns the proximal half of the wing. This apparently brings with it the shift of the main forks toward the apical end of the wing, the shift, in the distal direction, of vein R2+3, resulting in its climbing up the vein R4 (= capture of R4 by the vein R2+3), R2 running into R1, and in many cases becoming recurrent. Elongation of the first and often also the second anal vein. Reduction of the third anal vein. Further narrowing of the wing-base, resulting in a short petiole.
The mentioned processes, resulting in the formation of typical tipuloid wings have, as it has turned out, already taken place in the lower Jurassic, as is evidenced by Architipula clara (upper Lias) :
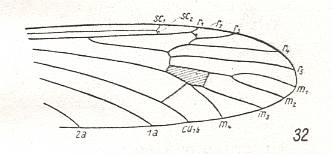
Figure 21 : Wing-venation of Architipula clara HANDL. Upper Lias of Mecklenburg (Germany). (After HANDLIRSCH, 1938, from HENNIG, 1954)
and also by Architipula radiata (lower Jurassic) :
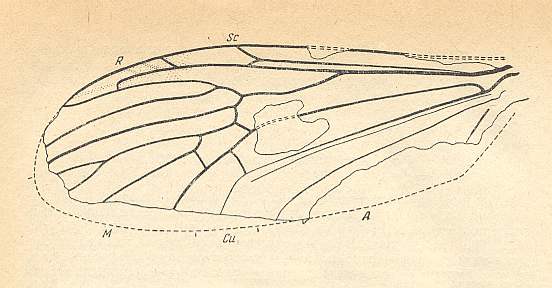
Figure 22 : Wing of Architipula radiata ROHD. from the lower Jurassic of Issic Kul, central Asia. PIN No. 371/403. ( The age of the Issic Kul fauna originally was held as being upper Triassic. Later, however, it was held as being Lower Jurassic). Length of wing 5.9 mm.
(After ROHDENDORF, 1962, from ROHDENDORF, 1964)
And because, as far as we know, no true Diptera have been found from times before the lower Jurassic, we can assume that all the fossil Diptera found in Issic Kul (central Asia) and in Mecklenburg (Germany) are among the oldest of Diptera. So the tipuloids found there (and having lived in the lower Jurassic) had relatively not long ago originated from certain mecopterous ancestors and had directly turned into their typical evolutionary direction (elongation of the wings, etc.). Once developing in this direction and diversifying into the several different tipuloid groups, they could no longer give rise to other Diptera, that is, they could not be the point of departure of, say, the Bibionoids and Fungivoroids. These latter have evolved from other mecopterous groups by directly reducing their wing-venation (for example their Radial Sector became at most 2-branched, and in many of them the trunk of the media weakened, further they underwent total reduction of the second anal vein, etc.). They did not evolve from tipuloids and also not from psychodoids (Also in their case (bibionoids and fungivoroids) the state of the venation as we see it in their recent forms was already present in lower and middle Jurassic times.).
This concludes our discussion of the wing-venation, its origin and transformations, of the superfamily Tipulidea (crane-flies). In the next document we will deal with the wing-venation of the superfamilies Psychodidea (moth-flies, sand-flies) and and Culicidea (mosquitoes and allies).
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XVIII.