

Further REMARKS on the noëtic interpretation of organic evolution.
In all up to now discussed phases of evolution the contact of the terebrants took place only with [still] developing, not yet sexually mature, stages of the host. Meanwhile, cases are observed of contact of terebrants also with fully-grown sexually mature hosts. Here we must realize the particular fact that this contact is, not only from the side of the terebrants, but also from the side of their hosts, being realized chiefly only between female individuals. The contact itself is normally of two types [that is, there exist two types of contact between adults, (1) phoresis : the female terebrant lets itself transport by the adult host to the places where egg-laying of the latter will take place, and where then these eggs are infected, (2) an infection of the adult host itself. Both cases are now expounded in detail.].
In some cases the connection between the adult components [of the contact] expresses itself in the form of so-called phoresis or transport by the female-host of the female-terebrant to the place where egg-laying [by the female-host] will take place, which eggs the transported terebrant then infects.
Such a kind of phoresis may be called "imaginal", to distinguish it from "larval" (transport of planidia, and comparable phenomena). We can further distinguish passive phoresis (as in the cases just mentioned) from active phoresis, which latter is observed almost exclusively in ants, where it is usually placed under the caption of caring for the young or adults. And every sort of transportation of the catch [as is done] by [true] wasps, does, of course, not belong here.
Such cases are mainly observed in serphoid terebrants (Serphoidea) of the family Scelionidae. As the best known example of phoresis serves the behavior of Rielia manticida Kieff. One usually finds the females of this scelionid under the base of the wings of praying mantids (Mantoptera, Mantodea (or, broader, Dictyoptera), Manteidae) or between the genital lobes of the end of their abdomen. As soon as the female mantid is to lay eggs the Rielia leaves its hiding place on the mantid's body, and descends to the foamy mass that forms the ootheca (egg-purse) of the mantid and lays in it its eggs. Also another serphoid terebrant, not closer determined, probably also belonging to the Scelionidae, was found on the body of an adult female of the bug Anoplocnemis curvipes F. from the family Coreidae (Hemiptera). When the latter began to lay eggs the terebrant laid its eggs on the egg-clutch. Certain authors later observed the presence of females of Scelio and Lepidoscelio on the body of Orthoptera (grasshoppers, locusts, and the like), especially Acrididae, where they develop in their egg-purses, although other species of these same genera can do it without phoresis.
It is interesting that similar "phoresic" behavior is sometimes observed also among the "shiny terebrants". Thus, one observes females of the chalcid Anastatus bifasciatus Fons. (family Eupelmidae) on the abdomen of the unpaired silk-worm butterfly Lymantria dispar L., awaiting the egg-laying by the butterfly. Also the females of another chalcid, Oligosita xiphidii Ferr. (of the family Trichogrammatidae), were found attached on the hind-wings of the bush-cricket Xiphidion longipenne de Haan (Tettigoniidae). Similar behavior show callimomids of the genus Podagrion, which infect, as do the Rielia's, eggs of mantids.
In all these cases, thanks to such transportation, the female terebrant reaches not only through the right way the place where the host will lay its eggs, but also at the right moment [namely] when the carried-along eggs of the host are not yet covered by firm protective shells. So, exploiting a relatively large and strong host as a means of transport turned out to be a very convenient adaptation of the very small terebrant-egg-eaters.
A second type of contact with adult preys is assumed by a series of relatively large terebrants belonging to different systematic groups. Although these terebrants manage without phoresis, they obtain essentially the same result, namely laying their eggs directly into the body of the host -- usually into the abdomen of the female not yet in a condition to lay eggs. As was already mentioned earlier, the evaniine Zeuxevania splendidula Costa (Evaniidae) lays its egg into the egg-pack of a cockroach. However, there exists an interesting report according to which that same evaniine (or a biological subspecies of it) has been derived from the adult cockroach Loboptera decipiens Géné, which was held in a test-tube without ootheca [egg-purse]. This fact evidently indicates that the egg of the terebrant was carried into the female cockroach which was not yet in a position to form an ootheca.
Parallel with this we must also speak about the ichneumonoid terebrants of the subfamily Pimplinae, of which certain representatives (the genera Polysphincta, Acrodactila) in their larval state live as ectoparasites on freely moving spiders. This, apparently, totally unexpected transition from a development on insects to parasitism on spiders had, as it may be understood, as its origin the predatory life of the larvae of the Pimplinae (such as in Zaglyptus, Tromatobia), going after egg-cocoons of spiders. A clear hint to this gives the behavior of the terebrant Zaglyptus variipes Crav., which, according to NIELSEN, 1935, kills the female spider and then lays its egg into its egg-cocoon. But it turns out that this terebrant, according to data of another investigator (MANEVAL, 1936), is able to develop on the body of the spider itself, and consequently already representing a different biological species -- similar as in the mentioned Zeuxevania.
From these examples we can see how easily typical egg-eaters, formerly having developed at the expense of egg-clutches of the hosts, could become parasites (more precisely carnivores), now living at the expense of the adult stages of these same hosts. This ease of having transformed one form into the other was determined, of course, by the situation in which the feeding of the terebrant larvae in essence did not change. Here only the behavior of the adults has changed : the latter now lay their eggs onto the host-females which are not yet in a condition to lay eggs, and [the terebrants laying their eggs] thus not on the already laid egg-clutches of these hosts.
This peculiar way of changing one maternal instinct into another was widely distributed in terebrant evolution. But there is reason to believe that it has played not less an important role also in the [evolutionary] establishment of a series of higher forms [true wasps]. From what has been expounded, also other cases of infection by terebrants of more or less fully-grown stages of various hosts will now be understood. Here, not only the stage of the prey, undergoing the attact of the terebrant, is important, but also the very part of the prey's body and the precise point where the ovipositor of the attacker is sunken into, that is, the organs which are to be destroyed in the prey, and even the place from where the developed terebrant or its larva exits [are important]. In this connection the behavior of the terebrant-braconids (Braconidae) of the subfamily Euphorinae is especially interesting. It is remarkable that the great majority of their species develops in adult beetles of various families : in leaf-eaters (Chrysomelidae), lady beetles (Coccinellidae), weevils (Curculionidae), mealbeetles (Tenebrionidae), and bark-eaters (Ipidae), and some [of the terebrant-Euphorinae] in grass-bugs of the families Pentatomidae and Miridae. It is observed that certain species or genera have specialized on definite preys of the mentioned families. Best known in this respect are the euphorines of the genus Perilitus (Dinocampus).
According to data of OGLOBLIN (1913), Perilitus terminatus Nees. very tenaciously hunts lady beetles, especially the 'seven-spot' (Coccinella septempunctata L.) and the 'variable' (Adonia variegata Goez.). The terebrant hunts down the running-away beetle, holding its ovipositor bent under its [own] body, whereby the apex of the ovipositor almost reaches the terebrant's head. After having identified the right moment, it pushes its ovipositor still more forwardly and drives it into the leathery hinge of the prey's abdomen, in one case from above, in another case from below. The very small terebrant egg having ended up in the beetle's body, swells up to such an extent that it becomes up to a thousand times larger. A mere cursory inspection will not reveal anything indicating the presence of a terebrant larva inside the beetle. The latter continues moving and devouring aphids as a completely normal insect. Only before the very exit of the parasite it becomes less mobile. For the rest, in the case of infection of the smaller species Adonia variegeta, the beetle, containing a larva of Perilitus in one of its last developmental stages, can easily be distinguished from healthy beetles : Its abdomen is strongly stretched, the apices of the elytra are drawn apart from each other, and the wings are relaxed and project from the sides of the body. But, despite such far-reaching changes in the organism, the insect remains mobile and gluttonous as usual. All this points to the fact that the internal organs of crucial importance to the beetle do not suffer in an essential way. Indeed, from the data of OGLOBLIN it becomes clear that the larva of P. terminatus chiefly feeds on the fat-body of the beetle, whereby the latter [the [fat-]body] is so much changed that its condition allows us with certainty to decide whether the beetle is infected or not. About the condition [fate] of the reproductive organs of the prey OGLOBLIN does not make statements. But according to other data the ovaries of infected females were very often damaged. Also in adult female weevils (Curculionidae) (Sitona sp.), infected with another species of terebrant, Perilitus rutilus Nees., the ovaries are atrophied, while in the males the sexual system is still in a functional condition upon the exit of the parasitic larva. Upon exiting the beetle, the larval parasite settles itself longitudinally between the beetle's legs and weaves a cocoon, with its threads partly spinning round the beetle too. A remarkable reaction is observed in the coccinellid Hippodamia with respect to this cocoon. When the latter is removed the beetle furiously runs around in search of the cocoon. Having found the latter, it sits down on it and tries again to spin its legs into the loose silk tissue that surrounds the cocoon.
There is an indication that Perilitus coccinellae lays its eggs not only in adult coccinellids but also in their larvae and pupae. Such an assertion is held to be unproven. Nevertheless, it is completely possible, because the different developmental stages of the prey are here usually found together in the same [ecological] conditions. At least in the experiments of CUSHMAN, 1913, P. americanus Riley attacked many adult coccinellids and their larvae (Megilla maculata de Geer, and others). However, in them, only in one adult beetle the development of the terebrant larva was seen. See next Figure.
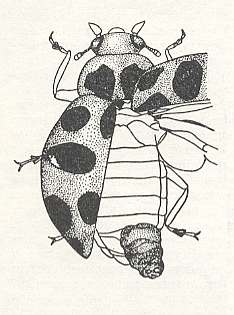
Figure 1 : The fully-grown larva of the terebrant Perilitus americanus Riley exits from the abdomen of a coccinellid [the original subscript reads : leaf-eating] beetle. (After CUSHMAN, 1913, in MALYSHEV, 1966)
Another representative of the terebrant-Euphorinae, Microctonus brevicollis Hal., hibernates in its larval stage in the adult leaf-eating beetles Haltica ampelophaga Gues. Meanwhile, its summer generation exclusively develops in larvae of the mentioned leaf-eater. And so, one and the same terebrant species, in its alternating generations, turns out to stand as it were in different stages of evolution. But it is interesting that in other species of the same genus Microctonus such a complexification in development is not observed. Thus, according to data of KURDJUMOB and ZNAMENSKY (1917), Microctonus sp., after having hibernated in the cereal leaf-eaters halticines (Phyllotreta vittula Redt., Chaetocnema aridula Gyll.), starts to infect these 'fleas' in the spring in their egg-laying period, when the first portion of them are already laid, and the rest being already completely ripe for laying. In such conditions " the larva of the parasite disturbs the ripening of the later eggs, causing a castration of the beetle and reduces the number of eggs actually laid by it ". Both summer generations of this terebrant develop again in adult beetles of a new generation, which [beetles] soon after exiting from their cocoons fly off in all directions and then hibernate.
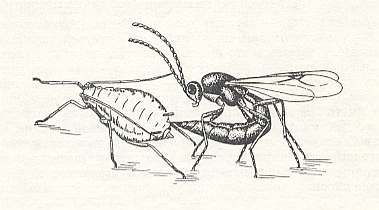
Figure 2 : The female aphidiid Lysiphlebus testaceipes Cress. lays an egg in an aphid.
(From BERLAND, 1951, in MALYSHEV, 1966)
Thus, in the case of infection of nymphs of the common grass aphid (Toxoptera graminum Rond) by the aphidiid Lysiphlebus testaceipes Cress. the aphids reach maturity but do not produce offspring. In another case, of the infection of aphids by the terebrant Aphidius rapae Curt., the development of the embryos in the body of the aphid (Myzus, Aphis) is delayed during the time of ripening of the egg of the parasite, and later these embryos completely disintegrate. In the same way also the development of the winter eggs is delayed. After first having destroyed germs and eggs in the body of the aphid, the larva of the parasite then consumes all its content. The terebrant concludes its development usually in the emptied body of the aphid. Only the larva of Praon exits from the eaten-empty aphid and makes a cocoon under it. The phylogenetic connection with the oophagous phase is also in this case easily established in view of what has been expounded.
From all the examples presented, it is clear how widely was followed the above described path of the evolution of maternal instincts of the terebrants. But soon we will meet with the different, the higher forms, which [also] reflect the imaginally-parasitic phase of evolution of the Hymenoptera.
[Anticipating, we present the next Figure] :
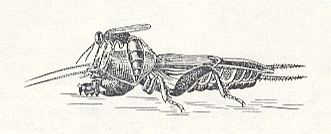
Figure 3 : The female of Larra anathema Rossi. directs its abdomen under the fore-leg of the mole-cricket Gryllotalpa gryllotalpa L. (Orthoptera), in order to lay an egg under it.
[The genus Larra belongs to a subfamily of the digger-wasps, Sphecidae (Aculeata)] (After MALYSHEV, 1966)
And thus, as a result of the accomplished analysis of the behavior and way of life of the various terebrants it becomes clear that all above discussed basic lines of evolution of them depart from the original inquilinoid phase -- in some cases directly, in other cases through the oophagous phase, itself having originated from it, or through a phase derived from the oophagous phase. And this is true even of those phases that were last put forward -- the immediately-parasitic and the imaginally-parasitic phases. Such a conclusion totally complies with the thesis presented earlier according to which the terebrants as an independent systematical unit originated from gall-producing Cephoid saw-flies.

[So all the, in this and the foregoing documents, described evolutionary phases (organic strategies) generally have not evolved one from the other such that it would result in a single evolutionary line, starting from the original inquilines and ending at the imaginally-parasitic phase. On the contrary, there has been several more or less parallel lines starting from the inquilines. And in all this the oophagous strategy plays a pivotal role in the evolution of the Suborder Terebrantia. Noëtically seen (which will later be worked out more fully) we can say that many different terebrant strategies -- now as immaterial patterns in the Implicate Order -- cannot, as it seems, be formally derived from each other, but can be formally derived directly from the original inquilinoid strategy, while many others cannot directly be derived from it, neither from each other, but can be so derived from the oophagous strategy, which itself can be directly derived from the original inquilinoid strategy.
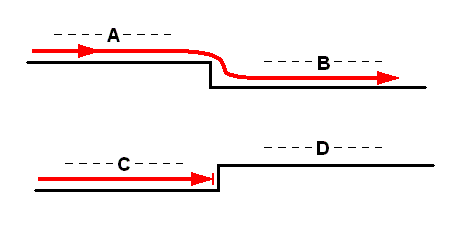
Figure 5 : Diagram spatially illustrating formal derivability and underivability of noëtic patterns (here organic strategies) in the Implicate order.
A, B, C, and D -- noëtic patterns (organic strategies). Red curve -- noëtic trajectory, expressing actual derivation and non-derivation.
Pattern B can be formally derived from pattern A (and is also actually so derived, as indicated by the noëtic trajectory). Pattern A cannot formally be derived from pattern B.
Pattern D cannot formally be derived from pattern C, but the latter can be derived from the former (but is not actually so derived -- there is no noëtic trajectory going from D to C). The noëtic trajectory passing through pattern C is, at least in the direction of pattern D, a dead-end course.
The uni-directional course of the noëtic trajectory, together with the noëtic derivability and non-derivability, expresses the irreversibility of organic evolution.
Now we have concluded the exposition of the Imaginally-parasitic phase of Hymenopterous evolution, and with it we have concluded the exposition of the evolution of the second suborder of the Hymenoptera, the Terebrantia.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part XLVI.
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV