

e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam ) Preface to the whole Series on Organic Evolution
In Fourth Part of Website we have dealt extensively with inorganic beings such as crystals, and compared them with organic beings. We compared the categorical make-up of the Inorganic category-layer with that of the Organic category-layer (by considering the status of the categorical NOVUM of the latter layer).
In the present Series on Evolution (evolution in general, and the evolution of the insect Orders Diptera and Hymenoptera) we will gradually develop a "noëtic theory of organic evolution". And in this we will also again pick up ideas concerning the Implicate Order and the existence of category-layers (where "categories" are invariably nothing else than If / Then constants).
Our noëtic theory of evolution strongly opposes the conventional evolutionary theory based on random genetic mutation and natural selection (neodarwinism). And, as has been said, the development of the mentioned theory, in the documents on evolution (dealing with the Orders Diptera and Hymenoptera) in Present Part of Website, takes place gradually, and in the course of it certain viewpoints and suppositions will be slightly altered without explicitly mentioning it. Only near the end of our exposition of the evolution in the Order Hymenoptera (wasps, ants, bees) our noëtic theory of evolution is becoming more complete. So to anticipate this, the reader may consult the second half of part LXc of the present Series beginning with : " Summary of Noëtic Theory" (so far developed).
But let us now continue with the present document.
Introduction (to present and following documents)
After having dealt with the proposed ontological background of the evolutionary process in general terms, we will now delve into the very facts directly relating to organic evolution as it actually has taken place. And because we cannot cover the evolution of all organisms, we have restricted ourselves to that of the Class of Insects, which, after all, form the largest and most diversified group of animals on Earth. As in all organisms, their evolution is --as we suppose -- driven by continued adaptation, accomplished in given insect species, to more and more different environments or to particular aspects of them, resulting in the occupation of specific ecological niches.
In all this we will begin with the evolutionary diversification as it has taken place in the Order Diptera (two-winged insects). This Order comprises a number of so-called Infraorders, which we will discuss one after the other, beginning with the Infraorder Tipulomorpha, that is, the crane-flies and their relatives.
REMARK :
As regards the classification and the supposed phylogeny of Diptera (and of all Insects for that matter) the reader may consult several of the many entomological textbooks. He or she will discover that the classification and phylogeny of Insects is far from settled. Here we will largely follow the classification as propsed by ROHDENDORF, 1964, Istoritsjeskoje razwitije dwukrilich nasjekomich [The historical development of the two-winged insects] (in Russian), translated into English (sometimes, unfortunately, with errors or omissions) as
The historical development of Diptera, 1974, the University of Alberta Press. We follow his classification because it is based, in addition to certain morphological features, on the original ecology of the taxa. But of course, also in this classification the determination (establisment) of many higher-ranking taxa and their relations to other taxa of the system is far from certain.
But our objective is not to discover the 'true' evolutionary pathways actually having been followed by insects and to present yet again a new classificatory system or genealogical tree of them, but to investigate possible ways of historical development, as suggested by ROHDENDORF and other authors, and see how the general features of these possible ways do or do not fit into our very general theory of the evolutionary process, where this process is expressed in terms of the Explicate and Implicate Orders.
(end of Remark)
When discussing taxa and subtaxa of the order Diptera, emphasis is being laid on the original habitat of the taxon under discussion, that is, the new habitat that was entered and colonized by (one or more populations of) the ancestral species ( = the stem species of the taxon), which transition resulted in the origin of the taxon. This original habitat -- (original) environment in a somewhat narrower sense -- cannot, of course, directly be observed (because its becoming colonized by the ancestral species has happened in the distant past) but can be guessed from the taxon's ecology as it is today. And indeed it is the ecology of the taxon, and that is the ecology of its species, that will be reported in the pages to come.
Following the mentioned book of ROHDENDORF (1964), and also his 1946 book about the evolution of the land-midges (such as fungus-gnats) Evoljutsjia krila i filogenez dlinnoysich dwukrilich Oligoneura (Diptera, Nematocera) [ Evolution of the wing and the phylogeny of the long-antennate two-winged insects Oligoneura (Diptera, Nematocera)] (in Russian), but also (following) the book of OLDROYD, 1964, The Natural History of Flies, which is (also) a book about the way of life of the species of the Order Diptera, we will report and discuss the known ways of life (natural histories) of the species of the various taxa of the Order Diptera, beginning with the taxon (infraorder) Tipulomorpha as a whole, followed by its subordinated taxa down to the family level. We will trace back the original habitat of the taxon and will observe in which way the ecology of the taxon has diversified in the course of its evolution. All this will then be translated into the language that is about the underlying noëtic reactions and the projection of their products.
Let us paraphrase ROHDENDORF, 1964, p. 21, where he speaks about how we must interpret and evaluate systematic categories -- taxa -- in order to set up a natural system of animals, in the present case a natural system of Diptera. Such a system ought to reflect the result of the historical development of them (that is of the Diptera) :
The basis of the correct solution of the problem of the system of subordinated categories or taxa of organisms, said differently, of the system of the given group, must be the determination [evaluation, interpretation] of the systematic category or taxon as a unity, which is characterized by definite conditions of existence, expressed by similar structures, functions and ontogenetic development [= individual development, including postembryonic development from larva to adult] which are determined by common origin, and which [unity, taxon] includes subordinated (of lower rank !) systematic categories or populations of individuals. Such an interpretation implies that the consideration of the systematic category must be all-round [= being undertaken from all sides] and thus not only one-sided morphologically. The determination [establishment] of every taxon, be it a species, genus, family, or whatever other systematic category, must involve not only a clarification of morphological features, but necessarily also a characteristic of the idiosyncrasy of its conditions of existence, history, and phylogeny. Practically this boils down to the evaluation, that is, to the apprehension, of the functional significance of the observed morphological features -- the characteristic "diagnostic properties" of the given group. Only in this way we will open up the essence of that very phenomenon [the taxon] and with it we will discover the way leading to the clarification of the specific features of its [that is, of the taxon's] conflicts as [of the clarification of] the historic process of development.
( ROHDENDORF, p. 21)
Agreeing with ROHDENDORF, we hold that animal evolution is driven by the fact that animal species tend to colonize as much as different environments as possible, resulting in the ecological isolation of populations of some given original species, which (isolation) in turn results in the splitting up of this species. Something like this must also be the case with plants and all other organismic groups that exist on this planet. So we say that it is an intrinsic property of life to diversify as much as possible. And only such a type of life can survive ecological catastrophes (which will take place from time to time and from place to place) that wipe out certain environments or make them unsuited for certain organisms to live in. The life that we know, already exists for millions of years, so it must be of this type, that is, it must be such that it intrinsically strives to diversify itself as much as possible. And it does so by continuously colonizing new environments (or new for a given species) and occupying newly defined ecological niches.
We are not the only one having such an ecological view of evolution. Something similar one can read in the book The Collapse of Chaos by COHEN & STEWART, 1994, where "context" stands more or less for "ecological".
There will be many others too, and one of them is KRIVOSJEINA, Ontogenez i evoljutsija dwykrilich nasjekomich [ = Ontogenesis and evolution of the two-winged insects], 1969. Here we reproduce a diagram from this book which outlines the evolutionary directions taken by the taxa, mainly families, of the order Diptera, as a result of colonizing different environments.
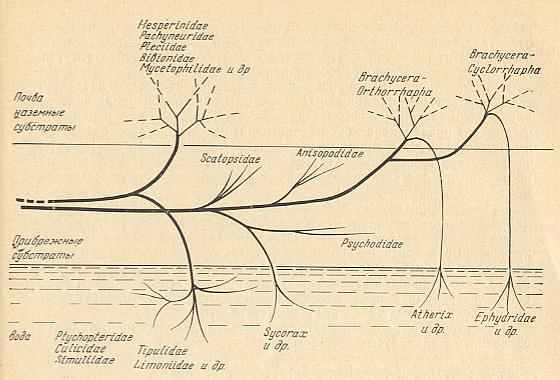
Figure above : Scheme of the directions taken by the historical development of Diptera.
The upper part of the diagram represents : Soil, terrestrial substrates.
The middle part of the diagram represents : costal substrates (that is, banks of water-basins or rivers and the littoral part of the water).
The lower part of the diagram represents : Water.
After KRIVOSJEINA, 1969.
Before we start discussing the ecologically-determined evolutionary pathways of the infraorders of the Diptera, we pause to expound the basics and terminology of insect wing-venation. The diagram that follows does not depict a wing that is representative of a particular (larger) group of insects, neither is it the wing of a particular insect species or individual. It just depicts the general scheme of longitudinal veins. This scheme, generally consisting of eight venational (sub)systems ( Costa, Subcosta, Radius, Radial Sector, Media, Cubitus, Analis, Jugum ) can be recognized in principle in all winged insects, altough in many insect groups some venational systems, or parts of them, have either shifted in a certain direction over the plane of the wing (say, in the direction of the anterior margin, or shifted to the wing's tip) or has been partially or completely reduced (Often the jugal system is absent, and often one or more branches of the Subcosta, Radial Sector, and Media have disappeared). Very often the venational system of the wings has become very specialized and is then, as a result, almost unrecognizable.
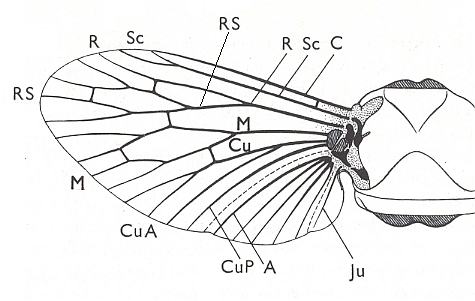
Figure 1 :
Generalized wing-venation of insects, showing the eight venational systems :
The wing itself is membranous, while its veins are hollow thickenings supporting the flexible wing-membrane. The external outline of the wing (its shape), its absolute size, its relative size, its [if it is a fore wing] coupling to the hindwing [if present], the presence of hairs on its membrane, veins or margins, and certain structural details of its venation, are supposed to determine the aerodynamic properties of the wing. As to the majority of the structural details of the wing-venation, this is not so sure : These venational details might be totally non-functional.
Within certain taxonomical groups the venational pattern is remarkably constant.
The main veins generally connect with the insect's thorax mediated by certain sclerites that form something like a hinge. The wing is moved by the flight muscles of the thorax. Often, that is, in certain groups of insects, the wing-beat frequency is very high, up to 500 or more wing-beats per second.
Because in the present and following documents we will have a lot to do with the structure of the wings in especially Diptera, we will reproduce the wing-venation of two representatives of the order (Diptera), that is, from two dipterous families. However, we must realize that the structure and venation of the wings vary enormously in the order Diptera. We here show two dipterous wings in which the venation is evolutionarily fairly completely preserved (in most Diptera many reductions of veins have taken place).
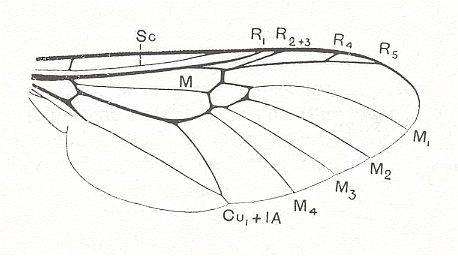
Wing venation of Geosargus (Asilomorpha-Stratiomyiidae) (Diptera).
1A = first anal vein (often denoted as A1, or An1 ).
R = radial system of veins. M = medial system of veins.
Cu1 (often denoted as CuA) = anterior branch of cubital system of veins (the posterior branch [ Cu2 , CuP ] is in Diptera either a fold or is absent altogether).
(After RICHARDS & DAVIES, 1977, Imms' General Textbook of Entomology)
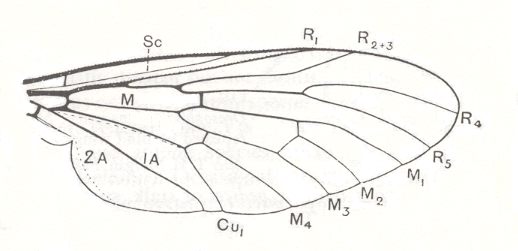
Wing venation of Rhagio (Asilomorpha-Rhagionidae) (Diptera).
1A = first anal vein (often denoted as A1, or An1 ).
2A = second anal vein (often denoted as A2, or An2 ).
R = radial system of veins. M = medial system of veins.
Cu1 (often denoted as CuA) = anterior branch of cubital system of veins (the posterior branch [ Cu2 , CuP ] is in Diptera either a fold or is absent altogether).
(After RICHARDS & DAVIES, 1977, Imms' General Textbook of Entomology)
In the above depicted wing-venation of two representatives of the order Diptera we can see features like the intermedial cell, that is, the cell lying at the center of the wing within the Medial system of veins. Further we can see the radio-medial cross-vein connecting the posterior part of the Radial system of veins with the Medial system. We can also see that the Medial system is connected by some sort of cross-vein with the Cubital vein (Cu1).
Functional interpretation and evolutionary evaluation of the features of the wings of the order Diptera
We introduce this discussion by presenting a figure which, diagrammatically, shows the general movement of the insect wing during flight (that is, of insects in general, not specifically of Diptera).
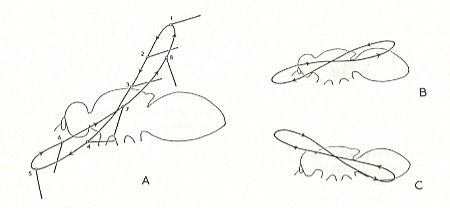
Wing movements in insect flight.
A -- forward flight, B -- hovering, C -- backward flight.
The successive positions of the wing are indicated by the numbers 1 - 8.
We can see that the down-beat (1-4) produces the up-lifting force because the wing-blade is held horizontally, with some inclination to produce a forward thrusting force, while the wing-blade is held more or less vertically (5-8) during the up-beat.
(From RICHARDS & DAVIES, Imms' General Textbook of Entomology,1977, partly after CHADWICK, 1953)
If we want to understand the evolution of organisms, we cannot succeed when only formally evaluating morphological features. Evolution has been, and still is, so complex and 'opportunistic' that without taking into account the specific biological functions of the investigated morphological (and physiological) features -- in all cases where they indeed do have biological functions -- the evolutionist will get entangled into a mass of necessary assumptions he must make -- that is, in a formal analysis of transformation of morphological features it is unavoidable to 'believe' certain alleged 'facts' -- about the direction of change of these morphological features. In a purely cladistic -- and thus formal -- approach this means for example that in the establisment of (in the sense of revealing) the monophyly of a given group of organisms (i.e. the fact that all its members, and only they, have ultimately originated from a single ancestral species) it must be assumed that the derived features possessed by its members and 'proving' its monophyly did not originate independently within this group, that is, did not originate and develop convergently within the group. In most cases this is a troublesome assumption (troublesome not generally, but with repect to the particular group and features under discussion), and its truth or falsity can only be ascertained on the basis of a knowledge of the evolution of that particular group and on the basis of a general understanding of the driving forces of evolution. And this general understanding can only be achieved on the basis of having actually investigated the phylogeny of a great many organisms. And presupposed by the cladistic approach to a particular group of organisms, that is to cladistically investigate the phylogeny of that particular group, is, precisely, knowledge of the phylogeny of that group. So a cladistic approach turns out to be more or less circular, it must apply principles that can only be unearthed after its investigations have more or less completed. So revealing evolution -- be it the evolution of just one chosen group of organisms or of all of them -- on the basis of just a formal analysis of certain morphological features will not succeed. This is, because such features do not stand alone. What is evolving evolutionarily is not such a single feature (although we do speak of it for convenience), and also not just a group of features, but a whole organism or even a whole taxon. Sometimes a given organ-system (such as the wings and the associated muscular system) will undergo regressive evolution, but that does not mean that the organism or the taxon itself evolved regressively (although that can be the case). The cause of such regression can be the simultaneous progressive development of another organ-system (say, the legs and associated musculature). Determining the progressive or regressive nature of the evolution of some given organismic species or taxon necessarily implies the evaluation of that animal (to stick with animals) species' way of life, its diet, and its natural habitat, and [implies] the evaluation and appreciation of these features as being a result of evolutionary transformations determining the evolutionary origin of that particular animal species (and of the higher taxon to which it will further develop). The colonization of a new environment or habitat can be made possible by special evolutionary changes -- adaptations -- and result in the origin of initially a new species which may later develop into a new high-level taxon. In this way it is evident that morphological features, insofar as they are important to systematics and evolutionary research, must be investigated as to their biological function. And, of course, features of as many different organ-systems [of the organism under investigation] as possible must be so investigated, leading to the correct determination as to what (qua qualitative content) the several organic species under investigation essentially are, and how, and especially, why, they have (evolutionarily) changed.
The wings of Diptera are structured in very diverse ways. When we compare the degree of diversity of the flight organs -- the wings themselves, the wing- and thoracic lobes, and the halteres -- in the different suborders [Nematocera, Brachycera-Orthorrapha, Brachycera-Cyclorrapha] we directly observe an interesting regularity. While the Nematocera [midges, mosquitoes, crane-flies, etc] possess wings that belong to no less than 13 separate characteristic structural types, the younger Brachycera-Orthorrapha [horse-flies, robber-flies, and the like] only show 6 types. Finally, the youngest suborder of Diptera, the Brachycera-Cyclorrapha [fruit-flies, blowflies, and the like], distinguish themselves by an even higher degree of uniformity of wing-structure : all diversity is expressed by only 3 types. If we inspect the respective numerical sizes of the suborders, as they are in the present [geological] epoch, then we see a reversed situation. The suborder Nematocera includes the lowest number of species. The number of Bracycera-Orthorrapha is larger. Finally, the Brachycera-Cyclorrapha is numerically the richest group. Thus we clearly see a reversed relationship between the diversity of the structure of the wings and the number of species of the suborders. The relationships approximately turn out to be such : In the suborder Nematocera one particular single type of wings is possessed by only some thousand species, in the suborder Brachycera-Orthorrapha by 2.7 thousand species, and in the suborder Brachycera-Cyclorrapha by 7.3 thousand species. This regularity natually explains itself by the different age of the suborders, that is, by the different [lengths of the] periods of their existence. On the one hand the more ancient suborders, as compared with the younger ones, have in their long period of existence attained a high degree of diversity : the number of declining relict groups is significantly larger in the ancient suborders. The presence of such relict groups also contributes to the diversity of the ancient suborders. On the other hand, in their long history the ancient groups could [unimpeded] enter into possession of a truly higher number of special ecological niches and could therefore more deeply be transformed under [and as a result of] the most diverse [environmental] conditions. In short, the more ancient groups characterize themselves by a significantly higher abundance of narrowly specialized forms as compared to younger groups.
The at present existing types of dipterous wings are diverse, and they are distinguished by the structure of the venation of the wing-blade and of the basiala [ = the most proximal part of the wing], as well as by other features -- shape, sizes, different coverings such as hairs, spines, or scales [these coverings can be present on the veins as well as on the wing-membrane].
Before going over to the description of the types, it is necessary to consider, in order of significance, the features by which the individual types are distinguished. The principal qualities that determine the properties of the wings as organs of beat or swing are their size, shape, and firmness.
The sizes of the wings must be considered from two points of view : as absolute, measured by dimensions of length and surface area, and as relative, measured by the ratio of (1) the length or weight of (usually) the wing, and (2) the length or weight of the body, sometimes the length of just the abdomen [ROHDENDORF then refers to a table that lists these magnitudes as they are found in a number of Diptera and in a few representatives of some other closely related insect orders (Mecoptera [scorpionflies], Trichoptera [caddis flies]). We will not reproduce this table, and also not the many other tables, also listing those magnitudes, that are presented by him].
The features of the function of flight, that is, the role played by the wing as an organ of swing, which is its principal significance, consisting in the creation of lifting power and pulling or drawing power -- all this determines the evaluation of the quality of the wing as a supporting organ which evaluation is expressed by the determination of the magnitudes of the load per unit of surface area. From this viewpoint the absolute and the relative sizes are equally important. Indeed, a certain mutual dependency of wing dimensions and weight of insect (which [dependency] corresponds to the ratio of surface area and volume, that is, to a ratio of square and cube) determines differences of the magnitude of load [exerted] on the wings of equal relative sizes, but of different absolute sizes [indeed because weight grows faster than surface area when linear sizes increase] [ When we have two perfectly similar flies A, and B, but B being a significantly magnified copy of A, then the load exerted on the wings of B is larger than that on the wings of A.]. And the other way around, different relative sizes of wings in insects, themselves of different size, can determine an equal load per unit of surface area of the wings [The large fly has even more large wings]. Therefore it is especially important to take into account the body size of the insect, and, more precisely said, it is especially important to take into account the direction in which the historical development of this or that group went. The increase of body size implies the necessity of a relative increase of wing size in order to prevent too large a load. But because the dimensions of the wings ( = their surface area) grow as the squares of numbers, and the body weight ( = its volume) by cubes, the load inescapably grows faster, and the muscular apparatus of the insect soon becomes too weak to do the job [ This is because the power of a muscle depends on the size of its transverse section, which is a surface area, which grows as the squares, not the cubes, of numbers].
The reduction of body size of the insect in the evolutionary process implies the reverse phenomenon : the load decreases faster than the decrease of the dimensions of the wings and the power of the muscular apparatus [minute insects can keep aloft easily, despite the correspondingly small absolute dimensions of their wings].
The next important quality of the wings is their shape, closely connected with their firmness and with, guaranteeing the latter, the skeleton of the wing -- its venation. While the dimensions of the wings were determined by the very simple relationships of surface area and body weight, and by the magnitude of the load, the shape of the wing is connected with the incomparably more complicated relationships of forces which are created during the activity of the wing, its swing. It is known that different qualities of flight, namely velocity, direction, navigability, are being determined by different properties of swing, its higher or lower frequency, by differences in bending of the wing membrane, by a different shape of the curve [trajectory] traced out by the wings at the time of swinging. All these features of the functioning of the wings of insects are still far from complete clarification [that is, by a detailed aerodynamics], and we are confined to evaluate the structure of the wings in a mere descriptive manner, taking into account only the very basic features of the wing beat [swing] and their expression in the [morphological] organization of the wing. As [being] such basic features of the wing beat, whose knowledge helps to discuss and evaluate the structure of the wing, must first of all be reckoned the position [in the sense of orientation] of the wing-blade, which is always slanted by a smaller or larger angle with respect to the direction of movement [of the wings], where the wing's anterior edge is always oriented forwardly. Another feature of the wing is connected with the mechanics of the wing beat, consisting of different velocities of different points of the wing which lie at different distances from the wing-base which itself moves slowest of all the remaining parts of the wing. Accordingly, different points of the wing experience very different pressures of the air, which is expressed by the correspondingly different degree of firmness [of the wing] at these points. When we have knowledge of these basic features of the wing-beat, we may, for instance, confirm that when in the structure of the wing sharply expressed features of costalization are observed (that is, intense thickening of the anterior edge, thickening of the veins of the anterior half of the wing and at the same time a weakening of the venation [of the posterior half] and a softening of the posterior edge of the wing), such wings experience especially high pressures of air during their swing, which [high pressures] always result from a high wing-beat frequency, a phenomenon which itself results from a specially powerful muscular apparatus and bears witness of the high degree of intensity of the process of flight. Together with this, when we observe a straightening-out of the anterior edge of the wing, further, the wing's elongation, the presence of a characteristic bend of the anterior edge not far from the wing tip, and, finally, a sharply marked-off apical part [wing tip], we conclude about the [high] velocity of the flight as a whole, because these features bear witness to the development of a large pulling force during the wing-beat.
Speaking about the shape of the wing, it is necesary to especially point to the structure of the anal region of the wing-blade and the basiala. These parts of the wing possess in the overwhelming majority of Diptera different features that have undoubtedly important functional significance first of all as sense organs of different sorts, sensoria (which is evident by their innervation), and partly exerting functions of controlling the air currents during the swing. The anal region of the wing forms, in the most simplest case, a special projection, the so-called anal lobe (lobus analis), that is, a more or less protruding elastic outgrowth of the wing-membrane, usually provided with a vein and long spinelets along its edge. Sometimes a tongue-formed narrow outgrowth is individualized behind [that is, still more close to the insect's thorax] the anal lobe, which is called the alula [winglet], not carrying veins and provided with a fringe of spinelets along its margin. Still closer to the thoracic wall, already at the posterior edge of the basiala, is sometimes developed an outgrowth, which along its margin is provided with a thick tracheal trunk and long spinelets. It is called the squama alaris [wing-scale]. Finally, totally proximally, attached to the very thoracic wall, lies a special membraneous structure carrying the name squama thoracalis [thoracic scale]. In the Diptera Nematocera the thoracic scales are not developed. Both latter structures, the scales, can rightly be interpreted as being derivatives of the jugal region of the wing, lying posterior to the system of anal veins [ For the jugal region of the insect wing see Figure 1 above , where this region is drawn together with its jugal vein ( Ju ) ].
The firmness of the wing is determined by the presence of a special skeleton of it -- its venation -- and by features of the membrane, that is the latter's greater or lesser thickness and elasticity. The venation of the wing first of all determines, as a rule, its general shape and especially the shape of the anterior margin. Precisely the venation of the wing, has, in addition to its mechanical role of supporting the membrane, no less an important significance as being a vascular system of the wing which allows the circulation of hemolymph and is a system of passageways for nerves and tracheae. The performance of these very different tasks [mechanical support, vascular system] is the job of the venation, and upon them depends its structure and those characteristic sights of a complex network of veins, that is observed in the wings of various insects. The wing-venation in Diptera turns out to be a fairly complex system. The veins, that compose it, do not only differ by their [different degrees of] extent and position relative to each other on the wing-membrane, but also by their relative thickness, and, especially, by their [vertical] position on the membrane ( The veins may protrude [ = each forming a ridge] from the upper and [at the same time from the] lower surface of the wing-membrane [thus, each single vein forming two ridges, one the upper side, one on the lower side of the membrane], or, more commonly, may only protrude from this or that side of the membrane, that is, being either "convex" [forming a ridge] or "concave" [lying in a groove] veins ). One can observe the most different stages of development of the veins : from very thick and firm to hardly visible almost disappearing traces. Finally, very often there are cases of the formation of new veins which appear in the form of stiffening of the wing-substance at places of developed folds or bends. Well known are the difficulties which arise at attempts of homologization and the precise comparison of the wing-venation of the different groups of Diptera. In practise it usually is very difficult to precisely establish the nature of certain veins, that is, having to consider them as secondary structures, or "real", primary veins. So much attention, devoted to such a kind of enquiry to find all kinds of indicators that make the understanding of these or those veins more precise, which we encounter in the works of many researchers (HENDEL, ALEXANDER, EDWARDS), is, it seems to me [that is, Rohdendorf], ungrounded. To me the many examples of complete reduction of veins, having been disappeared without a trace in the processes of the evolution of the wing, are convincing. Also supporting this opinion are the various cases of the appearance of new veins, which acquire a structure that is indistinguishable from the "real" veins. It is sufficient to remember the appearance of new veins at places of folds of the wing in Blephariceratidae, Deuterophlebiidae [relict Diptera], further, the stiffening of longitudinal folds of the wing in Simuliidae [blackflies], and the well-known "pseudo vein" [vena spuria] in Syrphidae [hoverflies]. The nature of such neo-formations [formation of new entities] is fully evident : They originate along those particular folds that should, for mechanical reasons, be solid and firm. Such a process wholly really takes place before our eyes, and we must [therefore] take it into account. Enquiry into the homology of veins, in the narrow sense of this expression, that is to track down the fate of the various systems of veins and their branches [as this fate is] in the history of the order Diptera, must proceed on the basis of the assessment of the equality or difference of only those particular veins that are neatly and mechanically definitly expressed. Every sort of alleged "dual" veins, folds and splittings of principal veins cannot form the basis for a revision of existing schemes and terminology of wing-venation. Much more important is the investigation of fossil material which often provides decisive evidence about the nature of given veins. This material also forces me to doubt the appropriateness and scientific value of enquiries into formal homologies in the wing-venation of Diptera without taking into account its functions.
Special attention must be given to the study of the basal part of the wing, its individualized part, by which the wing is articulated with the thoracic wall, that is, its basiala. See next Figure.
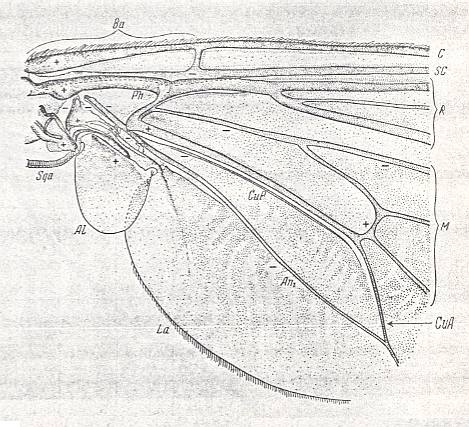
Figure 1a : Eremomydas bek SEM. ( Asilomorpha-Mydaidae). Proximal half of the right wing of a male. Top view.
Specimen Nr. 2002. Length of complete wing 14.5 mm. Of basiala 2.5 mm. Punctuation expresses stronger or weaker coloration and undulation of the wing-membrane.
Abbreviations : The veins are symbolized as usual. From top to bottom : C = Costa, SC = Subcosta, R = Radius (radial veins), M = media (medial veins), CuA = anterior Cubitus, CuP = posterior Cubitus, An1 = first Anal vein.
La = anal lobe, Al = alula (winglet), Sqa = squama alaris (wing-scale), Ba = basiala, Ph = phragma.
The signs + and - signify respectively the convexity or concavity of the given part of the wing.
The distal border separating the true wing-blade from the basiala runs about from the humeral cross-vein [the small cross-vein between Costa and Subcosta] to the boundary between the anal lobe (La) and the alula (Al).
(After ROHDENDORF, 1951)
This part of the wing experiences the highest mechanical stress, being some sort of conveying mechanism, passing over the forces generated by the muscular apparatus to the wing-blade. Essentially, by these two small parts [basiala, wing-blade] of the wing's skeleton the whole body of the insect is supported during flight. A basiala develops as an individualized structure only in those insects that have an active and intensive flight, and thus it is well developed in [many] Diptera. The structure of this part of the wing is fairly complex. It consists of various more solid skeletal structures, the 'handles' of veins and the parts of the membrane lying between them. Generally the whole basiala appears as a sharply folded, sometimes even a gashed organ and is meant to acquire maximal firmness while retaining maximal lightness and necessary suppleness. The venation of the basiala is peculiar : in it one may observe the result of various processes of reduction (the disappearance of many venational elements), as well as certain neo-formations, outgrows and widenings of veins. The whole structure of the basiala is geared to special mechanical demands : the transfer of the movements of the wing-sclerites [special individual sclerotized small plates, lying between the thorax and the wing-base] to the wing-blade, and the acquisition of minimal resistance resulting from air currents. See next Figure.
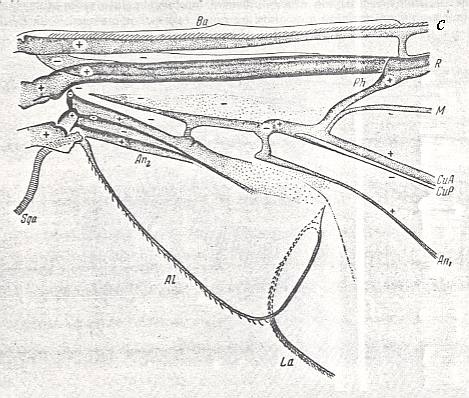
Figure 1b : Nemestrinus capito LOEW ( Asilomorpha-Nemestrinidae). Basiala of right wing of male. Top view.
Specimen 2001. Length of the whole wing 17.0 mm, of the basiala 4.5 mm.
For the abbreviations see previous Figure.
(After ROHDENDORF, 1951)
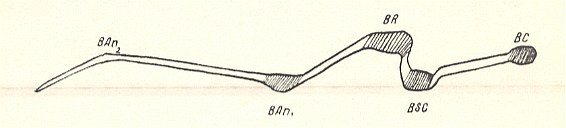
Figure 1c : Zelmira semirufa MEIG. ( Bibionomorpha-Ceroplatidae). Section through the wing at the level of center of the wing-base (basiala), that is, through the middle of basianalis 1 [base of the first anal vein]. The drawing depicts the relative thicknesses of the wing-membrane and the veins, and their mutual position.
Abbreviations : BAn1 = basianalis 1, BAn2 = basianalis 2, BSC = basisubcosta, BC = basicosta, BR = basiradius.
(After ROHDENDORF, 1946)
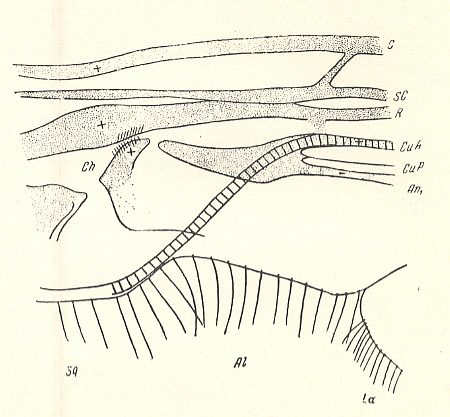
Figure 1d : Zelmira semirufa MEIG. ( Bibionomorpha-Ceroplatidae). Wing-base, basiala. Schematic.
Abbreviations as usual. Ch = chaetarium (chaetarium radiale and chaetarium anale).
(After ROHDENDORF, 1946)
[After this exposition of the structure of the basiala in dipterous wings by ROHDENDORF, 1951, it is, before going further, perhaps instructive to supplement it by an earlier discussion of the basiala by ROHDENDORF in his 1946 booklet on Diptera Nematocera Oligoneura (largely coinciding with the infraorder Bibionomorpha), pp. 22] :
First of all [as he writes there] it is necessary to consider the structure of the wing-base, in which lie the proximal parts ('handles') of the trunks of the principal veins. The wing-base naturally defines itself as that part of the wing which lies between the axillary sclerites, the epidemata, by which the wing articulates with the thorax, and, on the one hand, the level of the humeral cross-vein [between Costa and Subcosta], and, on the other hand, the indentation between the winglet (alula) and the anal lobe (lobus analis). The basiala is a complex organ, in its form far removed from being just a flat surface to which approaches the rest of the wing. See Figure 1c above .
The anterior part of the basiala consists of a more or less wide, and distally widening, flat, lying nearly horizontally, membrane -- the costal cell, which anteriorly is bounded by the costal vein, and which, when we more and more go towards this cell's posterior margin, gradually lowers, and passing over into the subcostal groove, in which lies the "concave" subcostal vein SC. Immediately posteriorly to SC lies the "convex", very strong radial vein R. Both these veins, SC and R, are closer to each other in the basiala, that is, proximally they gradually thicken, forming an irregularly shaped thickening. The proximal ends of C, SC, and R -- basicosta, basisubcosta, and basiradius -- while uniting [articulating] with the epidemata [the above mentioned axillary sclerites] significantly become thinner again.
The posterior part of the basiala is the most variable, and in its structure we observe in the various groups of the Oligoneura different types of specialization of the venation, principally boiling down to reduction of the basal parts of the anal veins. The convex vein of the posterior part of the wing-base [basiala] -- the second anal vein (An2) is well developed in the representatives of the family Bibionidae and relatives (See Figure 1e ). The proximal end of this vein (basianalis 2) is strongly enlarged and dilated, and lies closely to the base of the radial vein (basiradius). The place of [near] contact of basiradius and basianalis 2 is provided with a dense patch of short and firm spinelets [strong hairs] in the form of two brushes, which may be called chaetaria, the anal and radial (chaetarium anale, chaetarium radiale). Both these chaetaria (brushes) at the bases of the radial and second anal vein are able to tightly touch each other. Having this overall structure, their mutual movement is strictly delimited by the definite orientation [direction], namely "along with the nap". In the other direction the spinelets [strong hairs] fixate them to immobility.
ROHDENDORF adds as a note the following :
It is possible to speculate on the real meaning of the described structure : The spinelets forming the radial chaetarium are distally directed, while the spinelets of the anal chaetarium are directed proximally. While the wing moves forward the chaetaria come in contact with each other, and the anal part of the wing with the second anal vein will be fixated. While, on the other hand, the wing moves backwards the chaetaria move away from each other, and the wing eases and folds [backwards along the abdomen, when the insect is not flying].
The immediate connection of An2 with the [anal] chaetarium described above is well expressed only in Bibionidae and Penthetriidae. See next Figure.
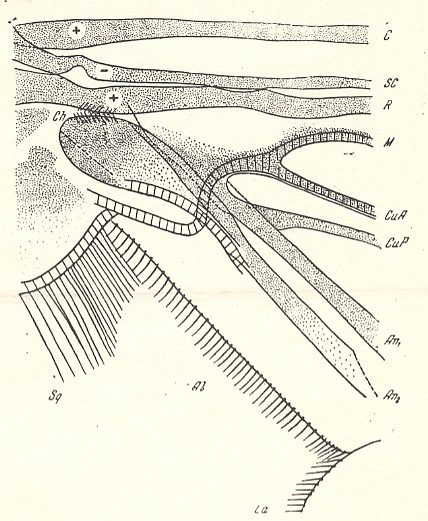
Figure 1e : Bibio japonicus DUDA. ( Bibionomorpha-Bibionidae). Wing-base, basiala. Schematic.
Abbreviations as usual. Ch = chaetarium (chaetarium radiale and chaetarium anale).
(After ROHDENDORF, 1946)
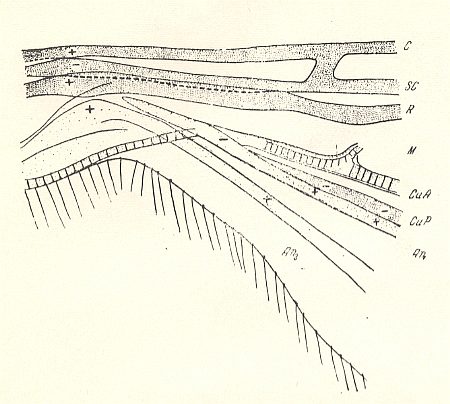
Figure 1f : Pachyneura fasciata ZETT. ( Pachyneuridae). Wing-base, basiala. Schematic.
Abbreviations as usual.
Here we have an example of a weakly developed basiala, that is, lots of elements are missing.
(After ROHDENDORF, 1946)
In the Fungivoridae [ = Mycetophilidae], Ceroplatidae, and others, the anal chaetarium is wonderfully expressed, but, on the other hand, has lost every connection with the weak An2. More often, however, the latter is totally reduced and of it is left only the chaetarium. See Figure 1d above .
This concludes our reproduction of ROHDENDORF's exposition of the structure and possible function of the wing-base (basiala) as he had laid it down in his 1946 booklet on the history of the Nematocera Oligoneura (Diptera).
We now continue with ROHDENDORF's introduction to the structure of dipterous wings (covering the whole order Diptera) from a functional point of view, as he wrote it in his 1951 book.
Apart from the features which guarantee the mechanical qualities of the wing as swing organ, the wing possesses a vast system of sense organs, sensoria, which lie at the ends of nerve branches going out from branches of neural trunks that pass through [ROHDENDORF writes along] the majority of the main veins. The nervous system of the wing is studied still [1951] very little, although existing data indicate the important significance and great differences in the development of nerves and sensoria in the wings of different groups of Diptera.
A special role is played by the various forms of wing coverings -- hairs, spinelets, and scales. Hairs or microtrichia usually cover the whole surface of the wing, being present not only on the veins, but also on all of the membrane. Rarely some parts of the membrane are without hairs. The sizes of the hairs are small : their length varies from 5 to 25 mu, usually around 12-15 mu. Spinelets or macrotrichia are, as a rule, only present on the veins and usually reach sizes that vary from 40 to 75 mu. In certain groups of Nematocera spinelets are also present on the free membrane. Sometimes, on the veins of the anterior margin -- the costal vein and the common trunk of the radial veins -- lie significantly larger spinelets, having lengths of several hundreds of microns. As a special transformation of spinelets one must interpret every kind of scale -- special flat, pointed or serrated along the distal margin, stalky structures of various sizes, sitting in rows on longitudinal veins of the wing, sometimes covering nearly the whole wing with a dense covering and forming a broad fringe at the posterior margin.
Functional evaluation of the described coverings formed on the wings -- hairs, spinelets, and scales -- is not completely clear. Spinelets as a rule are sense organs : to their bases usually reach out nerve endings. Especially this is true of relatively large spinelets lying on the anterior wing margin. All other structures, such as hairs, scales, and minute spinelets, undoubtedly play a certain role in the aerodynamics of the wing, apparently influencing micro-aero-turbulence-producing events that are created during the swinging of the wing. To such an assessment, although not verified experimentally, leads the consideration of the distribution of hairs, spinelets, and scales, which are especially abundant and regularly distributed at places of strongest effluent air currents (hind margin, distal part of the wing [wing tip] ). See next Figure.
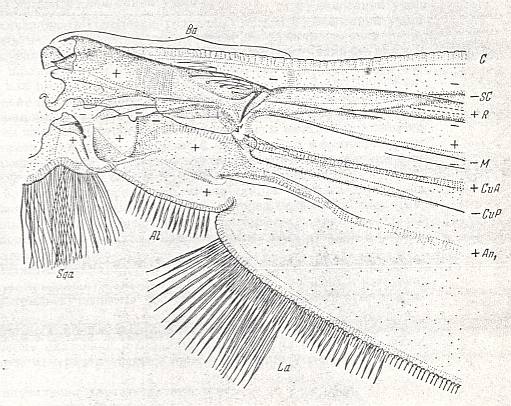
Figure 1g : Theobaldia alaskaensis LUND. ( Culicidae).
Basiala and base of wing-blade of right wing of male. Top view.
Specimen nr. 2570. Length of whole wing 6.5 mm., of basiala 0.75 mm. Macrotrichia and scales not drawn.
For abbreviations see Figure 1a above .
(After ROHDENDORF, 1951)
The probability of such an assumption is therefore very high. The spinelets of the wing have special significance as indicators of the nature of the veins, i.e. letting us identify this or that vein on the basis of the presence or absence of spinelets on them. As was already indicated above, it is not possible to successfully find out the homology of the various veins on the basis of an evaluation of these or those features of them while at the same time not evaluating the functional role of these veins, that is, considering them as supporting elements of the wing. Therefore the presence or absense of spinelets on the veins [i.e. on this or that vein] definitely does not determine [in the sense of uncover] the nature of the vein as longitudinal or cross vein, or its belonging to this or that particular system [of veins, for instance belonging to the medial system or to the radial system] : the strengthening of the vein, a change of its function, also change its structure, resulting, for example, in the development or reduction of its spinelets. This we can clearly see in the structure of the cross-veins rm [supposed to connect the radial and medial system] and mcu [supposed to connect the medial and cubital system], which usually are devoid of spinelets, but in the case of their strengthening and transformation into functioning longitudinal venational parts, they are provided with well-defined spinelets (for instance, rm in Lycoriidae [ = Sciaridae], mcu in Tipulidae and Liriopeidae).
This concludes our reproduction of ROHDENDORF's introduction to the study and evaluation of the functional types of wings in the order Diptera, as he had written it in his 1951 book Organi dwizjenija dwukrilich nasjekomich i ich proischozdjenje ( The organs of locomotion of the two-winged insects and their origin ).
Preliminary explanation and interpretation of the origin and meaning of the various wing types in the order Diptera.
In the foregoing theoretical documents of present Part of website we have extensively discussed in what way new higher-ranking taxa originate. The origin of such a taxon has to do with the 'invasion' of a new type of environment by its ancestral species, which has adapted to it, and which has consequently split up into new species each occupying a different ecological niche in that same new environment. All these species together form the new higher-ranking taxon. The new environment has become the habitat of the taxon's species. The "new environment", becoming the "new habitat" is not confined to, or defined by, a particular geographic region, but is a new type of environment or habitat. And going to live in a new environment will evolutionarily lead to the origin of special adaptations often expressed in a set of morphological features that characterize the new taxon as a whole.
In the case of animals belonging to the insect-order Diptera one such set could be the morphological structure of the wings (reflecting a specific flight-regime), which, together with other sets of features, also possessed by these animals, characterizes a whole taxon within the order Diptera. The special structure of the wings of the flies of this taxon, different from that of other dipterous taxa, can be historically connected with the colonization of a[n] [at the time] new environment by the ancestral species of the taxon. In cases such as this, the morphological differences in wing structure existing between (1) the species of this given dipterous taxon, connected with a particular type of environment, and (2) the species of another dipterous taxon which is connected with another type of environment, are then understood to be one of the many evolutionary effects of major ecological changes. All this has been already discussed earlier in general terms and does not bring with it major theoretical problems. Such problems (and thus challenges for a general theory) will, however, emerge as soon as we consider -- as an example, illustrating something more general -- the very common case of actually existing significant differences of wing structures within a group (not necessarily a taxon) of Diptera, of which, however, the members live in the same (type of) habitat and seem to have an identical way of life, as larva and as adult (the latter looking for the same nutritive substrate for their larvae as do the other members). In such a case we cannot trace these differences back to some major ecological shift. But they do exist. In what comes next we will try to explain -- in general terms -- also these differences.
In Part V of the present series of documents, [this series] dealing with the evolutionary processes having taken place in the insect order Diptera, we extensively deal with the life habits of the larvae of Diptera. Indeed, in Diptera the larval life is the most important. The adult often only feeds on flowers (to obtain energy-providing substances for their flight muscles) or is aphagous. Only a minority of dipterous adults are preditors or suck blood. So the evolutionary changes in Diptera are largely due to a change of larval nutritive substrate. This means that the precise ecological niche of every species must in some way be different. So this specific ecological niche is mainly characterized by the nature of the larval nutritive substrate. However, we know that for instance rotting wood (containing, in addition to disintegrating wood material, also fungi ) turns out to harbor larvae of many different species of Diptera. So the ecological niche of these different Diptera, as regards their larval nutritional substrate, seems to be identical. However, we think this is not so. Larvae of different species of Diptera living in rotting wood will use different (precisely defined biochemically) nutritive substances present in it, or, when using the same substances, process (that is, digest) them differently or more efficiently as compared to their fellow inhabitants, or the larvae of different species live in rotting wood of different species of tree, or they live in rotting wood finding itself in slightly different stages of disintegration, or if not all this, larvae of different dipterous species populate the rotting wood in slighly different periods of the season. In all these cases the precise ecological niches of the larvae of different species are different. Even in cases where all the mentioned more or less external differences (species of wood, stages of its disintegration, periods of populating the wood) are absent, subtle differences in biochemical relations of larva and immediate habitat (rotting wood) remain, and therefore corresponding differences in ecological niches of different dipterous species remain.
We will concentrate on the latter case when considering the adult life. Of course the precise habitat of the adult also belongs to the precise ecological niche of the given species of fly. The nature of adult life of a dipterous insect is mainly expressed by the nature of its organs of locomotion (and, in contrast to many other insects, generally not so much on the nature of their mouthparts, to mention just some other organ system). The organs of locomotion are the legs and the wings, and there is a complicated interplay between the development and refinement of running (or digging) and of flying. In most cases, as regards Diptera, the wings are the most important organs of locomotion. ROHDENDORF, in his 1951 book on the organs of locomotion in Diptera, shows that in Diptera unexpectedly many different types and subtypes of wings have been developed. And as can be seen, the differences of these types -- expressed in many morphological features -- must be functional differences judging from the subtle 'elaborateness' of the corresponding features of the wings : the complex and mechanical structure of the basiala, the pattern of the wing-venation, the wing-coverings and the wing appendages. So the differences of these features as these differences are observably and definitely present in the different species or in the different higher-ranking taxa of Diptera, cannot be the result of some sort of random morphological variation. They must express essential differences obtaining between these species. And what other diffference would they express than a difference in flight-regime? Indeed, as we shall see later, these differently constructed wings must reflect different flight-regimes. That there can exist a great many different flight-regimes at all (in contrast, probably, to those of birds) is clear when we realize that insects are very small animals. For them the atmospheric air is a more or less viscous medium to move in, and the smaller the insect is, the stronger viscous this medium is for it. So we hold that in Diptera there exist a great many types (and subtypes) of flight-regimes, permitting different kinds of flight in different species of Diptera ( This is equivalent to saying that the morphological features of the wings of different species of Diptera are functional features).
When considering the above case of different dipterous species (sometimes belonging to even different higher-ranking taxa) occupying as larvae ecological niches that are almost completely equal and only differing slightly in their specific biochemical nature, we will expect differences to be present in the larvae, maybe in their mouthparts, in their sense organs or in the morphology of their intestinal tract, and of course in their physiology, that is, the differences of the precise ecological niches of the larvae are expected to be expressed in the very nature of these larvae. But how can the mentioned subtle differences -- biochemical in nature -- of the various precise ecological niches (as compared to one another) of the larvae of different species -- living (in our case under consideration) in precisely the same habitat -- evolutionarily affect the flight-regimes of the corresponding adult flies? The answer is that they don't. The subtle (biochemical) differences obtaining between the very similar but nevertheless different ecological niches of the various taxonomically different larvae will become (evolutionarily) expressed in these larvae. They will be expressed as larval adaptations to a precise ecological niche. And because, in our case, the very habitat of these various larvae is exactly the same, it cannot provoke or demand differences in the morphology and behavior of the corresponding adult flies. More specifically, it cannot demand differences in flight-regime, because the adults -- in their flight -- will look for the same type of micro-environment or substrate where to lay their eggs. If there are differences in flight-regime they must (evolutionarily) come from the adult life itself, for example in relation to the presence or absence of a swarming habit. In a broad sense then we can say that, in the present case here considered, the larval structures (and chemistry) will evolutionarily evolve in depency upon the larva's precise nutritive substrate (its chemical and physical properties), that is, from specifically its proper environment. In the same way we can maintain that, also in the present case here considered, the adult features, and especially the precise structure of its wings, depend on its (that is on the adult's) proper environment. But while what to mean by "proper environment" is clear in the case of the larva, it is not so clear in the case of the adult fly. What is the 'environment' of the adult fly that supposedly evolutionarily determines its specific flight-regime, a flight-regime differing from that of other species of fly although their corresponding larvae live, in the present case under consideration, in highly similar habitats?
Of course the environment, as meaning the micro-landscape (and its microclimate) with all its relevant biological factors, in which (landscape) the adults, belonging to the dipterous species under consideration, live, is exactly of the same type for all these species, and in it is present the appropriate nutritive substrate for the larvae of all of them. This implies that a given particular individual environment will be the habitat for larvae and flies of a number of different dipterous species. But, as has been said, for the larvae there exist in their supposedly similar habitat (for example rotting wood) subtle differences, differences ultimately of a biochemical nature. An in the same way we expect that also for the adults there must exist in their supposedly similar habitat (woodland) subtle differences. But, again, what are these 'subtle differences'? We can trace these differences when we (also) take into account the more abstract aspects of the habitat of the adult flies (as we already did with respect to the larval habitat).
In our supposed case adult flies of different species are moving (where we first of all think of flying) around in allegedly a same habitat (a same with respect to micro-landscape, microclimate, and biological factors such as the presence of predators). For a given dipterous species one aspect (out of many) of its natural habitat is the presence in this same habitat of precisely those other dipterous species that are also naturally connected with this type of habitat. For adult flies of the given species this means that the environment ('Umwelt') of them consists, among other things, in the presence of adults of certain other species that are, like those of the given species, intrinsically connected with the same habitat. Well, in order for the individuals of these different species to maintain their intrinsic specific differences, maintain them even when they, as adult flies, naturally live in the same habitat, they must have (at least) a different way of moving around in this habitat (although looking for the same substrate for their larvae, in which substrate the females will lay their eggs). And this means that -- so many things being equal -- they, that is, the different (dipterous) species, must fly according to a (more or less correspondingly) different flight-regime, that is to say, they must be such as to fly around in a different way. And this is made possible by the existence of structural specific differences (visible when inspecting one species after another) in their flying apparatus proper, namely their wings (making at the same time necessary the presence of certain differences in the structural nature of the flight-muscles, of their innervation, and of their physiology). So if we consider the environment ('Umwelt') with respect to all its aspects, including its abstract aspects, then we can say that differences of environment as they are present for different species living in the same habitat will evolutionarily have determined the differences as they are now present in these species, that is differences that distinguish these species from one another. And one of these so determined differences are the specific structural differences as they indeed occur in the wings (in their venation, their shape, their basiala, and their covering), and which guarantee different flight-regimes.
Differences in flight-regime can come about as a result of the influence of many factors. In most cases they are large-scale factors such as the appearance of new types of vegetation (such as the appearance of flower-carrying plants having nectar as a source of food for adult flies) or (the appearance of) new types of animals which can be chased for their blood. By these large-scale factors whole new types of insects, and therefore new types of large-scale structural features, may evolve. But there are also -- and this is the case we are discussing here -- small-scale factors that, it is true, do not cause the origin of new types of insects, but can cause subtle differences in the flight-regime and in the corresponding morphological structural features (making possible that regime) within a group of more or less closely related dipterous species.
In the above (rather difficult) discussion we have revealed -- only in general terms -- precisely these small-scale factors. And we hope that we have explained the presence of differences in the structural features of wings of different, but often closely related, dipterous species that, in spite of having these different wing-structures, seem to show the same way of life and populate the same habitat. This phenomenon we will especially encounter when we investigate the flies of the infraorder Myiomorpha (fruitflies, blowflies, etc.) and some of their allies.
Because in Diptera, and especially in Diptera, we see an enormous diversification of wing-structure, expressing different flight-regimes, we can hold that generally in dipterous insects flight is a very dominant feature, and this means that development of different flight-regimes must be a very significant determining factor in their evolution (alongside the widening of the range of larval substrates). That's why we will devote much attention to the wing structure of representatives of the order Diptera, especially because this type of feature is relatively well accessible to investigation and evaluation.
When we have scrutinized many examples of the above described phenomenon of difference in wing-structure of species having a similar way of life, we will attempt to describe it in noëtical terms, that is in terms of the Implicate Order, noëtic reactions, their products, and the projection into the Explicate Order of these products. Indeed, the study of biological evolution, as it has actually occurred in the Earth's history, will help us to test, to modify if necessary, and to develop further, our metaphysical theory of the Implicate and Explicate Orders. On the other hand, the necessity of fitting-in the phenomena of biological evolution into a general theory forms by far the greatest challenge for any proposed allegedly general metaphysical theory of the structure and essence of Reality.
Further elaboration of the theory that purports to explain the differences in wing-structure in more or less closely related Diptera.
What has been said about the different wing-structures present even in Diptera that have a similar way of life (and similar body-sizes) can be worked out still further.
Here we consider the habitat of the winged fly of a given species. This habitat is partly determined by the presence of the appropriate substrate in which its larvae have to develop. But this habitat of the adult fly is also determined by factors having to do with the possibility of finding a mate (sometimes by way of swarming) and in many cases with the presence and accessibility of food (nectar, blood) for the winged phase.
Now we can say that the environment is perceived by the insect largely by means of its eyes and antennae, that is, by sight and smell. In this way the insect extracts as it were from its environment a certain definite aspect, which becomes its environment ( its habitat-in-the-most-narrow-sense), "its" here used in the sense of "the species's". And we all know that organs of sight and smell cover different (often of course partly overlapping) ranges of light (wavelength) and smell (chemistry) in different organisms (for example bees can see colored patterns in flowers that we cannot see). So it is to be expected that also already between different species, or at least between different groups, of insects, and thus also between different species or groups of diptera, there exist differences in the ranges of perception by the visual (sight) and olfactory (smell) organs, that is, by their eyes and antennae. So different winged diptera will experience different aspects, or the same aspects differently, even in the same overall environment. And exactly what they perceive in this environment is their precise habitat. Of course this precise habitat is not some spatially local place, not a single unique locality, but a precisely defined type of micro-physiognomy (micro-landscape), and thus a precisely defined micro-habitat. Such a micro-habitat can be present at many geographically different locations. It is a certain specific aspect of the overall type of environment, an aspect to which the sense-organs of the insect (of a given species) are specifically geared.
Now it will be clear that the way of flying about in the micro-landscape as it is perceived and defined by the insect's sense-organs, must be compatible with that specific type of sensory perception by the insect. Certain flight-regimes will undoubtedly disturb the insect's perception of its micro-habitat, and thus the flight-regime must be adapted to that micro-habitat, it must integrate with the insect's sensory organs, that is, with its eyes and antennae. And in the case of such an adaptation the new flight-regime does not necessarily need to involve an aerodynamic improvement of the wing-structure (venation) and shape, but often just a change of it. The new wing-structure makes the flight of the insect to be better adapted to the [perception of the] new micro-habitat. Often it is 'better' only in this particular sense. And only by having acquired such an adaptation -- along with adaptations in other organ-systems -- the adult fly (the winged insect) can perform its overall function which consists in finding food (nectar, blood), in finding a mate, in geographically expanding the (new) species' range, and for the female in finding the appropriate substrate for the development of its larvae.
Of course there are cases that demand a genuine aerodynamic improvement of flight (for example faster and more stable flight), such as in the origin of robber-flies (Asilidae) which prey upon flying insects and which have to carry their prey during flight. So their wings represent a real mechanical improvement with respect to those of their immediate ancestors. But the more subtle (and smaller) differences between the wings of asilids point to qualitative differences of the asilid species as compared with one another, or, related with this, to qualitative differences of their micro-habitats.
Interpretation in terms of the Explicate and Implicate Orders.
A given species of fly represents a definite qualitative content. This content is, as a result of the process of injection ( from the Explicate [ = physical] into the Implicate [ = noëtical, i.e. thought-like] Order), present in the Implicate Order as a noëtic entity, that is, as an immaterial thought-like enfolded entity. And as qualitative content of the given species of fly it defines, and thus determines and delimits, the respective qualitative contents of several potential ecological niches, where one of them has become actual. And because these potential and actual ecological niches are qualitatively defined and delimited by the qualitative content of the given species of fly they too can be injected into the Implicate Order and exist there as noëtic entities. And, further, according to our metaphysical theory (expounded in Part I-IV of the Dynamic Metaphysics [nomological] Series), with the transition of an original species of fly into a new species we have to do with a noëtic reaction taking place in the Implicate Order between the qualitative content of the original species -- as such a noëtic reactant -- and the qualitative content of one of its potential ecological niches -- this content also being a noëtic reactant -- resulting in a noëtic reaction-product. This noëtic reaction-product is the qualitative content of the new species of fly still existing as a noëtic entity in the Implicate Order (that is, not yet as a material entity existing in the Explicate Order). When conditions in the Explicate Order are right, this noëtic content will (as a copy) be projected into the Explicate Order ( It might also be assumed that the whole course (i.e. all stages) of the noëtic reaction as such is, together with its product, projected into the Explicate Order ). This means that the projected noëtic contents will be unfolded along space and time dimensions (they become physical), which we (in the Explicate Order) observe as the evolutionary development of certain morphological or physiological adaptations of the new species of fly to its new ecological niche, a development which generally takes much time. And one of these adaptations can be seen in transformations of the wing-structure, especially of its venation. The new wing-venation then is a co-product of the mentioned noëtic reaction. It is fully integrated with the rest of the fly's organization. It is part of the adaptation to the new ecological niche (in the narrowest sense), because it is the morphological expression of a new flight-regime enabling the fly to move about precisely in this new niche, that is, in conformity with this niche. And for the flight-regime to be in conformity with precisely the new niche means the existence of a qualitative compatibility between the way of flying and the nature of the environment precisely as perceived by the fly. And so evolution of flies, as driven by ecology, results -- among other things -- in sequences of transformations of wing-venation (often consisting in reductions and/or shifts of veins) as such [these sequences] not necessarily leading to an aerodynamically improved and refined flight, but merely to a different flight, that is a flight better adapted to certain subtle aspects of the new ecological niche. In such a way, I think, we must interpret the changes in wing-venation as will be discussed further down in the context of the distribution of functional wing-types over taxa of Diptera.
The difference of the flight of Diptera as compared to that of some other insects.
In Diptera flight is what generally counts. We can deduce this from their having brought to ultimate perfection the phenomenon of dipterygia. The latter is a process observable in many insects (such as wasps and bees) and consists of the gradual reduction of the hind-wings and their functional coupling with the fore-wings. In such cases we see large, elongated and strong fore-wings and much smaller, weaker, and shorter hind-wings. In Diptera this process has led to the complete loss of the hind-wings which are replaced by so-called halteres (one at each side) which almost certainly play a role in stabilizing the flying insect. The Diptera have evolved a great many functional types of flight-regimes and corresponding wing-structures.
In the order Hymenoptera, saw-flies, wasps, bees, and (winged) ants, the process of dipterygia has led to the diminution of the hind-wings (not to their total loss) and their coupling with the fore-wings by means of a row of small hooks. We can interpret this process here as being progressive, yielding able flyers in all the groups evolutionarily coming after the saw-flies (wasps of which the larvae are caterpiller-like plant-eaters), except the smaller parasitic wasps whose wings are regressive, belonging to the type of pseudo-feather-wingedness (like the gall-midges in Diptera). In accordance with this it seems that in Hymenoptera only a small number of functional wing-types has been developed. In Diptera, on the other hand, the evolution of wings has led to the establishment of a great many functional wing-types : in many cases to narrow-specialized wings, in some cases to regressive wings or even to a wingless condition of one or both sexes, and in other cases to progressive wings.
( End of the g e n e r a l exposition and discussion of functional wing-types in Diptera.)
Additional discussion of the wing-base in Diptera, its functional and systematic meaning.
ROHDENDORF has investigated the wing-base in Diptera in his 1946 and 1951 writings. Since these years there has been written some more about it, especially by Willi HENNIG (a specialist on Diptera and on a general theory of phylogenetic systematics) in the year 1968. I consider his discussion so important that I will give it in full here. It is taken from his Kritische Bemerkungen über den Bau der Flügelwurzel bei den Dipteren und die Frage nach der Monophylie der Nematocera, ublished in "Stuttgarter Beiträge zur Naturkunde", Nr. 193, 1968.
HENNIG, in all his works, tries to reveal the purely phylogenetic relationships of insects among one another that should result in a genealogical tree of them and of their various groups. For him, phylogenetic relationship is based on recency of common ancestry. This implies that in groups of related organisms (or taxa) -- monophyletic groups -- the organisms that make up such a group are not just morphologically, physiologically, and behaviorally, similar, as in any purely typological approach, but are g e n e t i c a l l y connected to each other by having all (and only they) descended from a particular ancestral species. And although we now know that there is no always holding strict correlation between a (particular) gene or (particular) groups of genes, on the one hand, and a (particular) feature or (particular) complex of features, on the other, we are still convinced that a purely phylogenetic taxonomic system of some group of organisms, set up according to HENNIG's phylogenetic principles, will reveal an important and interesting aspect of that group. And we know that yet another aspect of a group of organisms is revealed by a purely typological taxonomic system, namely the very essence or qualitative content of this group and its typological subtaxa, expressed in their ecology and their way of life, expressed, that is, made possible, by certain morphological and physiological features. Such a typological system (as we see it in ROHDENDORF for example) is just as interesting and important as is a phylogenetic system, but it may not call itself such a system, that is, it may not call itself a phylogenetic system, because it simply isn't such a system. It is a system that expresses qualitative connections between species or higher taxa, not necessarily genetic connections, that is, connections in terms of similaries in genomes as a result of genetic descent. It will be interesting to set up an ancestral tree, purely based on genetic descent, and then superimpose onto it (without changing it) the qualitative relationships as found by typological systematics, and then describe it in terms of the Implicate Order and noëtic reactions. We will not here dwell any longer on this interesting subject (we do that later!), but start with HENNIG's discussion (reproduced as such, but with additions and clarifications) of the functional and systematic meaning of the structure of the wing-base in Diptera :
Among the many open questions still existing concerning the system of Diptera, the most important one is about the monophyly of the "Nematocera" [the complex of gnats, black-flies, mosqitoes, and crane-flies]. In Europe it is still customary to distinguish in the Order Diptera two suborders, viz., the Nematocera and Brachycera. By the collective of American authors (Stone et al., 1965), presenting a catalogue of North American Diptera, is mentioned yet a third suborder, the Cyclorrapha. There is, however, no doubt that the Cyclorrapha together with the "Brachycera" of the mentioned catalogue (the so-called "orthorraphous Brachycera" of other systems) form a monophyletic group, which should in the phylogenetic system also have a name. The traditional name of this monophyletic group is "Brachycera" and we will therefore use it in what follows. The Cyclorrapha in the system then remain a subgroup of the Brachycera.
It is far more difficult to determine whether also the "Nematocera" form a monophyletic group, which as such would then be the sister-group of the Brachycera. Only if this were so it would be legitimate to distinguish in the order Diptera two suborders -- Nematocera and Brachycera. To me (Hennig) no more recent author is known who explicitly holds that the Nematocera and the Brachycera are sister-groups. On the contrary, in all recent -- and also in older -- designs of the ancestral tree of Diptera several subgroups of Nematocera are held as closely related to the Brachycera, where then the "Nematocera" as a whole are drawn as just a paraphyletic group.
The "Nematocera" can be relatively clearly divided into 4 monophyletic groups [We must of course realize that HENNIG's groups and their names do not necessarily coincide with the groups as established by ROHDENDORF] :
Tipulomorpha (containing the families Trichoceridae, Cylindrotomidae, Limoniidae, and Tipulidae).
Psychodomorpha (the families Tanyderidae, Ptychopteridae, Psychodidae in the broadest sense. Probably also Blephariceridae and Deuterophlebiidae. Possibly also Nymphomyiidae).
Culicomorpha (the family group Chironomidea -- including Thaumaleidae -- and the family group Culicidea).
Bibionomorpha (families of the fungus- and gall-midges, including Anisopodidae, Cramptonomyiidae, Bibionidae, Pachyneuridae, and Perissommatidae).
The question that is asked is then : Is it possible that some of these groups have close genetic relationships with the Brachycera? In my work about the wing-venation and system of Diptera ( HENNIG, 1954, p. 377) I said about this : " The only feature in the wing-venation that could indicate that the Brachycera cannot be the sister-group of all Nematocera, is the reduction of 2a [= second anal vein, also written as A2, or A2 ]. With respect to this feature the Brachycera go together with the Psychodiformia, Bibionomorpha and of course the Culiciformia. But one should ask whether we here have to do with true synapomorphy ["synapomorphy" means that one or another d e r i v e d feature (= apomorph feature) is possessed by all the members of a group (and only by these), indicating that this group might be monophyletic [= descends from one single ancestral species]]. This feature [reduction of 2a] is too insignificant to use it as a basis for far-reaching conclusions [The feature could represent merely so small a morphological change that it could easily evolutionarily originate over and over again many times and independently, that is, it could have been originated in genetically unrelated groups, meaning that these groups cannot -- on the basis of such a feature -- together [said to] form a single monophyletic (super)group.] "
However, a more precise investigation shows that we have here not at all to do with an "insignificant" feature. On the contrary, it shows that the reduction of the second anal vein [2a] is connected with a fundamental [evolutionary] transformation of the wing-base.
The [historical] development of the wings in Diptera can only be understood when we depart from the [insect Order] Mecoptera [This is a small, but very old, insect order, related to the Order Diptera, consisting of Scorpion-flies and relatives, and still possessing four wings]. The next Figure depicts the basal part of a wing of a representative of the Order Mecoptera.
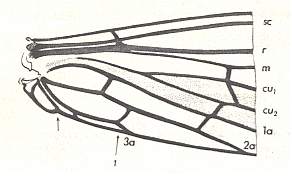
Figure 1h : Basal part of a wing of Panorpa communis L. ( Mecoptera). The left arrow indicates the distal boundary of the Neala ('wing-scale').
(After HENNIG, 1968)
It is perhaps not superfluous to point to the fact that in naming the wing-scale there is quite a confusion. The wing-scale is apparently homologous to the, since MARTYNOV, so called "Neala", which, as a derived feature, belongs to the basic plan of the Neoptera, having from there taken over by the Diptera in their basic plan [The Winged Insects, Pterygota, are divided into two main groups, viz., the Paleoptera -- insects, such as dragonflies, that cannot fold their wings during rest, and Neoptera that can do so]. In contrast with this structure [the wing-scale], the "thoracic scale" and the "alula" (see below) are newly formed structures in Diptera, not yet belonging to their basic plan.
In comparison with the Mecoptera -- which might be the sister-group of the Diptera -- the fore-wing in Diptera possesses the following derived features :
ROHDENDORF [in his work of] 1958/59 [This is a German translation of ROHDENDORF's 1951 book on functional wing-types in Diptera (On the present website we have used throughout the original Russian text)] sees in "the coming close to each other of the two cubital branches" "the most characteristic wing feature of a primary two-winged insect". " The firm longitudinal fold" formed by the two, now lying close to each other, cubital branches, "divided off the posterior part [of the wing from its anterior part], which now acquired the ability to swing under an angle with the rest of the wing-blade during the wing-beat". This "important evolutionary accomplishment" was, according to ROHDENDORF, in turn "determined by the refinement of flight, namely the increased wing-beat frequency" [It looks to me [JB] rather that things have occurred the other way around : Morphological transformation of the wing, such as the described mechanical separation of its posterior part, has made it possible to develop a higher wing-beat frequency and in this way to refine flight.]. " During the wing-beat the swinging posterior part of the wing-blade is now playing an important aerodynamic role. This turned out to be an essential advantage with respect to the tenaciously atavistic wing forms of the Paratrichoptera [a fossil insect Order, supposed to have led to the origin of the Order Diptera] and other Mecopteroidea ...".
I [HENNIG] consider it to be very probable that ROHDENDORF has discovered the functional significance of a feature that until now has played [in morphological and systematic discussions] only a formal role in the characterization of the Diptera. At the same time it seems to me that the described feature of the dipterous wing is closely connected with the next one of its features.
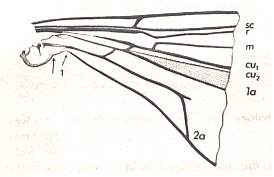
Figure 1i : Basal part of a wing of Trichocera sp. ( Trichoceridae). The left arrow indicates the distal boundary of the Neala ('wing-scale'). The venational stump attached to 2a is an anomaly. But it indicates that the sharp curvature of the second anal vein in Trichocera is a secondary feature. The with 1 marked arrow indicates the distal boundary between the neala and the wing proper.
(After HENNIG, 1968)
It is possible, that a sclerotized tract, -- coming from the same point [the point from which the anal veins originate], which then initially runs along the boundary between the wing-scale and the wing proper, and finally runs along the posterior wing-margin and is then connected with the second anal vein by a cross-vein-like sclerotization, -- can be interpreted as being a rudiment of the third anal vein. For us, however, this question is without essential significance [because no matter how the reduction of the third anal vein was accomplished, it is reduced in all Diptera]. It does not need to be discussed further.
These three described features are, in their original form, present only in the Tipulomorpha (see Figure 1i, above ). In all other Diptera ( Psychodomorpha, Culicomorpha, Bibionomorpha, Brachycera) the wing is at least in one respect transformed further :
At the posterior wing-margin a deep incision was formed, which, lying about opposite from the humeral cross-vein of the anterior margin, separates a narrow wing-stalk from the wing-blade. The second anal vein is reduced. It, when present, does not, or hardly, reach beyond the described incision.
ROHDENDORF (1958/59) had called the wing-stalk "basiala", and he interprets the differentiation of the wing in those two parts, basiala and wing-blade, as representing a general characteristic of the dipterous wing. The as Basiala indicated "part of the wing experiences the largest mechanical load [in the sense of stress]. It is a special transmission device, which passes the forces from the muscular apparatus on to the wing-blade .... Only in insects that possess an active and intensive flight the basiala is developed into a special structure, and thus also in Diptera".
These insights of ROHDENDORF are without doubt correct and important. However, one should supplement it by the fact that a true basiala is not yet developed in Tipulomorpha. Here (see Figure 1i, above ) we can only speak of a "basiala" when we give the basal region of the wing, which is in all other Diptera further developed into a basiala, as it were in an anticipating fashion, already this name. But a differentiation between these wing regions, basiala and wing-blade, is not yet present in the Tipulomorpha : both passing on to each other without a boundary.
The formation of a sharp boundary between basiala and wing-blade is in most Diptera also expressed by another feature : While in Tipulomorpha the Media is at its base connected only with the Cubitus (both longitudinal veins originate from a common trunk) there is in all other Diptera also a cross-vein-like connection with the Radius, and this connection is apparently formed by a sclerotization of a transverse fold. SEGUY (1959) calls this connection "arculus", ROHDENDORF calls it "phragma". Both names are not particularly fortunate because they are also used for totally different not homologous structures.
The question how closely the formation of a phragma is connected with the differentiation between wing-stalk and wing-blade hasn't yet been answered. It needs further meticulous comparative investigations.
In many Tipuloidea is in the region, where in other Diptera a phragma is developed, a clearly expressed fold present. This fold forms a transverse connection between R and Cu, and on it begins the medial trunk. ALEXANDER draws in his pictures of wings of various Tipuloidea at the location of this fold often a continuous cross-vein. In several such cases I could determine that the upper part of this "cross-vein" -- between M and R -- actually is a just a fold. On the other hand, however, it is questionable whether a true cross-vein, a real phragma, can be assumed to be present in the basic plan of all Diptera not belonging to the Tipulomorpha. One should, at least until now, reckon with the possibility that a true phragma in the sense of ROHDENDORF as a sclerotized cross-vein in Diptera -- perhaps also in some Tipulomorpha -- has been formed a number of times convergently [that is,independently]. Also the occurrence of reverse processes -- reverting from the sclerotized cross-vein to merely a fold in the membrane -- must be reckoned with.
Be that as it may, in many Diptera in which the differentiation between wing-stalk (basiala) and wing-blade has taken place, the following elements together form a line or zone which can be interpreted as the boundary between basiala and wing-blade : (a) humeral cross-vein [between Costa and Subcosta], (b) phragma, (c) the basal connection, flowing from the phragma, between M and Cu, (d) the end of the second anal vein (2a), and (e) the incision at the posterior wing-margin, indicated in the next Figures by an arrow marked with "2".
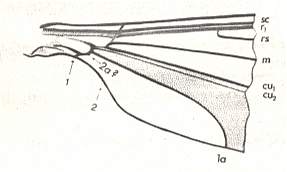
Figure 1j : Basal part of a wing of Ptychoptera sp. ( Ptychopteridae [ = Liriopeidae] ). The arrows with the respective marks "1" and "2" in following five Figures indicate the boundaries of the wing-stalk (basiala). At 1 lies the distal boundary between the neala and the wing proper.
(After HENNIG, 1968)
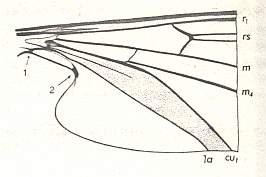
Figure 1k : Basal part of a wing of Liponeura bilobata LOEW. ( Blephariceridae).
(After HENNIG, 1968)
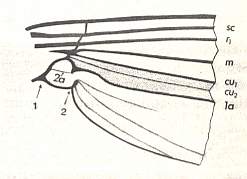
Figure 1l : Basal part of a wing of Culiseta annulata SCHRANK. ( Culicidae).
(After HENNIG, 1968)
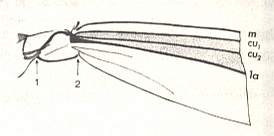
Figure 1m : Basal part of a wing of Anatopynia sp. ( Chironomidae [ = Tendipedidae] ). In this Figure only the posterior half of the wing is drawn : The radial area and the parts anteriorly to it are omitted.
(After HENNIG, 1968)
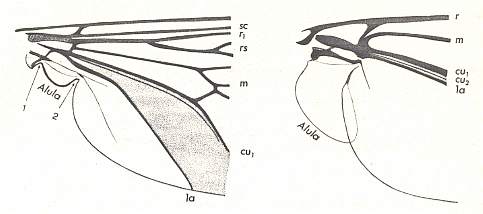
Figure 1n :
Left : Basal part of a wing of Anisopus sp. ( Bibionomorpha).
Right : Basal part of a wing of Philopota sp. ( Brachycera, Acroceridae).
(After HENNIG, 1968)
Accordingly, we can think of the first developmental steps of the wing in Diptera as follows :
In the ancestral group of the Diptera, after it having branched off from the forefathers which they have in common with the Mecoptera, things led to the loss of the hind-wings (at their places the halteres appeared) and thus to the transition of the flight function to the fore-wings alone. Moreover, -- according to ROHDENDORF connected with the increase of wing-beat frequency -- the passing on of the muscular force [to the wing] was concentrated on the anterior part of the wing. The posterior part retained -- still according to ROHDENDORF -- as a result of the reduction [desclerotization] of Cu2 and its shift toward Cu1, the ability to passively avoid the increased air-resistance. The transmission of the muscular forces to the anterior part of the wing was supported by a narrowing of the wing-base as a result of the reduction of its posterior region : Connected with this is the reduction of the third anal vein (or its coalescence with the posterior wing-margin?).
Up to here the development in the last common ancestors of the recent Diptera had taken place, and the recent Tipulomorpha have, in their basic plan, not yet gone beyond it.
A further step along this way was the still stronger differentiation between the already narrowed wing-base, which became the true basiala, and the wing-blade, as a result of (1) an incision at the posterior wing-margin, (2) reduction of also the second anal vein, and (3) the formation of the phragma. This situation can be assumed as being the basic plan or point of departure for all the remaining Diptera not belonging to the Tipulomorpha (as regards the formation of the phragma with the just mentioned restrictions).
If these ideas are correct, then the assumption naturally comes up that there exists a sister-group relationship between the Tipulomorpha and all other diptera. To test this, we will have to prove first of all that the Tipulomorpha are a monophyletic group, and secondly, that the totality of all other diptera, which we will, in what follows, call simply "Oligoneura" ( = Psychodomorpha + Culicomorpha + Bibionomorpha + Brachycera), also forms a monophyletic group. This should be done by negating the assumption that the described wing features of the Oligoneura have been formed in this group many times independently, convergently, and that we can, where it is possible, demonstrate the presence of also other derived features which are independent of the wing structure.
REMARK :
The basic plan of a given monophyletic group of organisms, in the sense of HENNIG, is the set of morphological features that is present in the most original ( 'primitive' ) members of the group. The corresponding morphological features of the other members of the group must be such that they represent further developments or reductions of these basic-plan-features.
To base the monophyly of the Tipulomorpha is not difficult. As derived [that is, apomorphic] features of the basic plan of the Tipulomorphs we can mention for example :
The ending up of R2 in R1 [that is, the first branch of the Radial Sector (R2) runs into the Radius (R1)] : The anterior branch of the Radial Sector is never free [that is, it never runs all the way to the wing-margin ending up in the Costa]. See Figure 2a, below . [This feature -- as any such derived feature -- must have been possessed by the most recent common ancestor of the Tipulomorpha and then inherited by its descendants and as such retained in the most primitive of them while transformed or disappeared in the other descendants].
The mandibles of the adults are reduced.
In the larvae are at most (namely in the basic plan of the Tipulomorpha, as can be seen in the Trichoceridae) two pairs of spiracles [ = respiratore openings] retained : The larvae are amphipneustic. Most often they are even metapneustic. See for this HERE , 3 = amphipneustic, 4 = metapneustic. In the Figure the spiracles are indicated as black dots.
Surely also the lengthening and narrowing of the wings in Tipulomorpha can count as a derived feature. With it is apparently connected the ending up of R2 in R1. Further, in Tipulomorpha many forkings of the longitudinal veins, the endings of all longitudinal veins (except that of 2a in the basic plan), and the markedly diminished discoidal cell (shaded in Figure 2a, below ), are transposed toward the apical half of the wing. The very common -- but repeatedly independently formed -- shift of the base of R2+3 onto R4 (see again the Figure, but there R3 is reduced, and see also Xiphura atra ( Tipulidae) HERE ) corresponds to the same general tendency to shift important elements of the venation towards the apex of the wing.
A comparable lengthening and narrowing of the wing is in many Diptera, and also in other insect groups (for instance Odonata [dragonflies] ), accompanied by a certain lengthening of the abdomen. Even in one and the same dipterous family, as in the Conopidae, one can observe that the forms with a long petiolate abdomen have long and narrow wings, while stocky species with a short broad abdomen possess broad wings. Surely, we cannot assume such a stocky abdomen, as we see it in many Brachycera, to be [already] present in the basic plan of the Diptera. But the abdomen in this basic plan may also not have been so long and slender as in the Tipulomorpha. This is evident by the fact that the formation of a particularly slender body shape is reflected in a lengthening of the legs. Also ROHDENDORF (1958/59, p. 443) characterizes the legs of the "Tipuloidea" [think of Daddy-long-legs] as "peculiar specialized extremities ..., that possess a high degree of prehensility and at the same time having a clearly limited ability to run". Apparently, with the development of such kind of legs is connected also the loss of the tergal depressor muscle of the trochanter [a segment belonging to the base of the insect leg] ( TDT-muscle : SMART, 1959) in the mesothorax [middle segment of thorax] : According to GRANT (1960) this is a starter muscle, which is present in all Diptera that begin flight with a leap. It is absent in all long-legged Nematocera as in the Tipulomorpha who do definitely not leap when flying off. According to SMART the TDT-muscle is present in some Psychodomorpha, Culicomorpha, and Bibionomorpha, but, apparently, never in Tipulomorpha.
SMART, whose muscle investigations are certainly very deserving, draws from the distribution of the TDT-muscle highly impossible conclusions :
"In the Nematocera the loss of the TDT-muscle is probably of ancient date. It is possible that the group lacking the TDT muscle is monophyletic, i.e. originating from ancestors possessing a CS muscle [=Coxosubalar-muscle] but which lost the TDT muscle. The nematocerous families in group I of Table 4 [that are the families Simuliidae, Sciaridae, and Psychodidae including Nemopalpus ] are probably representative of the ancient original Diptera stock possessing both the CS muscle and the TDT muscle. Further comparative studies may confirm the singularity of the Diptera comprised in group I of Table 4 and convince dipterists that they do in fact represent, albeit in a much modified form, the ancestors of the Diptera. If this happens, then it may be necessary to detach them from the Nematocera and set them apart as a new suborder of Diptera."
( p. 359-360 )
Apart from the fact that a group ("group I of Table 4") cannot be made to represent a new suborder of the system [merely] on the basis of original [primitive, plesiomorphous] features (the presence of the TDT-muscle and of the CS-muscle), everything goes against the supposition that the lack of the TDT-muscle can be interpreted as a synapomorphous feature of those groups that do not possess it. In the history of the Diptera the muscle is expected to have been independently reduced rather often. But this does not mean that it must also hold for the Tipulomorpha. All by itself the lack of this muscle cannot demonstrate the monophyly of the Tipulomorpha. But in connection with the fact that there are also other features that indicate the monophyly, the absence of the TDT-muscle gains significance in this respect.
Special significance can be attributed to the presence or absence of the TDT-muscle as a result of the new findings of the physiologists (for a popular exposition, and for literature, see NACHTIGALL, 1968), according to whom this muscle also serves to crank up the flight-engine (which is the set of indirect flight-muscles). The indirect flight-muscles receive their task to alternately contract not from stimuli coming from the brain, but from mechanical stimuli, of which the first come from the TDT-muscle. These findings, obtained from the study of Calyptrates [ = the highest flies such as blow-flies and house-flies], cannot, however, hold for all Diptera (which all do fly by using indirect flight-muscles), because the TDT-muscle is absent in many of them. According to SMART (1959) it is also absent in Glossina and Gasterophilus [both Calyptrates], that is in genera that are closely related to those that were investigated by the physiologists.
For the supposed sister-group of the Tipulomorpha, which we want to call "Oligoneura", we have already mentioned as a derived feature the formation of a true basiala. Before we will test this feature, we try to answer the question whether there exist other derived features of the Oligoneura. [ HENNIG (pp. 7) then discusses some morphological features of the body that might be such derived characters. We [JB] will not follow it here, but continue with HENNIG's discussion of the basal part of the wings in Diptera.]
The question whether the formation in Diptera of a basiala which is clearly differentiated from the wing-blade has happened more than once, can, according to me [HENNIG] with a fair amount of certainty be answered with no.
The genealogical tree of Diptera designed by ROHDENDORF (1962) contradicts, it is true, this opinion. If this tree were correct, and if at the same time our supposition holds that the Tipulomorpha (having the above scetched content, not that which is connected with this name by ROHDENDORF!) have preserved relatively original features in the structure of the wing-base, then a differentiation in the wing between basiala and blade, as it was described above, must have been taken place at least four times independently [We must realize that ROHDENDORF's 'genealogical tree' is in fact no such tree even when he would so call it. It is a typological system that classifies e s s e n c e s of taxa, resulting in t y p o l o g i c a l relationships, not genealogical relationships. So from such a system one cannot conclude that a certain morphological transformation (or reduction) happened only once or many times in the history of the group.]. This is, as will be shown, very improbable. ROHDENDORF has, to back up his genealogical tree, not come up with valid arguments, valid in the sense of genealogy ["Stammbaumforschung"] (or phylogenetic systematics, which ultimately means the same thing ). And although he has contributed essentially to the understanding of the wing-structure, he has failed to recognize rightly the important developmental step, lying between the Tipulomorpha and all remaining Diptera. But rightful recognition of this step is the very condition for the question to be asked whether it has happened only once or more than once anyway.
ROHDENDORF subsumes under the heading "primitive (tipuloid) traction type" the Tipuloidea (=Tipulomorpha in our [HENNIG's] sense), Liriopeoidea (=Psychodomorpha), Pachyneuroidea (a group which probably belongs to the Bibionomorpha), and the Nemopalpidae ( a subgroup of the Psychodidae s.l.). The similarity of these groups rests on convergence [ = development of a given structure (or its reduction) having taken place, genealogically independently, in several different genealogical lines]. More precisely said : Only in the " Tipuloidea" the absence of the differentiation between basiala and wing-blade is p r i m a r y [that is, original, meaning that this differentiation has never been there before]. In all other mentioned groups it has been lost s e c o n d a r i l y. This is supported by (among other things!) the fact that the reduction of the second anal vein, which is connected with the formation of the basiala, has not become undone again.
At first sight, this opinion seems to be contradicted by the alleged fact that the second anal vein has, in the Oligoneura, different lengths [in different representatives of the group]. This could indicate a repeated independent reduction of this vein and corresponding with it a repeated independent formation of the basiala. In reality the seemingly different lengths of the second anal vein are to a large extent a confusion : The second anal vein is in all Oligoneura rudimentary and much more weakly expressed than the first anal vein. It never extends into the wing-blade. This even, and especially, holds for those groups in which the incision, which, at the posterior wing-margin, originally separated the basiala from the wing-blade, was secondarily lost (as, for instance, in the Ptychopteridae, see Figure 1j, above ). In many Figures, that can be found in the literature (also in such of my [HENNIG's] own earlier works) things are different in this respect. Here the "second anal vein" often almost reaches the posterior margin of the wing. This is especially evident in a Figure of ENDERLEIN (1936) of Anopheles [Culicidae], where it (signified as "ax") is drawn as being as long and as strong as the first anal vein. In reality it is especially in Culicidae clear that the alleged extensive rudiments of the second anal vein in the wing-blade are mere folds. The posterior margin of the basiala is here somewhat curved, resulting in a weakly expressed alula. At the location where this alula comes together with the posterior margin of the wing-blade exists a sort of bump, and this bump is the point of departure of a manyfold of folds, which extends into the otherwise flat surface of the anal lobe of the wing-blade. In Culiseta and other Culicidae clearly three such folds are visible. See Figure 1l, above . In other cases there exist only two of them. See Figure 1m, above . Sometimes one of these folds is a little bit darker than its surroundings. But according to me [HENNIG] we have, in all these cases, to do only with a similar (but by far not so conspicuous) phenomenon as we see in the sclerotization of a cross-fold of the wing surface resulting in a phragma. With the second anal vein all these folds have nothing to do. They result from tensions which originate in the confines of the anal lobe in connection with the formation of an alula. Therefore they are absent in Blephariceridae and Tanyderidae, in which an alula is primarily absent.
A very strongly developed alula at the hind margin of the basiala is especially widely distributed among Brachycera, see Figure 1n, right image, above . In the literature I [HENNIG] have until now not found a hypothesis about the function of the alula. It seems to me that it is connected with a secondary broadening of the wing-base. In the forms in which the differentiation between basiala and wing-blade had been formed for the first time there apparently existed a strong opposition between a narrow wing-stalk and the broader wing-blade, see Figure 1k, above . This could have been unfortunate, especially for forms with a stocky body. Thanks to the development of an alula a broadening of the wing-base was accomplished once more secondarily, without the necessity to again neutralize the concentration of the force transmission in the wing-stalk [That is, the forces will still be transmitted to the a n t e r i o r part of the wing-base].
We then would have in Diptera a similar development as is known to be the case in Odonata [an insect-order consisting of Zygoptera (slender dragonflies) and Anisoptera (true dragonflies)] : In both cases the original forms were forms with a broad wing-base (paleozoic Odonata, respectively, the Mecoptera-like ancestors of the Diptera). The second step was the formation of a wing-stalk (in Odonata : Zygoptera, in Diptera : primitive Oligoneura, where this process had begun already in the Tipulomorpha). Finally, a secondary broadening of the wing-base (in the Odonata : Anisoptera, in Diptera in various groups independently by the development of an alula).
In the development of a very large alula ( Figure 1n, right image, above ) the rudiment of the second anal vein, still preserved within the confines of the basiala, might have gained new significance. In any case there exists in all forms with a strongly developed alula at its base a heavily sclerotized longitudial rod, which without doubt has been formed, at least for a part of it, by a secondary strengthening of the second anal vein in order to support the alula, see again Figure 1n, right image, above , and also the wing of Rhynchocephalus fasciatus (Brachycera, Nemestrinidae) HERE .
These facts and considerations show that there is no need to derive forms having a strongly developed second anal vein at the base of the alula from ancestors in whom this vein was better developed than for instance in the direct ancestors of the Psychodomorpha and Culicomorpha.
A certain significance has the fact that, in order to accomplish a secondary broadening of the wing-base, there was formed, at the posterior margin of the basiala, an individualized, separated from the wing-blade, and therefore independently mobile, flap, namely the alula. And this probably to support the resting-position of the wings : In the Brachycera, who place, when at rest, their wings together horizontally over and above the abdomen, one can see the alula -- if present -- one at each side of the scutellum [ = one of the dorsal sub-shields of the thorax] standing vertically directed upwards, that is, making a right angle with the remaining wing-surface. Were the wing up to its base gradually, that is, without incision, broadened, then such a resting position of the wings horizontally above the abdomen would not be possible because of the scutellum.
The alula can, without doubt, be reduced again. In that case we see, also in many Brachycera, the wing-stalk at the posterior margin gradually passing over into the wing-blade without incision or any other discontinuity.
The fact that the alula does not belong to the basic plan of Diptera and also not to that of the Oligoneura [that is, (even) the first Oligoneura still didn't have an alula], forces us from now on to more cautiously to investigate the question whether the absence or weak development of the alula is, in the given case, an original feature or a derived feature.
Unfortunately the further [historical] development of the wings in Oligoneura is, apart from some very general features, not yet completely clear. ROHDENDORF (1958/59) sees here two tendencies in conflict : The tendency to obtain a strong traction force (accomplished by the lengthening of the wings) and that to obtain an increase of lifting force (accomplished by an increase of wing-beat frequency and a broadening of the wing-blade). It is, however, at least for me, not always clear in what way the conflict between these opposing tendencies and the interference of further factors, of which there are undoubtedly many, is reflected in the not less than 16 functional types and 50 subtypes of wing-structure which ROHDENDORF distinguishes. Many type boundaries run right through clearly monophyletic groups. In itself this is completely legitimate, for certainly one and the same functional type can have been developed independently and polyphyletically in most different kinship groups. But in such extensive type schemes, especially when one has to rely on mere assumptions as to evaluate factors of which the function of an organ depends, there is always the danger that one dubiously ends up closely to the old-fashioned idealistic morphology. According to me also ROHDENDORF's scheme has not escaped from this danger, and perhaps one can, with respect to it, say the same thing as what I have brought against HERTING's scheme of ovipositor types : If one bases such a scheme on other "type features", then one obtains a classification of types which better corresponds to the system of monophyletic groups, without the types necessarily loosing their character of being "functional types" (HENNIG (1968), p. 10 ). [According to me, one should interpret typological taxa -- often (mistakenly) figuring in a so-called "genealogical scheme or tree" -- and also functional types as qualitative units of Nature, while true phylogenetic taxa are genealogical units which can figure in a true genealogical scheme or tree. And it would enhance our insight into Nature when we would superpose typological taxa and functional types onto a true phylogenetic (that is, genealogical) system of a given group of organisms. Later on we will indeed do such a thing.].
We are now, finally, ready to discuss the consecutive groups of Diptera, their evolution, life-habits, and morphological and taxonomic structure.
The system of Diptera : An extensive discussion of the higher-ranking taxa of the order Diptera as established mainly from an ecological and functional point of view.
After having expounded -- in a general way -- the functional aspect of the wings of Diptera, and its importance to an evolutionary understanding of these insects, we can now begin the systematic treatment of the Diptera especially from an evolutionary and ecological viewpoint.
So let us now then discuss the infraorders of the Order Diptera.
ROHDENDORF, 1964, distinguishes 13 such infraorders :
Figure 2 : Tipula paludosa Meigen ( Tipulidae, male), general view ( length of body 20 mm).
After Lindner, 1928.
Figure 2 depicts a typical member of the Tipulomorpha. It belongs to the family Tipulidae, that is, the long-palped crane-flies or 'daddy-long-legs'.
The Tipulomorpha form a large complex consisting of 8 superfamilies :
The most characteristic ecological feature of the Tipulomorpha as a whole is their connection with an aquatic environment in which their larvae live, although some species have returned to a terrestrial but still damp environment.
Where we, in the following discussion concerning the colonization of particular environments, speak about conflicts, we consider them in a more or less loose or broad sense without strictly distinguishing between (1) the ultimate internal conflicts -- as discussed in Part IV of the foregoing series -- and (2) the higher-level conflicts that are derived from them.
As has been expounded, evolution of organisms mainly consists of the solution of conflicts, conflicts that arise when organisms are going to colonize a new environment or a new aspect of their regular environment. Ultimately such a conflict boils down to a so-called internal conflict, that is a conflict between, or within, basic life-processes such as respiration, feeding, locomotion, etc. The solution of such a conflict depends on the prevailing external conditions in which the organisms under discussion presently live. We assume that such an internal conflict is, in the Implicate Order, present as some noëtic virtual pattern and that it as such is the noëtic context of some particular noëtic reaction. When the product of this reaction is projected into the Explicate Order we experience it as the development of some adaptation which as such appears to us as the solution of the conflict.
We will now discuss the supposed origin of the infraorder Tipulomorpha in terms of the solution of such conflicts.
Conflicts and determining tendencies in the historical origin of the infraorder Tipulomorpha.
Consideration of the features of the Tipulomorphs allows us to unearth the main characteristics of this infraorder of two-winged insects, which determine it as a whole. In virtue of this it becomes possible to find those conditions, those conflicts, [that were present] in the historical development, at which [conditions, conflicts] took place the origin and formation of this group of Diptera. The basic conflict in the history of the Tipulomorphs almost certainly originates as a result of the colonization by the first forms of an aquatic environment, that is the transition of the larval phase from conditions of terrestrial soil to the aquatic environment of fresh-water basins. This transition has turned out to be an important event in their [initial] history, which had determined the solution of that particular conflict that was basic to the first two-winged insects, which [conflict] had originated as a result of the larvae of these first Diptera inhabiting the layers of terrestrial substrates, namely [that conflict consisting of ] the necessity of developing more appropriate features of locomotion of the winged form, because of the restrictedness, localness, and of the temporary nature of such environments of the larvae and the necessity for the winged phase to be able to find them. This conflict was, in the case of the origin of the Tipulomorpha, solved not by actually improving the organs of locomotion (wings and legs), but by a change of habitat : Indeed, the transition of the larvae from a terrestrial environment to an aquatic one -- which can be an extensive, and therefore easy to find, environment -- solved the conflict. But, as a result of this there quickly appeared new demands, first of all those that are connected with the realization of respiration in an aquatic environment. And at the same time the very principal of all biological conflicts became aggravated for the poorly mobile apodous larvae in the open aquatic environment -- insufficient amount of food. The solution of this conflict co-determined the origin of the Tipulomorpha where the determining features turned out to consist of the perfection -- adaptation -- of the respiratory system of the larva (first of all the development of an amphi- or metapheustic condition -- that is certain degrees of reduction of the number of pairs of spiracles), or the development of a predatory way of life. The locomotion of the winged phase did not undergo perfection (because of the absence of a long necessary search for substrates onto [or into] which to lay eggs, as a result of the vastness and thus of their being omnipresent of the appropriate substrates, such as the banks of aquatic basins, boggy areas, etc.). The insufficient amount of food for the larval phase in the new environment, that is, the aquatic environment, originally determined the conservation and [further] developing of the feeding of the winged phase. And all these features of the mentioned conflicts which determined the origin of the Tipulomorphs sharply distinguish this infraorder from the other infraorders, first of all from the infraorder Bibionomorpha which lives simultaneously with it.
A taxon has a definite formal (qualitative) content, which, however, can, and often will, evolve further. The origin of a taxon, here the Tipulomorpha, starts with its, later to be called, ancestral species entering, in the context of beginning to occupy or colonize, a new potential ecological niche of its regular environment or entering an altogether new environment. When it has, in the form of one or more of its populations (not a single individual) entered the new potential habitat part of it will be more or less internalized which here means that at least some aspects of this new habitat form a qualitative content that is -- as potential habitat -- added, but not yet integrated, to the formal content of the species (while the formal content of the actual (original) habitat of the ancestral species was already incorporated in the species' formal content). Precisely because it is so added to the species's formal content the qualitative content of the new potential habitat is definitely delimited (by the formal content of the ancestral species) and only then is indeed a formal content.
The formal content of the ancestral species of the Tipulomorpha, is, as a result of injection (enfoldment), noëtically present in the Implicate Order. And now also the formal content of the new potential habitat (because it has become a formal content) is injected into the Implicate Order, that is, the formal content of the aquatic environment in which -- as seen in the Explicate Order -- one or more populations of the ancestral species have entered. Being now noëtically present in the Implicate Order, a noëtic reaction will take place between these two noëtic entities resulting in a noëtic reaction product. The directing noëtic context of this reaction is formed by the noëtic content of the conflicts involved, in fact by the content of their respective terms. The projection (unfoldment) of this noëtic reaction-product into the Explicate Order is seen as the evolutionary -- that is, historical -- development of adaptations of the larvae with respect to their respiratory system and of adaptations in the behavior of the adult flies to lay their eggs in the aquatic environment.
Of course all this, that is, the assumed presence and solution of the conflicts, as well as their interpretation in terms of the Implicate and Explicate Orders, is still more or less vague and hypothetical, but in spite of this well worthy of being presented here as a possible way of the origination of the Tipulomorpha from the (phylogenetically) first Diptera.
As has been said, the infraorder Tipulomorpha consists of 8 superfamilies (each consisting of a number of families). See the list above .
This largest superfamily of the Tipulomorpha, the Tipulidea, contains 6 families of very unequal sizes (number of species) :
We will now consider the families of the Tipulidea one by one with respect to their habits and origin.
The family Tipulidae are known as 'daddy-long-legs', and also as the 'larger crane-flies', or the 'long-palped crane-flies'. See Figure 2 .
Ecologically they are closely related to the similarly looking but generally smaller Limoniidae, that is, the short-palped crane-flies. Not very much is known about the details of the adult life of both families. They sit about on exposed surfaces, but they are not such ardent sun-lovers as are many flies. Rather one assiociates crane-flies with cool, damp places, and a great many escape notice because they are resting under leaves and between the stems of grasses. The main occupation of adult crane-flies (both families), as of the majority of adult flies, are mating and egg-laying. Feeding is less important, and probably water is the most pressing need, helped out with a little sugar from the nectar of flowers. Many crane-flies, including the Tipulidae have the face drawn out into a rather asinine snout, with the mouthparts at its tip. There are no mandibles (upper jaws), and the maxillae (lower jaws) are modified and reduced, so that neither chewing nor piercing is possible. The maxillary palpi are usually prominent, and it is from these that the division into long-palped (Tipulidae) and short-palped (Limoniidae) crane-flies is taken. They obviously serve to detect food by smell and touch. The snout is adapted to flower-feeding. Generally, the evolution of a flower-feeding snout (proboscis) in Diptera has not been entirely without a future. In some families, those in which mandibles and maxillae have been retained as part of the sucking-tube, it has been possible to adapt the proboscis for piercing the skin of vertebrates and sucking their blood. This is a step that adult crane-flies (Tipulidae, Limoniidae) have never taken. They presumably lost their mandibles and maxillae at an early stage. See for all this, OLDROYD, H., 1964, The Natural History of Flies.
The next Figure depicts the wing-venation of a representative (according to HENNIG, 1954) of the Tipulidae :
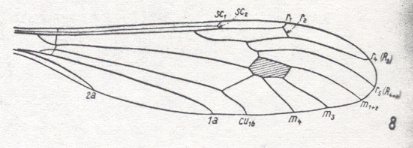
Figure 2a : Wing-venation of a member of the Tipulidae.
8 -- Idiotipula confluens ALEX.
(From HENNIG, 1954, after ALEXANDER, 1921)
The larvae of crane-flies (both families) can nearly always be recognized by having a single pair of spiracles posteriorly, surrounded by a disc of fleshy lobes. Among their very varied larval habits, crane-flies still retain examples of the various trends that we will be able to follow in Diptera as a whole. All larvae of crane-flies have posterior spiracles only, or none at all, and this is the prime adaptation to life in water, or in a medium where respiration is difficult.
Larval habits of Tipulidae.
The larvae of Tipulidae are rather plump and fleshy, the posterior spiracles surrounded by a crown of six lobes. The well-known 'leather-jackets' are among the most terrestrial of crane-fly larvae. They feed principally just below the surface of the soil, eating the roots of plants of all kinds. Although terrestrial, and provided with a tough skin, leather-jackets are moisture-loving (OLDROYD, 1964).
If we assume that the original habitat first colonized by the ancestral species of the infraorder Tipulomorpha was the aquatic environment bringing with it the development of the necessary adaptations to it, then we must envisage the terrestrial habits of the leather-jackets as a return by some members of the developing taxon (the infraorder Tipulomorpha or, narrower, the superfamily Tipulidea) to a terrestrial habitat. But the actual ecological niche in this terrestrial habitat now occupied by those Tipulidae having leather-jacket larvae is not expected to be the same as the one or the other niche that was occupied by members of the first Diptera, neither will all other terrestrial ecological niches that later became occupied by other members of the infraorder Tipulomorpha be the same as the one or the other that were occupied by members of those first Diptera. This means that evolution is, in addition to morphological features, also irreversible with respect to ecological niches, that is, although a given habitat or environment can evolutionarily be re-colonized by animals, they will never re-occupy exactly the same ecological niche.
Many Tipulidae develop in moss on dry rocks and in decaying wood of fallen logs and the stumps of felled trees. Species from a number of genera breed in rotting wood of varying degrees of softness. Most of them, species of Tipula, Dictenidia, and others, including the beautiful orange and black Ctenophora, have the usual simple ovipositor, and can only deposit their eggs on the surface, or push them into soft pulp, but Tanyptera, another beautifully colored red and black fly, has a slim, pencil-like ovipositor, and looks very much like one of the ichneumons (parasitic wasps) that lay eggs in similar places. As its appearance suggests, it lays eggs in wood that is less decayed, and is the extreme in the direction of a wood-boring fly. This is a larval habitat that has not been greatly exploited by Diptera, like it is by Coleoptera (beetles), though a few isolated flies have colonized it (OLDROYD, 1964, p. 34-35).
The larvae of the genus Tipula itself have a variety of habitats besides those in which they eat the roots of plants such as grass. In fact one species or another can be found in all the habitats from decaying wood, through moss, earth, and mud, to a more or less completely aquatic life. Even the aquatic larvae breathe air of course, and can remain submerged only for a limited period (OLDROYD, 1964).
It is clear that the family Tipulidae (and the same goes for the family Limoniidae) shows a radiation into a very diverse spectrum of habitats and ecological niches, and this makes it hard, to judge from recent members of it, to determine what precisely the original ecological niche, or even the original habitat for that matter, of the family was. But the metapneustic condition (spiracles only at the posterior end) of the larvae of all the known species indicates that the original habitat of the family was aquatic. From there other habitats were colonized, namely the mentioned terrrestrial habitats. And as it seems, the family Tipulidae is still flourishing today, that is, it is not evolutionarily a relict group. The same can be said of the next family of the superfamily Tipulidea, the Limoniidae.
The second family of the superfamily Tipulidea, namely the family Limoniidae, that is, the short-palped crane-flies, is even larger, as to the number of species, than the previous family, and also its ecological versatility has gone a step further. Like we did with respect to the family Tipulidae, we rely for the habits of the family Limoniidae on the mentioned book of OLDROYD (1964).
We can note that in their biology and habits the short-palped crane-flies cover a range as wide as, indeed wider than, that of the daddy-long-legs proper. We can see again the same situations, and similar larval adaptations to meet them. The two groups (Tipulidae and Limoniidae) have certainly evolved independently and often on parallel or convergent lines.
In general we can say that the short-palped crane-flies have all the versatility of the long-palped ones, and more. In place of the genus Tipula, sampling all the habitats with one species or another, we have Dicranomyia going even further by having the only leaf-mining crane-fly.
The short-palped crane-flies have, by one of its members, specialized further their adaptation to an aquatic life. We know that the large, open spiracles at the tip of the abdomen of the larva, themselves a modification making it easier to breathe in watery places, compel the larva to reach the air periodically, even if only at long intervals. If this is prevented, then diffusion of nitrogen from the open spiracles will gradually flood the tracheal system. This limitation has been overcome in a small group of crane-flies, the genus Antocha and its relatives, by closing the tracheal system completely. Exchange of oxygen takes place by diffusion through the thin cuticle of tracheal gills, lobes at the tip if the abdomen where tracheae are abundant. The pupae have long-branched filaments of a similar function arising from the thoracic spiracles. These larvae live in well-aerated water, often rapidly moving, much the same as we shall see later in the black-flies of the family Simuliidae.
Another direction in which the short-palped crane-flies have gone further than their bigger relatives is in moving towards a carnivorous diet. As we have seen, the larvae of the Tipulidae are essentially vegetarian eating moss, algae, diatoms, rotting and macerated wood, and living cells from the roots of growing plants, and even trees. It has been reported that these larvae will on occasion eat small animals, even earthworms, if they encounter them in the course of feeding, but this is probably not a significant part of their diet. The family Limoniidae, however, includes many species whose larvae are wholeheartedly carnivorous, especially in the tribes Hexatomini and Pediciini. Their mandibles are curved and sharp, and are used to attack other small animals such as dragonfly nymphs, larvae of other flies, and small worms. They can take prey almost as long as themselves and sometimes are cannibalistic on their own species. They tear and swallow their prey, and may have a roughened area in the gullet that helps them to keep it down, like the backward-facing teeth of sharks and snakes.
Carnivorous larvae, of course, are immensely more active than vegetarian ones. They are slender and wormlike in shape, in strong contrast to the fleshy, obese, rather repulsive leather-jackets.
The next Figure depicts some larvae of the family Limoniidae :
Figure 3 : General view of larvae of Limoniidae. Side view.
1 -- Metalimnobia quadrimaculata L.
2 -- Discobola annulata L.
3 -- Ula bolitophila Loew.
4 -- Epiphragma ocellaris L.
5 -- Lipsothrix errans Walk.
6 -- Elephantomyia sp.
After KRIVOSJEINA, 1969.
The study of the historical development of the remaining families of the superfamily Tipulidea, which are clearly relict groups, is, like the first two families discussed above, also hampered by the insufficiency of available data. But the very pecularity of these insects which is an evident consequence of their relict nature, already allows us to indicate the way to answer the questions about their history and in very general terms express a supposition about the nature of conflicts that determined their origin and their long survival as relict forms ( ROHDENDORF, 1964, p. 44). So let us discuss these families one by one.
Figure 4 :
Ptychoptera contaminata L., Ptychopteridae, male, general view (length of body 9 mm).
After LINDNER, 1928.
Considering the characteristic features of the family Ptychopteridae first of all the extraordinary features of the larva of these Tipulidea arrest our attention, which (larvae) possess, in addition to a free, more or less independent rather primitive head capsule, extremely elongated posterior spiracles in the form of telescopic tubes [which in fact carry these spiracles] : These features of the larval organization express the significant expansion of the ecological range, that is, the ability to populate deeper layers of a small aquatic basin, the possibility for the larva to move more freely and being more independent of the surface film of the aquatic basin. Another feature of the larvae and especially of the pupae of the Ptychopteridae consists in the robustness of the cuticle, sometimes provided with a tight spiny armouring. Its protective function is clear. This recent group of Tipilidea is more or less remote from all other families. It is a remnant of certain Jurassic groups which are absent in the Cenozoic era ( ROHDENDORF, 1964, p. 44).
While the habitat of the larvae of these flies is the same, the specific ecological niches occupied by them are expected to be different. Also the specific details of the mentioned adaptations are expected to be different. We can suppose that the different species, although perhaps eating the same materials of that habitat, make use of different nutritive substances present in those materials.
The characteristics of the other family, poor in number of species, the Cylindrotomidae, also allows to indicate the pathway of its historical development, partly similar to the previous family. Basically, as is evident, the cuticle of the larva has changed, which acquired a very tight protective cover consisting of fairly long thin outgrowths, which cover the whole body. See Figure 5.
See also next Figure.
Figure 6 : General view of larvae of Cylindrotomidae. Side view.
1 -- Phalacrocera replicata Schin.
2 -- Liogma glabrata Wied.
After KRIVOSJEINA, 1969.
At the same time, the Cylindrotomids are interesting by the shifting of the larval habitat away from the aquatic environment. These insects (larvae) live in overgrowths of moss and can thrive in these conditions totally outside the aquatic environment. This family is relatively close to the Limoniidae and probably is, as is the previous family, a derivative of the Mesozoic Architipulidae ( ROHDENDORF, 1964, p. 44).
This family, also known by the name Petauristidae, are the winter-gnats. These insects are characterized (ROHDENDORF, 1964, p. 44-45) on the one hand by a series of ancient features (as with the structure of the larva which possesses a simple independent head, and as with the winged form which possesses elongated tipuloid wings with a primitive little mechanized wing-base (described in ROHDENDORF, 1951, p.49, Fig. 22)), and on the other hand by a very peculiar ecology (OLDROYD, 1964, p. 39) : They carry to an extreme the liking of the crane-fly for cool, damp conditions. Although they occur in summer in such places, they are overlooked, or confused with true crane-flies, in the winter they come out and dance in the open on every sunny day. They are also common in caves, gorges, and grotto-like places, and in mines. Their swarms are male mating swarms, and the larvae (Figure 5) breed in decaying vegetable matter of a compost-like nature.

Figure 7 : Larva of Trichocera sp., Trichoceridae. Dorsal view.
After KRIVOSJEINA, 1969.
REMARK : We should be aware that an "ecological niche" is here -- on this website -- understood to be something very abstract : It is not some concrete terrain or environment, say a certain piece of forest, and also not even one or another type of forest, or type of whatever terrain or environment. It is one of the many possible specific aspects of a given type of environment or habitat. Its definition is mainly biochemical and physiological because it is connected with one definite organic species (or a population of its individuals) of which it is the ecological niche. So things like the season of activity of the organic species occupying a given ecological niche, the nature of the nutritive substances in the materials present in its habitat, the occurrence and nature of predators and parasites in this habitat, the temperature and moisture regime of the latter, those particular microclimates of the habitat that are relevant to the organic species, the nature of the usually separated 'sub-habitats' of larva and adult and their integration, all this are characteristics of the ecological niche of some given organic species (or of a population of individual representatives of it).
Flies of rather small size.
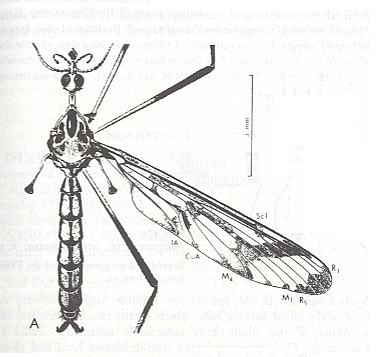
Figure 8 : Eutanyderus oreonympha, Tanyderidae.
The length-measure (vertical line segment) expresses : 5 mm.
After COLLESS, in RICHARDS & DAVIES, Imm's General Textbook of Entomology, 10th edition, 1977.
And the larva of the genus Protoplasa is depicted in the next Figure :
Figure 9 : Protoplasa fitchii Osten-Sacken ( Tanyderidae), larva, side view.
After HENNIG, 1948-1952, magnified.
The Tanyderidae are somewhat like the Ptychopteridae. It is a small group of 25 [or more] species (OLDROYD, 1964) of which the larva is known in only one (Figure 9). Protoplasa fitchii is one of the rarest of crane-flies, found only in North America. It is featured in textbooks because the adult fly has five branches to the radial vein of the wing (that is, the radius (R), and the 4 branches of the radial sector (RS)) : a hypothetically primitive arrangement almost never found in any real fly.
Functional type and subtypes of the wings of the representatives of the s u p e r f a m i l y T i p u l i d e a.
( The description of functional types and subtypes of wings is taken -- with additions and changes -- from ROHDENDORF 1951.)
The representatives of the superfamily Tipulidea all have wings that belong to the
Primitive traction (tipuloid) wing type.
Description of the type (and its subtypes) as such :
To begin with, ROHDENDORF adds as a note :
In naming the types I wanted to express the most important and the very characteristic functional features of the wings of the given type. Of course, the presented names do absolutely not indicate the exclusiveness of the trait, as expressed in the name, for the type [that is, other types could also possess this trait], but only indicate the type's most characteristic feature.
Representatives of the type.
To the primitive traction (tipuloid) wing type -- one of the most primitive types, [the representatives of] which are closely related to the ultimately first forms, namely to some Mecopteroidea (namely the fossil Paratrichoptera) which evolutionarily have led to the order Diptera -- today belong wings of the representatives of the families Tipulidae, Limoniidae, Liriopeidae [ = Ptychopteridae], Trichoceridae [ = Petauristidae], all belonging to the superfamily Tipulidea of the infraorder Tipulomorpha, further, of Pachyneuridae, as superfamily Pachyneuridea also belonging to the infraorder Tipulomorpha, and, finally, of the relict family Nemopalpidae belonging to the superfamily Psychodidea, also a superfamily of the Tipulomorpha). So we see that the primitive traction (tipuloid) wing type is not just confined to the superfamily Tipulidea. It is also present in some other superfamilies of the infraorder Tipulomorpha.
[ The systematic position of the family Pachyneuridae is uncertain. It could be that this family does not belong to the infraorder Tipulomorpha (and then, consequently, not to the superfamily Tipulidea) but to the infraorder Bibionomorpha. Functionally, of course, their wings do belong to the present type].
Here we present some Figures depicting wings of this type.

Wing-venation of a member of the Tipulidae.
8 -- Idiotipula confluens ALEX.
Interpretation of wing-venation (i.e. the identity of the individual veins and cross-veins) according to HENNIG, 1954.
(From HENNIG, 1954, after ALEXANDER, 1921)
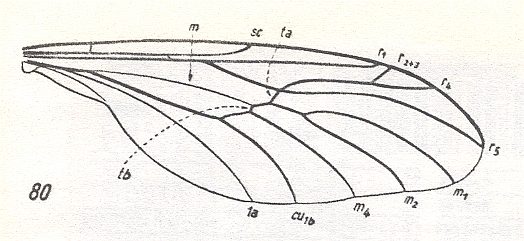
80 -- Wing of Pachyneura fasciata ZETT.
Interpretation of wing-venation according to HENNIG, 1954.
(From HENNIG, 1954, after DUDA, 1930.)
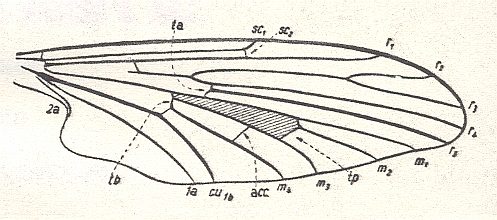
Wing of Protoplasa fitchii O.S. ( Tanyderidae).
Interpretation of wing-venation according to HENNIG, 1954. The venation of Protoplasa could be close to the original venation of the wings of the first Diptera.
(After HENNIG, 1954.)
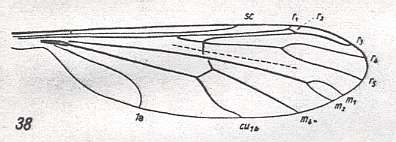
Wing of Liriope scutellaris MEIG. ( Liriopeidae [ = Ptychopteridae] ).
Interpretation of wing-venation according to HENNIG, 1954.
(After HENNIG, 1954.)
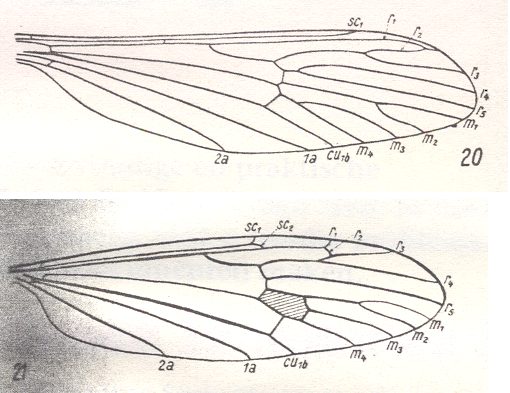
20 -- Wing of Tricyphona protea ALEX. ( Limoniidae). (From HENNIG, 1954, after ALEXANDER, 1927).
21 -- Wing of Limnophila ferruginea MEIG. ( Limoniidae). (After HENNIG, 1954.)
Interpretation of wing-venation according to HENNIG, 1954.
Wing of Nemopalpus zelandiae ALEX. ( Liriopeidae [ = Ptychopteridae] ). [according to ROHDENDORF Nemopalpus belongs to the family Nemopalpidae].
Interpretation of wing-venation according to HENNIG, 1954.
(From HENNIG, 1954, after ALEXANDER, 1927.)
Dimensions of the wings.
Shape of the wings.
The wings are always elongated -- their width three times, sometimes even four times smaller than their length. The anterior wing-edge is straight almost over two thirds or even three fourths of the [anterior] edge, rarely only over its half (Pachyneuridae). After this the edge starts to bend and to form the wing tip, its apex. The basiala is not clearly individualized [that is, not clearly distinguished from the wing-blade], and consists of a more or less expressed constriction. Transverse anastomoses and phragma as a rule not expressed. Sometimes there are only transverse veins [within the confines of the supposedly basiala] ( Petauristidae [ = Trichoceridae], see next Figure).
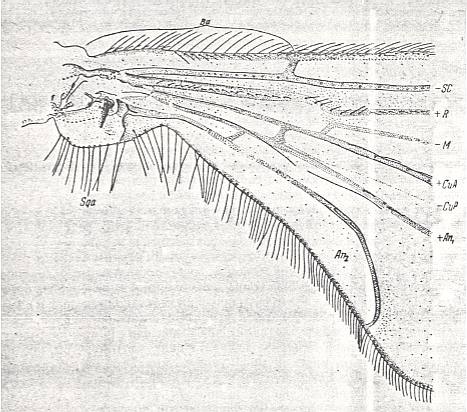
Petaurista maculipennis MEIG. ( Petauristidae [ = Trichoceridae] ).
Base of right wing of male. Topview.
Specimen Nr.2534. Length of wing 7.35 mm. Length of part starting from the level of the base of the costal vein to that of the humeral cross-vein [the cross-vein between Costa and Subcosta], supposedly delineating the basiala, 0.94 mm.
For the abbreviations, see Figure 1a above . The brace (in upper part of figure) with the sign Ba indicates the extent of the supposed basiala.
(After ROHDENDORF, 1951)
Sometimes, in the most specialized forms ( Tipulidae), the basiala is transformed into a complex tube-like construction. Rarely there is a germ [or rudiment] of a phragma ( Liriopeidae [ = Ptychopteridae], see next Figure).
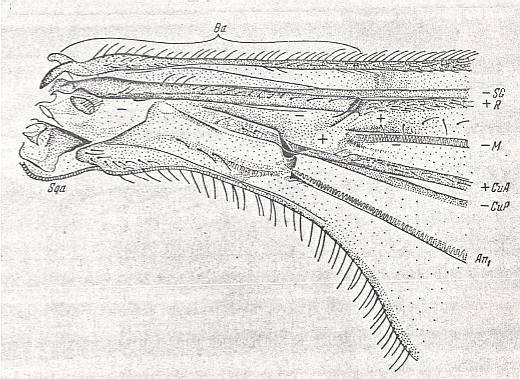
Liriope contaminata MEIG. ( Liriopeidae [ = Ptychopteridae] ).
Base of right wing of male. Topview.
Specimen Nr.3684. Length of wing 7.50 mm. Length of part starting from the level of the base of the costal vein to that of the humeral cross-vein [the cross-vein between Costa and Subcosta], supposedly delineating the basiala, 1.35 mm.
For the abbreviations, see Figure 1a above . The brace (in upper part of figure) with the sign Ba indicates the extent of the supposed basiala.
(After ROHDENDORF, 1951)
The anal lobe is weakly expressed. The alula and thoracic scale are always absent. There is a germ of the wing-scale in the form of a projection of the hind margin of the basiala.
Skeleton of the wing.
See Figures above [ It should be clear that not all features mentioned in what follows can be expected to be found in these Figures. This is because there is a lot of variation, even within a single type of wing].
The wing-venation is rich, very little reduced, and [as such] comparable with other Mecopteroidea [containing the order Diptera, Trichoptera (caddis flies), Lepidoptera (butterflies and moths), Mecoptera (scorpionflies), and one or more fossil orders], especially with certain Paratrichoptera [a fossil Mecopteroidean order]. Costal, Subcostal, and Radial veins always run parallel, having become more or less near to each other, and extending up to the place of curvature of the anterior edge, that is, up to where the apex of the wing starts. It is only this feature that guarantees [a degree of] costalization of the primitive traction (tipuloid) wings. It [that is, costalization] is almost not expressed by the rest of the wing's structural features (apart from the presence [where indeed it is] of the anal lobe ! ). [Costalization of the wings can be realized in different ways. Generally we can say that costalization means that the wing-skeleton concentrates on the anterior half of the wing. It has either shifted towards that half (and from there, often towards the wing-base), or the posterior half of the venation is partly reduced or weakened and/or the veins of the anterior half have thickened. So the wing-blade has, in the case of costalization, qualitatively differentiated into an anterior and posterior half. It is clear then that the development of a large anal lobe, totally or almost devoid of (strong) veins, contributes to this differentiation and thus to the wing's costalization. The same goes for the development of a fringe of long hairs along the hind margin of the wing.]
The structure of the venation of the wing-blade shows almost no sign of whatever [form of] displacement of veins [meant is here shifting of veins toward the wing's fore margin], which generally run parallel to each other and end up at the apical part of the hind margin. Only the cubital and anal veins [will sometimes] fan-like move away from each other (See Figure above ). The general number of branches of the radial system (up to 5) and of the medial system (up to 4) is the greatest of all wing types. There are cross-veins such as the basic ones, namely the radio-medial cross-vein (rm) and the medio-cubital cross-vein (mcu), as well as extra intermedial cross-veins, which determine the presence of closed cells. The costal vein runs along the whole wing margin, it goes around the whole wing including the anal lobe. Only sometimes the wing's hind margin is devoid of the costal vein, so at the Pachyneuridae.
The venation of the basiala consists of not-individualized 'handles' of the main veins (See Figure 1f above ), sometimes coming very close together and moved over, one above the other, forming in this way a characteristically tube-like basiala ( Tipulidae). Wing membrane firm. Sometimes, in the most specialized forms ( Tipulidae), in which the wings are strongly elongated, a characteristic transformation of the venation took place, consisting in the shifting of the forks of the medial veins to the distal part of the wing. This process is peculiar and in essence widens [in the direction of the wing's longitudinal axis] the basal [in text : basialar] part of the wing, being provided with the parallel trunks of the veins, which usually occupy only a third of the wing [See Figure above ]. In Tipulidae, on the other hand, more than half of the wing [length] is occupied by those veins [See Figure above ]. The mechanical significance of this process is still not completely clear. Probably a sharp individualization of the apex of the wing is taking place here, which apex is able to swing relative to the rest of the wing membrane. The swing axis lies along the line of forks of the medial and radial veins.
Coverings of the wing.
The wings are completely covered by [a] dense [layer of] very short hairs (microtrichia). Spinelets are sometimes developed on the main longitudinal veins and on certain cross-veins (the medio-cubital), while being absent on the wing membrane. Scales on the wings [like we see them in Culicidae] are absent.
The nerve system of the wing is studied only in one species of Tipulidae and consists of two main nerve branchings, many times splitting up and sending nerves along the main veins of the basiala and of the front part of the wing (costal, subcostal, radial, and, partly, medial, veins). These nerves consist of neurons, nerve cells, being placed below the base of hairs and spinelets. In addition, in certain parts of the wing (at the end of the anterior edge before the apex, and at the boundary of the basiala and the wing-blade, and especially at the base of the basiala on its [part of the] subcostal and radial veins) there are accumulations of sense organs, sensoria, which are densely innervated [that is, in which many nerve endings can be observed]. The hind part of the wing, the greater part of the cubital and anal veins, including the anal region of the wing, are devoid of nerves.
Functional characteristic.
The representatives of the primitive traction (tipuloid) type have a rather slow flight, which has no relation with getting food (hunting for prey, the sucking of nectar in flight [that is, sucking nectar from a flower while hovering above it], etc.). The flight usually is rectilinear, rather ponderous. Sometimes these insects fly while alternately going up and down, that is, performing so-called "dances" connected with sexual behavior (for example the winter-gnats of the genus Petaurista [ Petauristidae [ = Trichoceridae] ] and others). The wing-beat frequency is known only of one species of Tipulidae and equal to 44-73 beats per second. It seems to me that these numbers are even a bit too high, and that in reality insects with primitive traction (tipuloid) wings perform flight in which the wing-beat frequency is lower. The little biological significance of the flight function for the representatives of this type, among other things, is made evident by existing cases of reduction of the flight-ability, the existence of short-winged or even wingless species, which are clear derivatives of forms that had wings of the primitive traction (tipuloid) type.
History of the type and its transformations.
Primitive traction wings were possessed by representatives of the most ancient diptera which were discovered in already lower- and middlejurassic sediments. Rohdendorf adds as a note :
The by Tillyard described at the time as being a dipterous insect Permotipula TILL. from permian sediments in Australia belongs to a peculiar family of the closely related [to the order Diptera] mecopteriod order Paratrichoptera, in whose representatives, just like in Diptera, occurred processes of reduction of the wing-venation.
Such were the peculiar Eoptychopteridae, Eolimnobiidae, Tanyderophryneidae and the closest relatives of the recent Tipulidae, the Architipulidae. In [geologically] recent times this type contains comparatively different forms from many families, having in common a rich venation and a weakly individualized basiala. The [historical] differentiation of this type consisted in the separation of different forms of wings which are characterized by the development of a peculiar strengthening of the base of the wing, without, however, having worked out an individualized basiala. The determining processes leading to the formation of the individual subtypes of the tipuloid wings consisted in the increase of the size of the body and in a moderate refinement of flight in the direction of increase of tractional force which expressed itself in the elongation of the wings [as we se it in the most specialized members of the type, the Tipulidae]. We are still not in a position to present a complete overview of all the forms of tipuloid wings and can only distinguish the most conspicuous subtypes :
1. Conditionally, we must set, as the first subtype of (primitive traction) tipuloid wings the original [ancestral] form of the dipterous wing, which undoubtedly was most closely related to precisely the primitive traction (tipuloid) wings. This subtype is purely hypothetical, not illustrated by whatever concrete examples.
2. A special subtype of the primitive traction wings, which branched off from the original wing-forms of Diptera is represented by architipuloid wings. This subtype is characterized by a moderate increase of size, by an elongation of the wings, and with it in the wing-venation a shift of cross-veins and of forks of the medial and radial veins in the direction of the wing apex was developed. The greater part of the basal half of the wing is occupied only by the straight [rectilinear] parts of the longitudinal veins. The basiala does not undergo mechanical refinement. In it there have only taken place some reductions and mutual approaches of the basal parts of veins.
To this subtype belong many species of the jurassic family Architipulidae (see HERE for a figure), Tanyderophryneidae (see HERE for a figure), tertiary and recent Limoniidae (See Figure ABOVE ), Cylindrotomidae, Tanyderidae (See Figure ABOVE ), and Nemopalpidae (See Figure ABOVE ).
3. The tubular subtype of the primitive traction type of wings, which is a direct derivative of the previous subtype, is represented by wings of tertiary and recent Tipulidae (See Figure ABOVE ), which are characterized by an increase in size, a shift of forks and cross-veins in the direction of the apical part of the wing, and especially, a strong narrowing of the wing-base, in which the basal parts of the veins closely approach each other and partly one covering the other, and in this way forming a characteristic tubular structure.
This tubular subtype corresponds to the form of flight apparatus of insects earlier (1949) called by me paddle-shaped two-wingedness (copedipterygia) and undoubtedly is a very peculiar specialization of wings of large dipterous insects, which show certain analogies as regards the wing-beat mechanics with some other insects (the scorpionflies of the family Bittacidae and dragonflies of the family Coenagrionidae).
4. Another derivative of the architipuloid subtype were wings of the jurassic Eolimnobiidae and tertiary and recent Liriopeidae [ = Ptychopteridae] in which the process of shift of forks of medial veins and the shift of cross-veins towards the apex of the wing went slowly. At the same time in the basal [i.e. proximal] part of the wing several refinements of the venation took place and the presence of a phragma could be observed (that is, the unification by means of a transverse vein or fold, of the bases of the radial and cubital veins, see Figure above ).
This subtype, which can be called liriopeoid, in addition is characterized by the increase of size, not, however, becoming especially large.
5. From the original dipterous wings (the conditional first subtype of tipuloid wings), in addition to the wings of the architipuloid subtype, undoubtedly forms of wings branched off which were characterized by a refinement of the wing-beat, that is, the increase of its beat frequency, whereby the general size did not increase and the wings were not lengthened. This subtype, which can be called eoptychopteroid, is in fact only little known, but it is very interesting, because it appeared as [evolutionary] source for all other forms of dipterous wings.
The only known representatives of this subtype are [the] members of the family Eoptychopteridae, whose rare species are found in jurassic sediments. Most characteristic for this subtype is the absence of the shift of radial and medial forks towards the apex of the wing. The eoptychpteroid subtype formed the basis not only of the other types, but also the source of a special form of the primitive traction (tipuloid) wings, which we will now discuss.
6. The special diptera of the family Pachyneuridae, which in the recent fauna exists as a relict group, are characterized by clearly expressed processes of reduction of the wing-venation, which processes have the character of costalization [processes], namely in this particular case of the lightening of the posterior half of the wing [with "lightening" is here meant : making (the posterior half) free of (strong) veins]. See Figure above [In a figure of the wing, like this one, we must realize that in drawing it the emphasis was layed on the pattern of the wing-venation, not on possible differences of strength and thickness of the veins]. However, the structure of the wing-base (see Figure 1f, above ) shows not only the absence of an individualized basiala, but also the absence of any partial changes whatsoever of the proximal parts of the veins, which remain very similar to those of other representatives of the tipuloid type, to which these wings almost undoubtedly belong. They must be distinguished as a separate specialized, pachyneuroid, subtype. This subtype may functionally be characterized as a first "attempt" to work out costalized wings on the original still little refined basis of the tipuloid type.
This concludes our exposition of the character, content, and history of the superfamily Tipulidea, the first superfamily of the dipterous Infraorder Tipulomorpha.
In the next document we will expound the character, content, and history of the second superfamily, that is of the Chironomidea.
Will be supplemented if necessary . . .
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part III.
Back to Aristotelian metaphysics Part I
Back to Aristotelian metaphysics Part II
Back to Aristotelian metaphysics Part III
Back to Aristotelian metaphysics Part IIIa
Back to Aristotelian metaphysics Part IV
Back to Aristotelian metaphysics Part V