

We have now arrived at the true ants. They can be considered to form one single large hymenopterous family, the Formicidae. Thousands of species have been described. The following subfamilies were distinguished at the end of the 1960's :
Ponerinae, [some] genera : Termitopone, Megaponera, Odontomachus, Leptogenys (a brand of driver ants), Hypoponera, Stigmatomma, Ponera.
Myrmeciinae, bulldog ants, genus : Myrmecia.
Myrmicinae, node ants, [some] genera : Myrmica, Myrmicaria, Solenopsis (thief ants), Carebara, Strongylognathus, Epimyrma, Crematogaster, Anergates (socially parasitic ants), Pheidole, Messor (grain ants), Pogonomyrmex (harvest ants), Atta (leaf-cutter ants).
Formicinae, scale ants, [some] genera : Formica (forest ants), Raptiformica, Serviformica, Polyergus (obligatory slave-making ants ['amazones'], Lasius (pasture ants), Acropyga, Camponotus, Colobopsis, Oecophylla (weaving ants), Myrmecocystus (desert ants), Proformica.
Dolichoderinae, gland ants [some] genera : Azteca, Leptomyrmex, Iridomyrmex.
Pseudomyrminae (Pseudomyrmecinae),
Dorylinae, army ants (driver and legionary ants), [some] genera : Eciton, Anomma, Dorylus.
Cerapachyinae, also army ants.
Leptanillinae, also army ants.
I expect that still more subfamilies have been described since. We'll meet them when consulting more recent literature.
Before proceeding with the true ants (following MALYSHEV, 1966), let us produce some illustrations of them :
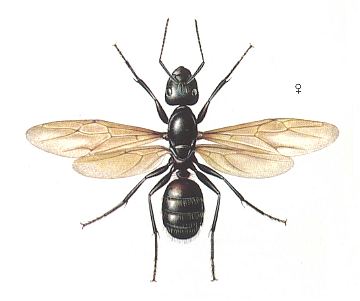
Figure 1 : Camponotus ligniperda, Family Formicidae, Ants, Subfamily Formicinae, Suborder Aculeata. Queen. 7 - 14 mm.
(After SEVERA, F., in ZAHRADNIK, J., Thieme's insektengids voor West- en Midden-Europa, 1977)
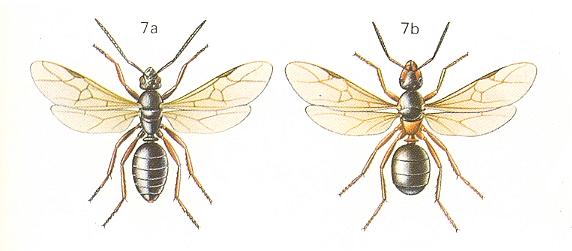
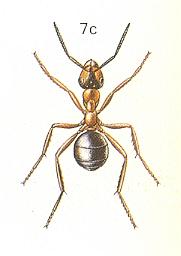
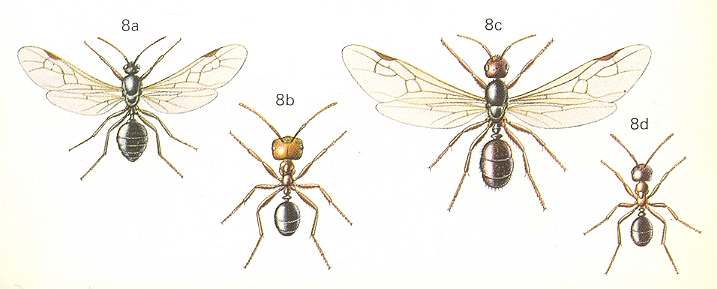
Figure 3 : Messor barbara L., Family Formicidae, Ants, subfamily Myrmicinae, Suborder Aculeata.
8a - male. 8b - soldier. 8c - queen. 8d - worker.
(After CHINERY, M., in Elseviers insektengids voor West-Europa, 1983)
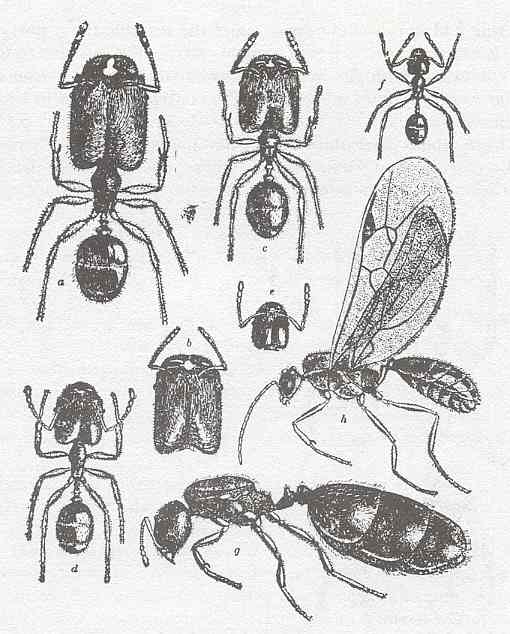
Figure 4 : Pheidole tepicana (= instabilis), Family Formicidae, Ants, subfamily Myrmicinae, Suborder Aculeata.
a - soldier. b-e - intermediate workers. f - typical worker (micrergate). g - deälated female. h - male.
(After WHEELER, 1910, in RICHARDS and DAVIES, Imms' General Textbook of Entomology, 1977)
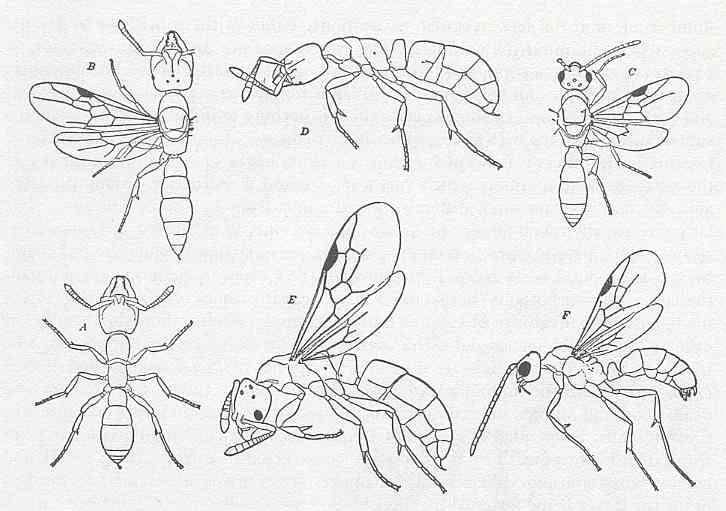
Figure 5 : Ponerine ants. A, B, and C, worker, female, and male, of Stigmatomma pallipes. D, E, F, worker, female, and male, of Ponera coarctata pennsylvanica. Family Formicidae, Ants, Subfamily Ponerinae, Suborder Aculeata.
(After WHEELER, 1910, in RICHARDS and DAVIES, Imms' General Textbook of Entomology, 1977)

Figure 6 :
Left : Dinoponera grandis. Family Formicidae, Ants, Subfamily Ponerinae, Suborder Aculeata.
Middle : Worker of the army ant Eciton vagans. Family Formicidae, Ants, Subfamily Dorylinae, Suborder Aculeata.
Right : Strongylognathus testaceus, (social parasite). Family Formicidae, Ants, Subfamily Myrmicinae, Suborder Aculeata.
(From Grzimeks Tierleben, zweiter Band, Insekten, 1969)
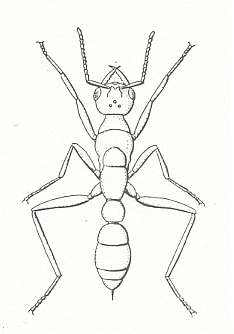
Figure 7 : A bulldog ant of the genus Myrmecia. Family Formicidae, Ants, Subfamily Myrmeciinae, Suborder Aculeata.
(From Grzimeks Tierleben, zweiter Band, Insekten, 1969)
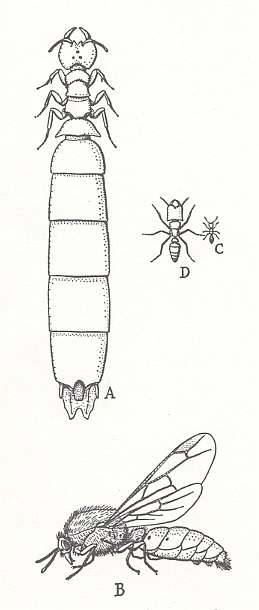
Figure 8 : The castes of the army ant of the genus Dorylus. Family Formicidae, Ants, Subfamily Dorylinae, Suborder Aculeata.
A - female, B - male, C - worker, D - soldier.
(From Grzimeks Tierleben, zweiter Band, Insekten, 1969)
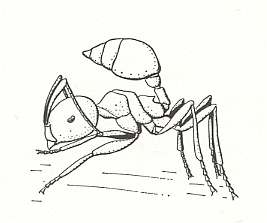
Figure 9 : When an ant of the genus Crematogaster is disturbed, it lifts its abdomen. Family Formicidae, Ants, Subfamily Myrmicinae, Suborder Aculeata.
(From Grzimeks Tierleben, zweiter Band, Insekten, 1969)
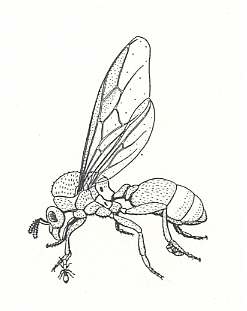
Figure 10 : A female ant of the genus Carebara vidua carries on her legs the minute workers with her in flight. Family Formicidae, Ants, Subfamily Myrmicinae, Suborder Aculeata.
(From Grzimeks Tierleben, zweiter Band, Insekten, 1969)
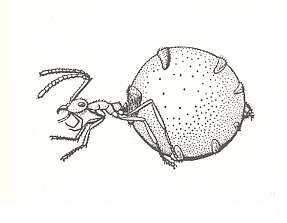
Figure 11 : A storage individual ("honey pot") of the genus Myrmecocystus. Family Formicidae, Ants, Subfamily Formicinae, Suborder Aculeata.
(From Grzimeks Tierleben, zweiter Band, Insekten, 1969)

Fuga formicalis
Again, before we continue with MALYSHEV's Phases of hymenopterous evolution (the final Phase of ant evolution), the "true ants" so to say, it is instructive to supplement the above pictures of them with a general description of the social structure of the colonies of the higher of these true ants. The important fact is that such a colony, community, or state, possesses a number of castes, that are morphologically, physiologically, and behaviorally different from each other (queen(s), males, several types of workers, and soldiers). Especially their distribution, that is, the (spatial) caste distribution, in the colony at any moment of its existence is significant, reflecting the state, that is the condition, of the colony as a whole. The second important fact is that the fundamental aspect of the colony, holding it together, is the mutual communication (and recognition of fellow members) between the individuals of a given colony, a communication that is, by means of highly specific chemical signals, by the transfer of food, or even, in many species, auditory signals. The third important fact is that such a colony is a (non-materially) layered entity, that is, we can distinguish in it a hierarchy of levels. The lowest level is that of the individuals themselves, that is, the individual ants. Then we have a number of emerging and/or disintegrating (again) "teams" in which the individuals together perform a certain task needed at the moment. And so we can go up (a few more levels) until we have reached the highest level, namely that of the complete colony seen as one single and functional whole.
This third fact, the hierarchy of levels in a given ant colony, precisely reflects the overall structure of it, and it was described by the mathematician-physicist-logician-computer scientist Douglas R. HOFSTADTER in 1979 (basing himself, of course, on accounts of myrmecologists [ant professionals] such as E.O. WILSON) in his superb book "GÖDEL, ESCHER, BACH, AN ETERNAL GOLDEN BRAID", on the fundaments of mathematics and logic, the function of language, and the meaning of intelligence, against the background of Gödels's Theorem, the art of Escher, and the music of Bach. In order to figure out what (human) consciousness and knowledge in fact are and what role is placed by their "hardware", the brain, HOFSTADTER compares the brain (and its functions and functioning) with an ant colony. In the brain we have a great many interacting neurons (brain cells) acting more or less together in one or another thinking process, that is, their combined actions make up the thinking process, without each individual neuron itself being able to think. The thinking process only emerges higher up in the hierachy of levels in the brain. So it is natural that neurons in the brain are compared with ants in an ant colony, and the brain as a whole with the ant colony as a whole. HOFSTADTER does this by way of a "quadrilogue" (or "tettilogue" to stick with the Greek) between four characters, the Tortoise, the Crab, the Ant-eater, and Achilles. [We may legitimately also call this a "dialogue", where "dia" means, not two, but "through", that is, a verbal exchange through a given group of participants]. The tettilogue is called Prelude . . . Ant Fugue (Chapter X), and in the part ". . . Ant Fugue" we find the description of the hierarchy in an ant colony (p.314 - 322 of the 20th-anniversary Edition, 2000).
Let us reproduce it here [where all the credit for this magnificent tettilogue goes, of course to Hofstadter!] (The four friends discuss things while listening to one of Bach's compositions, a fugue. They discuss whether an ant colony, an ant hill, and then also the brain, should be explained reductionistically, that is, explaining it from its lowest level, its ultimate constituents, or holistically, that is, from its higher levels. And, with respect to ants, Dr. Anteater is a specialist of course, so he has much to tell) :
.
Achilles : Well, I can vaguely see how it might be possible for a limited and regulated amount of ant consumption to improve the overall health of a colony -- but what is far more perplexing is all this talk about having conversations with ant colonies. That's impossible. An ant colony is simply a bunch of individual ants running around at random looking for food and making a nest.
Anteater : You could put it that way if you want to insist on seeing the trees but missing the forest, Achilles. In fact, ant colonies, seen as wholes, are quite well-defined units, with their own qualities, at times including the mastery of language.
Achilles : I find it hard to imagine myself shouting something out loud in the middle of the forest, and hearing an ant colony answer back.
Anteater : Silly fellow! That's not the way it happens. Ant colonies don't converse out loud, but in writing. You know how ants form trails leading them hither and thither?
Achilles : Oh, yes -- usually straight through the kitchen sink and into my peach jam.
Anteater : Actually, some trails contain information in coded form. If you know the system, you can read what they're saying just like a book.
Achilles : Remarkable. And you can communicate back to them?
Anteater : Without any trouble at all. That's how Aunt Hillary
Achilles : There must be some amazingly smart ants in that colony, I'll say that.
Anteater : I think you are still having some difficulty realizing the difference in levels here. Just as you would never confuse an individual tree with a forest, so here you must not take an ant for the colony. You see, all the ants in Aunt Hillary are as dumb as can be. They couldn't converse to save their little thoraxes!
Achilles : Well then, where does the ability to converse come from? It must reside somewhere inside the colony! I don't understand how the ants can all be unintelligent, if Aunt Hillary can entertain you for hours with witty banter.
Tortoise : It seems to me that the situation is not unlike the composition of a human brain out of neurons. Certainly no one would insist that individual brain cells have to be intelligent beings on their own, in order to explain the fact that a person can have an intelligent conversation.
Achilles : Oh, no, clearly not. With brain cells, I see your point completely. Only . . . ants are a horse of another color. I mean, ants just roam about at will, completely randomly, chancing now and then upon a morsel of food . . . They are free to do what they want to do, and with that freedom, I don't see at all how their behavior, seen as a whole, can amount to anything coherent -- especially something so coherent as the brain behavior necessary for conversing.
Crab : It seems to me that the ants are free only within certain constraints. For example, they are free to wander, to brush against each other, to pick up small items, to work on trails, and so on. But they never step out of that small world, that ant-system, which they are in. It would never occur to them, for they don't have the mentality to imagine anything of the kind. Thus the ants are very reliable components, in the sense that you can depend on them to perform certain kinds of tasks in certain ways.
Achilles : But even so, within those limits they are still free, and they just act at random, running about incoherently without any regard for the thought mechanisms of a higher-level being which Dr. Anteater asserts they are merely components of.
Anteater : Ah, but you fail to recognize one thing, Achilles -- the regularity of statistics.
Achilles : How is that?
Anteater : For example, even though ants as individuals wander about in what seems a random way, there are nevertheless overall trends, involving large numbers of ants, which can emerge from that chaos.
Achilles : Oh, I know what you mean. In fact, ant trails are a perfect example of such a phenomenon. There, you have really quite unpredictable motion on the part of any single ant -- and yet, the trail itself seems to remain well-defined and stable. Certainly that must mean that the individual ants are not just running about totally at random.
Anteater : Exactly Achilles. There is some degree of communication among the ants, just enough to keep them from wandering off completely at random. By this minimal communication they can remind each other that they are not alone but are cooperating with teammates. It takes a large number of ants, all reinforcing each other this way, to sustain any activity -- such as trail-building -- for any length of time. Now my very hazy understanding of the operation of brains leads me to believe that something similar pertains to the firing of neurons. Isn't it true Mr. Crab, that it takes a group of neurons firing in order to make another neuron fire?
Crab : Definitively. Take the neurons of Achilles' brain, for example. Each neuron receives signals from neurons attached to its input lines, and if the sum total of inputs at any moment exceeds a critical threshold, then that neuron will fire and send its own output pulse rushing off to other neurons, which may in turn fire -- and on down the line it goes. The neural flash swoops relentlessly in its Achillean path, in shapes stranger than the dash of a gnat-hungry swallow. Every twist, every turn foreordained by the neural structure in Achilles' brain, until sensory input messages interfere.
Achilles : Normally, I think that I'M in control of what I think -- but the way you put it turns it all inside out, so that it sounds as though " I " am just what comes out of all this neural structure, and natural law. It makes what I consider my SELF sound at best like a by-product of an organism governed by natural law, and at worst, an artificial notion produced by my distorted perspective. In other words, you make me feel like I don't know who -- or what -- I am, if anything.
Tortoise : You'll come to understand much better as we go along. But Dr. Anteater -- what do you make of this similarity?
Anteater : I knew that there was something parallel going on in the two very different systems. Now I understand it much better. It seems that group phenomena which have coherence -- trail-building, for example -- will take place only when a certain threshold number of ants get involved. If an effort is initiated, perhaps at radom, by a few ants in some locale, one of two things can happen : either it will fizzle out after a brief sputtering start --
Achilles : When there aren't enough ants to keep the thing rolling?
Anteater : Exactly. The other thing that can happen is that a critical mass of ants is present, and the thing will snowball, bringing more and more ants into the picture. In the latter case, a whole "team" is brought into being which works on a single project. That project might be trail-making, or food-gathering, or it might involve nest-keeping. Despite the extreme simplicity of this scheme on a small scale, it can give rise to very complex consequences on a larger scale.
Achilles : I can grasp the general idea of order emerging from chaos, as you sketch it, but that still is a long way from the ability to converse. After all, order also emerges from chaos when molecules of a gas bounce against each other randomly -- yet all that results there is an amorphous mass with but three parameters to characterize it : volume, pressure, and temperature. Now that's a far cry from the ability to understand the world, or to talk about it!
Anteater : That highlights a very interesting difference between the explanation of the behavior of an ant colony and the explanation of the behavior of gas inside a container. One can explain the behavior of the gas simply by calculating the statistical properties of the motions of its molecules. There is no need to discuss any higher elements of structure than molecules, except the full gas itself. On the other hand, in an ant colony, you can't even begin to understand the activities of the colony unless you go through several layers of structure.
Achilles : I see what you mean. In a gas, one jump takes you from the lowest level -- molecules -- to the highest level -- the full gas. There are no intermediate levels of organization. Now how do intermediate levels of organized activity arise in an ant colony?
Anteater : It has to do with the existence of several different varieties of ants inside any colony.
Achilles : Oh, yes. I think I have heard about that. They are called "castes", aren't they?
Anteater : That's correct. Aside from the queen, there are males, who do practically nothing towards the upkeep of the nest --
Achilles : And of course there are soldiers -- Glorious Fighters Against Communism!
Crab : Hmm . . . I hardly think that could be right, Achilles. An ant colony is quite communistic internally, so why would its soldiers fight against communism? Or am I right, Dr. Anteater?
Anteater : Yes, about colonies you are right, Mr. Crab. They are indeed based on somewhat communistic principles. But about soldiers Achilles is somewhat naïve. In fact, the so-called "soldiers" are hardly adept at fighting at all. They are slow, ungainly ants with giant heads, who can snap with their strong jaws, but are hardly to be glorified. As in a true communistic state, it is rather the workers who are to be glorified. It is they that do most of the chores, such as food-gathering, hunting, and nursing of the young. It is even they who do most of the fighting.
Achilles : Bah. That is an absurd state of affairs. Soldiers who won't fight!
Anteater : Well, as I just said, they really aren't soldiers at all. It's the workers who are soldiers. The soldiers are just lazy fatheads.
Achilles : Oh, how disgraceful! Why, if I were an ant, I'd put some discipline in their ranks! I'd knock some sense into those fatheads!
Tortoise : If you were an ant? How could you be an ant? There is no way to map your brain onto an ant brain, so it seems to me to be a pretty fruitless question to worry over. More reasonable would be the proposition of mapping your brain onto an ant colony . . . But let us not get sidetracked. Let Dr. Anteater continue with his most illuminating description of castes and their role in the higher levels of organization.
Anteater : Very well. There are all sorts of tasks which must be accomplished in a colony, and individual ants develop specializations. Usually an ant's specialization changes as the ant ages. And of course it is also dependent on the ant's caste. At any one moment, in any small area of a colony, there are ants of all types present. Of course, one caste may be very sparse in some places and very dense in others.
Crab : Is the density of a given caste, or specialization, just a random thing? Or is there a reason why ants of one type might be more heavily concentrated in certain areas, and less heavily in others?
Anteater : I'm glad you brought that up, since it is of crucial importance in understanding how a colony thinks. In fact, there evolves, over a long period of time, a very delicate distribution of castes inside a colony. And it is this distribution which allows the colony to have the complexity which underlies the ability to converse with me.
Achilles : It would seem to me that the constant motion of ants to and fro would completely prevent the possibiliy of a very delicate distribution. Any delicate distribution would be quickly destroyed by all the random motions of ants, just as any delicate pattern among molecules in a gas would not survive for an instant, due to the random bombardment from all sides.
Anteater : In an ant colony, the situation is quite the contrary. In fact, it is just exactly the constant to-ing and fro-ing of ants inside the colony which adapts the caste distribution to varying situations, and thereby preserves the delicate caste distribution. You see, the caste distribution cannot remain as one single rigid pattern. Rather, it must constantly be changing so as to reflect, in some manner, the real-world situation with which the colony is dealing, and it is precisely the motion inside the colony which updates the caste distribution, so as to keep it in line with the present circumstances facing the colony.
Tortoise : Could you give an example?
Anteater : Gladly. When I, an ant eater, arrive to pay a visit to Aunt Hillary, all the foolish ants, upon sniffing my odor, go into panic -- which means, of course, that they begin running around completely differently from the way they were before I arrived.
Achilles : But that's understandable, since you're a dreaded enemy of the colony.
Anteater : Oh no. I must reiterate that, far from being an enemy of the colony, I am Aunt Hillary's favorite companion. And Aunt Hillary is my favorite aunt. I grant you, I'm quite feared by all the individual ants in the colony -- but that's another matter entirely. In any case, you see that the ants' action in response to my arrival completely changes the internal distribution of ants.
Achilles : That's clear.
Anteater : And that sort of thing is the updating which I spoke of. The new distribution reflects my presence. One can describe the change from old state to new as having added a "piece of knowledge" to the colony.
Achilles : How can you refer to the distribution of differrent types of ants inside a colony as a "piece of knowledge"?
Anteater : Now there's a vital point. It requires some elaboration. You see, what it comes down to is how you choose to describe the caste distribution. If you continue to think in terms of the lower levels -- individual ants -- then you miss the forest for the trees. That's just too microscopic a level, and when you think microscopically, you're bound to miss some large-scale features. You've got to find the proper high-level framework in which to describe the caste distribution -- only then will it make sense how the caste distribution can encode many pieces of knowledge.
Achilles : Well, how DO you find the proper-sized units in which to describe the present state of the colony, then?
Anteater : Allright. Let's begin at the bottom. When ants need to get something done, they form little "teams", which stick together to perform a chore. As I mentioned earlier, small groups of ants are constantly forming and unforming. Those which actually exist for a while are the teams, and the reason they don't fall apart is that there really is something for them to do.
Achilles : Earlier you said that a group will stick together if its size exceeds a certain threshold. Now you're saying that a group will stick together if there is something for it to do.
Anteater : They are equivalent statements. For instance, in food-gathering, if there is an inconsequential amount of food somewhere which gets discovered by some wandering ant who then attempts to communicate its enthusiasm to other ants, the number of ants who respond will be proportional to the size of the food sample -- and an inconsequential amount will not attract enough ants to surpass the threshold. Which is exactly what I meant by saying there is nothing to do -- too little food ought to be ignored.
Achilles : I see. I assume that these "teams" are one of the levels of structure falling somewhere in between the single-ant level and the colony level.
Anteater : Precisely. There exists a special kind of team, which I call a "signal" -- and all the higher levels of structure are based on signals. In fact, all the higher entities are collections of signals acting in concert. There are teams on higher levels whose members are not ants, but teams on lower levels. Eventually you reach the lowest-level teams -- which is to say, signals -- and below them, ants.
Achilles : Why do signals deserve their suggestive names?
Anteater : It comes from their function. The effect of signals is to transport ants of various specializations to appropriate parts of the colony. So the typical story of a signal is thus : it comes into existence by exceeding the threshold needed for survival, then it migrates for some distance through the colony, and at some point it more or less disintegrates into its individual members, leaving them on their own.
Achilles : It sounds like a wave, carrying sand dollars and seaweed from afar, and leaving them strewn, high and dry, on the shore.
Anteater : In a way that's analogous, since the team does indeed deposit something which it had carried from a distance, but whereas the water in the wave rolls back to the sea, there is no analogous carrier substance in the case of a signal, since the ants themselves compose it.
Tortoise : And I suppose that a signal loses its coherency just at some spot in the colony where ants of that type were needed in the first place.
Anteater : Naturally.
Achilles : Naturally? It's not so obvious to ME that a signal should always go just where it is needed. And even if it goes in the right direction, how does it figure out where to decompose? How does it know it has arrived?
Anteater : Those are extremely important matters, since they involve explaining the existence of purposeful behavior -- or what seems to be purposeful behavior -- on the part of signals. From the description, one would be inclined to characterize the signals' behavior as being oriented towards filling a need, and to call it "purposeful". But you can look at it otherwise.
Achilles : Oh, wait. Either the behavior IS purposeful, or it is NOT. I don't see how you can have it both ways.
Anteater : Let me explain my way of seeing things, and then see if you agree. Once a signal is formed, there is no awareness on its part that it should head off in any particular direction. But here, the delicate case distribution plays a crucial role. It is what determines the motion of signals through the colony, and also how long a signal will remain stable, and where it will "dissolve".
Achilles : So everything depends on the caste distribution, eh?
Anteater : Right. Let's say a signal is moving along. As it goes, the ants which compose it interact, either by direct contact or by exchange of scents, with ants of the local neighborhoods which it passes through. The contacts and scents provide information about local matters of urgency, such as nest-building, or nursing, or whatever. The signal will remain glued together as long as the local needs are different from what it can supply. But if it CAN contribute, it disintegrates, spilling a fresh team of usable ants onto the scene. Do you see now how the caste distribution acts as an overall guide of the teams inside the colony?
Achilles : I do see that.
Anteater : And do you see how this way of looking at things requires attributing no sense of purpose to the signal?
Achilles : I think so. Actually, I'm beginning to see things from two different vantage points. From an ant's-eye point of view, a signal has NO purpose. The typical ant in a signal is just meandering around the colony, in search of nothing in particular, until it finds that it feels like stopping. Its teammates usually agree, and at that moment the team unloads itself by crumbling apart, leaving just its members but none of its coherency. No planning is required, no looking ahead. Nor is any search required, to determine the proper direction. But from the COLONY's point of view, the team has just responded to a message which was written in the language of the caste distribution. Now from this perspective, it looks very much like purposeful activity.
Crab : What would happen if the caste distribution were entirely random? Would signals still band and disband?
Anteater : Certainly. But the colony would not last long, due to the meaninglessness of the caste distribution.
Crab : Precisely the point I wanted to make. Colonies survive because their caste distribution has meaning, and that meaning is a holistic aspect, invisible on lower levels. You lose explanatory power unless you take that higher level into account.
Anteater : I see your side. But I believe you see things too narrowly.
Crab : How so?
Anteater : Ant colonies have been subjected to the rigors of evolution for billions of years.
Crab : No, but --
Anteater : Now that's MY point. No matter how big a bubble is, its owes its existence to processes on the molecular level, and you can forget about any "higher-level laws". The same goes for ant colonies and their teams. By looking at things from the vast perspective of evolution, you can drain the whole colony of meaning and purpose. They become superfluous notions.
Achilles : Why, then, Dr. Anteater, did you tell me that you talked with Aunt Hillary? It now seems that you would deny that she can talk or think at all.
Anteater : I am not being inconsistent, Achilles. You see, I have as much difficulty as anyone else in seeing things on such a grandiose time scale, so I find it much easier to change points of view. When I do so, forgetting about evolution and seeing things in the here and now, the vocabulary of teleology comes back : the MEANING of the caste distribution and the PURPOSEFULNESS of signals. This not only happens when I think of ant colonies, but also when I think about my own brain and other brains. However, with some effort I can always remember the other point of view if necessary, and drain all these systems of meaning too.
Crab : Evolution certainly works some miracles. You never know the next trick it will pull out of its sleeve. For instance, it wouldn't surprise me one bit if it were theoretically possible for two or more "signals" to pass through each other, each one unaware that the other one is also a signal. Each one treating the other as if it were just part of the background population.
Anteater : It is better than theoretically possible. In fact it happens routinely!
Achilles : Hmm . . . What a strange image that conjures up in my mind. I can just imagine ants moving in four different directions, some black, some white, criss-crossing, together forming an orderly pattern, almost like -- like --
Tortoise : A fugue, perhaps?
Achilles : Yes -- that's it! An ant fuge!
.
.
Tortoise : Pardon me, my friends. I am sorry to have interrupted. Dr. Anteater was trying to explain how eating ants is perfectly consistent with being a friend of an ant colony.
[In fact we know of ants being present only from the Cretataceous onwards, that is, they, as ants (ant colonies) are subjected to evolution some 100 million years or so (not billions).]
A few mechanisms were selected for, and most were selected against. The end result was a set of mechanisms which make ant colonies work as we have been describing. If you could watch the whole process in a movie -- running a billion or so times faster than life, of course -- the emergence of various mechanisms would be seen as natural responses to external pressures, just as bubbles in boiling water are natural responses to an external heat source. I don't suppose you see "meaning" and "purpose" in the bubbles in boiling water -- or do you?
[Here it is assumed that evolution is a material, mechanical, process, a mechanism. Sociality in ants has originated and developed further by the process of evolution. So every feature in an ant colony (such as signals and higher teams) has, as a type or specialization, originated by a blind mechanical process, and consequently they lack, as to their nature, any purposefulness. However, it is not a priori evident that evolution is just a mechanical process, allegedly without a series of intermediate levels. It might very well be a process that is such that it allows for a hierarchy of levels to exist in it. So we can view, and describe, evolution from one of its higher levels too. This is certainly possible when we hold that evolution takes first of all place, not in the material order, but in some immaterial order, which we called (following David Bohm in this respect) the Implicate, or noëtic Order. If that is correct, then evolution is definitively not a mere mechanical process.]
.
.
.
( HOFSTADTER, in "GÖDEL, ESCHER, BACH", p. 314-322 (20th-anniversary edition, 2000)
As we can see in the above tettilogue, lots of elements of the actual behavior of ants in a colony depend on the present condition of that colony as a whole. And on the lowest structural level of that colony, that behavior is d i r e c t l y determined by physical or chemical contacts between ants of a given signal (= lowest-level team) on the one hand, and ants of the immediate surroundings through which the signal passes. And such contacts also determine whether a given signal will stop somewhere and disintegrate or whether it continues its course. And, of course, all this individual behavior (resulting from contacts and interactions between ants), and the overall behavior of the given signal (whereto it is heading) resulting from it, is not 'written down' in the strategy of the given ant species, that is, in the noëtic description as it resides in the Implicate Order. This is, because a noëtic description cannot contain individual circumstances, encounters, etc. So the complete behavior will unfold only upon projection of this noëtic description into the Explicate Order. And such a projection of a given (new) strategy, for the first time, can only take place when the appropriate ecological context exists (in the Explicate Order), and when, in addition, the immediate evolutionary precursor (pre-stage) of this (new) strategy exists in that ecological context or directly adjacent to it. And having once been projected, the alternation of injection, reprojection, and reinjection continues as long as the ecological context remains such that it can keep on to receive the (new) strategy. And thus this (new) strategy cannot appear in the Explicate Order where ecological conditions are different, that is, not compatible with it. But it can also not appear there where the ecological conditions are appropriate but where no evolutionary precursor exists. This accords with the fact that on many islands several animal or plant species, although already existing elsewhere, do not exist on these islands despite the fact that these islands' ecological make up is fully compatible with the corresponding strategies of these species. Of course such a strategy, such a species, may appear on such an island when it has actually migrated to it, or blown to it by the wind, or carried by other things or organisms to it. The existence of such species there is then not a result of projection, but of actually having come there or being transported to it.
As has been said earlier, a given "strategy" in the Implicate Order, is a description (in fact a long string of symbols) that prescibes how this very description itself, being in the Implicate Order a mere immaterial Form, can exist, in the form of (similar) individual material enties, in the Explicate Order, and so obtaining ontological completeness.
We can express this same state of affairs also in different terms : Upon projection, a given noëtic strategy acquires meaning, that is, the (appropriate) ecological context (in the Explicate Order) decyphers (or decodes) the noëtic "message" coming from the Implicate Order. We can also say that the strategy is being "interpreted" by the ecological context, and therefore has obtained meaning. The result is that there is an isomorphism between the noëtic description and the corresponding part of 'reality' (= the Explicate Order). Here this "corresponding part of reality" is that particular part of the Explicate Order that qualitatively, and only qualitatively, corresponds to the given noëtic strategy ( = the noëtic description of such a strategy) that is projected into the Explicate Order. All local, temporal, and individual aspects of the strategy as we find the latter in the Explicate Order, are matters of this Order alone. They are consequences of the unfolding, along space and time dimensions, of an initially purely noëtic, that is, immaterial, pattern. Also the consequence of that unfolding is the fact that the single strategy is now represented by "individuals" physically separated from each other. In the collection of these individuals we find commonly possessed features. And it is these common features that express the strategy of the given animal or plant species, and, between (1) them, taken together, as they reside in the Explicate Order, and (2) the noëtic strategy, as it resides in the Implicate Order, there exists, as mentioned, an isomorphism, where the word "isomorphism" applies when two complex structures can be mapped onto each other, in such a way that to each part of one structure there is a corresponding part in the other structure, and where "corresponding" means that the two parts play similar roles in their respective structures. In the next 'intermezzoing' document we will further elaborate on these matters.
Let us now continue with MALYSHEV : The Secondary-Ant (Formicoid) Phase (true ants).
One distinguishes between two main methods of founding a community in ants : the independent (method), when the colony is founded by only a single individual -- a young fertilized female, and the dependent (method), when the colony is founded by several ant individuals. Both these methods are widely distributed and in one way or another represented among almost all recent ants.
The independent method in its most typical and general form, consists of the following. The young female foundress, after being fertilized in the nuptial flight, descends onto the ground, disposes of her four wings and looks for some small cavity under a log or stone or digs herself into the soil constructing there a small chamber. In the room found in this way or made, she walls herself off the outside world. Her large wing-muscles, now degenerating, and the fat body of the abdomen accumulated by her in the larval state in the maternal nest, now make up that stock of food that guarantees the ripening of the eggs in her ovaries and allow her to live through for a month (and sometimes for more than a year!), while being isolated from the outside world. After that she lays a package of eggs, feeds the larvae hatching from them with her saliva and cares for them until they transform into small ant-workers. It was established that she displays a special inclination to eat eggs deposited by her and the larvae to be nursed, and feeds them to those that are left. Apparently, only in this way she is in a position to provide the larvae with that food that they need and in this way leading the left-over larvae to maturity. When going out and strengthening, the young 'workers' dig an exit out of the chamber and take part in the catch of food for them and for their mother, and also help her to raise additional offspring.
Such a method of independent foundation of a colony by the ant female, Wheeler (1932) called "perfectly claustral method". It is observed in various ant species of the subfamilies Formicinae, Dolichoderinae, Myrmicinae, and Pseudomyrminae. As regards the remaining four subfamilies of recent ants, the method of founding a colony as applied by three exotic subfamilies of them -- Dorylinae, Cerapachyinae, and Leptanillinae -- are up to now [1966] not yet known. And as regards the eighth subfamily (the ponerines), relevant articles began to appear only recently ( The mentioned three subfamilies are often taken as families).
The ponerines (Ponerinae) forming a diverse group of primitive ants, are represented by 52 genera divided into 8 tribes and counting about 900 described species. See Figure 6 (left) and Figure 5 above. They are widely distributed, especially in hot countries, and most abundantly represented in Australia, where about 300 species, subspecies, and varieties are known (or 25 percent of the total number -- 1200 -- of Australian ants ( In the article about Russian ponerines ARNOLDI (1932) gives six genera of them, comprising all in all 10 species). From the ponerines, as is supposed, the remaining subfamilies of recent ants originated.
The first substantial treatises on the founding of a colony by ponerines were collected by Wheeler. They are mainly about the large genus Myrmecia (see Figure 7, above) which is endemic to Australia, counting about a third of all Australian ponerines. Some of these myrmecia's, the so-called "bulldog ants", reach a length of 3 cm. Looking like wasps, having long jaws and able to sting severely, the myrmecia's are the only (when not counting poisonous snakes) dangerous inhabitants of areas in Australia covered with bush. Some of them are able to jump, like small crickets. Others have clear colors, bluish with orange tip of abdomen, and the like. They strew large 'ant hills' on the soil, reaching in large species 1.5 m in diameter. The occupation of their nest is not great and usually does not exceed 150-200 individuals, but sometimes may reach 2000. In other ponerines the nest may not built by strewing and consists in total merely of a dozen individuals, looking similar to each other, among which the fertile female ("tsaritsa") is only slightly larger than the "workers".
A good example of the foundation of the nest by ponerines is, according to Wheeler, its foundation by Myrmecia regularis Crawley. After fertilization and shedding of the wings in the first monthes of the year, the female of this ant species digs under the margin of a stone, and sometimes a log, a large flat chamber and in it shuts herself off from the outside world. See next Figure.
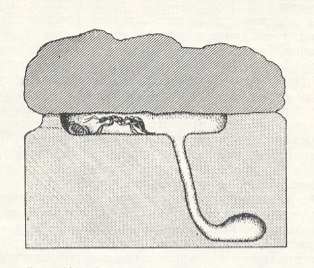
She also digs from the nest floor a canal running some distance in the soil and terminating with a small broadening. This is a refugium to which the female may escape the heat or hide in case of the smallest disturbance. In November and December (the beginning of the summer in Australia) she starts to deposit rounded eggs that are being strewn across the floor of the upper chamber, and thus does not lay them in heaps as higher ants do. Differently from the latter, in addition, the female is not completely confined to the chamber, but, as soon as from her eggs hatch larvae, she breaches through the outer wall of the chamber and exits from it, in order to hunt for insects to feed her larvae with them. Each time upon leaving the nest and upon returning to it she closes [and re-opens] the entrance of the channel that she makes in the outer wall. Apart from these foraging excursions for food for herself and her larvae, the remaining behavior of her as to the offspring is similar to that of the higher ants. Such a kind of independent way of founding a colony Wheeler characterizes as "imperfectly or intermittently claustral method" and holds that the "perfectly claustral method" is a latest highly specialized form of behavior of females of higher ants.
Sustained and methodical observations in nature on this female fixing and closing the nest would be interesting. But the investigator makes no mention of them. He merly advances indirect observations which are, however, little indicative.
The general assertions of Wheeler that the life and habits of the ants of M. regularis are typical of ponerines, and that the "intermittently claustral" method of founding a colony is more primitive than the "perfectly claustral" method, give rise, however, to doubt. It first of all concerns his chief conclusion that the "intermittently claustral" method is essentially similar to the method applied by social wasps of the type Vespa, and that "the ants are merely a peculiar group of social Vespoids" (Wheeler, 1932).
Referring to the viewpoint of Emery [I hope I have transcribed this name properly], according to whom the ants originated at the end of the Jurassic period, Wheeler reckoned it too moderate and held it more probable that the ants originated in the Triassic, "if not at the end of the Permian". Meanwhile, in such a remote period not only "social wasps", standing at the highest phase of vespine evolution, but also any wasps whatsoever, apart from real "half-wasps", did not yet exist. Therefore, a phylogenetic close relationship between ants and social wasps is not probable. To this improbability also points the above mentioned essential biological difference : All wasps, including the "social" ones, develop in cells constructed by them, while in the life of ants the cell is totally absent. In addition, in the "social" wasps all the individuals are winged, while the main mass of the occupants of an ant society -- "workers" -- are always wingless, and in some of them, especially in primitive ants ( Dorylinae (Figure 8 ), Leptanillinae, and some of the Ponerinae and Cerapachyinae), also the fertile females are wingless. All this indicates how remote the ants are from social wasps, and consequently it is not admissible to hold that the ants are "merely a peculiar group of social wasps".
Further, Wheeler points to the fact that ants are, as to their origin, a terricolous family, originated in pure xerophytic conditions, namely on dry continental plains and plateaus of the Mesozoic. As regards this, BERNARD, 1951, writes that we don't know at all which habitats were preferred by the first ants, which might be hygrophilous or, we don't know, xerophilous. Only one thing is certain : the lower genera of ants are, without exception, terricoles, while nesting in trees is characteristic only to higher forms of three out of eight families of them.
Describing the hymenopterous fauna of the steppe zone, Popov, with respect to Ponerinae, remarks that representatives of this relict subfamily are "connected with southern forests, that is, with moist biotopes" (1948). But things are even more definite. According to data of Morley, 1939, concerning the phylogeny of the ponerines, it turned out that the agressive wasp-looking Myrmecia are not the original group of the ponerines, but represent, evidently, merely a side-branch of them "with a dryed-out end", not having given the beginning of higher groups. As to the most primitive ponerines, having given rise to the remaining groups, we must reckon the Amblyoponini, which also Emery directly derives from hypothetical ancestors of the ponerines -- the "Proponerini".
Being small, peaceful and timid, and as to their appearance reminding one of scleroderms (see Figure 3, in part LVIII ), they [Amblyoponini], as also their close relatives, the Ponerini, are typical inhabitants of moist places. In the moist forests of Kolchida, for example, precisely such ponerines (Ponera coarctata K. Arn., P. eduardi For., Euponera ochracea Mayr.) find, according to Arnoldi, 1932, optimal conditions for their existence. On the shore of the Black Sea close to Batum, where the annual precipitation reaches 2400-2600 mm, these ants colonize moist shaded soil very densely and constitute an essential numerical component of the soil biocenosis of invertebrates. Ponera coarctata K. Arn. is also encountered in the sandy steppes near the Caspian Sea in its north-eastern corners of its area of distribution, but here it nests in small deciduous forests, often flooded by the mountain river Terekom.
Certain details with respect to the selection of nesting places by ponerines are very indicative. Thus, according to Wheeler, 1933, Amblyopone australis Erichson. are primitive, slow moving ponerines widely distributed in moist forests in Australia. Different forms of this amblyopone have similar habits. All of them live "under fallen rotting trees or within them, or in rather moist soil under stones". Also Lobopelta neutralis Forel. nests, according to Wheeler, under fallen tree trunks or, more rarely, under stones in fairly moist soil. These are, evidently, optimal living places for the ponerine, because in the adjacent sandy and in a lesser degree tree-covered places its nests are more rarely encountered. Here, in the case of settling under a log, the nest often is extended upwards into the wood when in it there are cracks, or channels gnawed by other insects. Once a nest was found "in moist soil under a log lying at the margin of a small pool", and in another case -- "underneath a log on the shore of a stream". Also the nests of Brachyponera lutea Mayr., widely distributed in Australia, are found in the moist black-soil of eucalyptus forests and underneath stones and logs lying on lake-shores.
Even in regard of the "wasp-like" Myrmecia recently it became known that they are most numerous, as to species as well as to individuals, in certain coastal areas of Australia, where the annual precipitation exceeds 625 mm. They are common also in the mighty moist eucalyptus forests of West-Australia, where they nest under fallen moist tree trunks. On the other hand, in the center of the continent, where precipitation reaches only 375 mm or even less, only one species, having especially adapted to arid conditions, has been encountered -- M. desertorum.
From these data it becomes clear that the amblyopones, as well as other primitive ponerines, indeed are moist lovers, and definitely not xerophiles (lovers of dryness), like the wasp-like Myrmecia, as was held by Wheeler. In these data, in addition, one should see an indication of a definite inclination of a series of primitive ponerines toward dead disintegrating wood, underneath which they not only eagerly settle, but in which they in one or another measure build their channels and chambers. From this, one must suppose, that settling by ponerines under stones is a derived adaptation. Accordingly, again rotting wood appears to be the milieu of living, beginning with the 'semi-ants' the scleroderms, then for the first (hypothetical) ants, and now for archaic ponerines. From this peculiar milieu a part of the first ants, as was noted already above, could easily pass over into the soil rich in rotting substances. Later some of them, that is, relatively few of them, gave rise to the true arboreal forms, which inhabit also today still hard wood of trees and shrubs, or their bark, leaves, and special cavities.
In this conception of the original habitat of the first ants two images, apparently excluding each other, are reconciled, namely that of Wheeler and that of Forel, of which the first held the ants as to their nature to be terricolous organisms, and the second held them to be arboricolous.
With respect to these, from the viewpoint of the author [Malyshev], the first ants may be called hemicolous, as being with respect to their habitat, intermediate between arboricolous and terricolous.
With respect to founding colonies in Amblyoponini, Ponerini, and related primitive ponerines there exist only a few publications. The nuptial flight from the nest of young winged individuals is observed in them, but in contrast to the higher ants, here mating in flight, apparently does not take place, but takes place on the ground or on various elevations where the females sit and to where males fly to them. It is held to be completely probable that sometimes young females shed their wings, when apparently by far not having reached maturity, in the parental nest. After having reached maturity, they, already in their wingless state, go outside and wander for some time in the surroundings until they are found and fertilized by males flying towards them. After mating, the males die, but the females hide somewhere.
Here are three observations by Wheeler, that indicate that the solitary female Amblyopone australis Erichson may found a colony.
[. . .]
Carrying larvae from one chamber, that had become [too] crowded for them, to another, larger one, by their mother-ponerine founding the colony, is a very interesting phenomenon. It points, first of all, to the fact how widely distributed the inclination is in ants to carry their young from one place to another. The habit to nurse (= to look after) the young is, consequently, intrinsic not only to the higher ants, but also to the most primitive of them, the ponerines. From there it is clear that this instinct has very deep roots, growing out at least from the ancestors of the recent ants, the first ants, discussed above, and possibly these roots extend even deeper.
Also widely distributed among ants is the habit of carrying adult individuals, one by the other, partly present also in ponerines.
In addition, the presence of this ability in ponerines [to carry the young] renders possible to clear up some, apparently not understood, phenomena in their life-activities. We know that it is not possible to hold that the female-amblyopone was capable to carry to its chamber such a large prey as, referred to earlier, a larva of a lamellicornid or of a black-bodied beetle. From here, taking into account the ability of the female ponerine to "look after" its larvae, it is easy to expect something else : She herself carried her, in need to be fed, larvae to appropriate for them provisions [instead of carrying these provisions to them], and it is even possible that she, like the scleroderm, founded the colony directly on these provisions. Therefore it would be interesting to carry out direct experiments in placing into the chamber of Amblyopone, or next to it, preys of this sort.
Here we may think of another case, not belonging, though, to the ponerines, but to the Myrmicinae, reported by Hölldobler, 1938. The female Myrmica laevinodis Nyl. carried her larvae from that chamber where they had hatched, to another, where the experimentor had placed food -- larvae of other ants, flies, or syrup. In other cases, when provisions were directly supplied to the first chamber, the female carried the larvae to them.
In the light of all this it is clear that in the habits of the female Amblyopone, feeding the larvae with a large prey, we see the atavistic trait of their sclerodermoid ancestors shine through -- rearing the offspring on large larvae of beetles that live in old rotting wood.
[. . .]
We may go with the assertion by K. and E. Haskins that the Myrmecia's (Ponerinae or Myrmeciinae, not to confuse with Myrmica's, Myrmicinae) "stand, as to their structure, as well as to their habits, very far away from all remaining recent ants". But very doubtful, on the other hand, is another of their positions, namely that Myrmecia "represent the greatest approximation of all living Formicidae to the original form of the ants". Here, clearly intending to confirm the latter, it is stated that not only the myrmecia's are so close to the original form, but "together so stand with them one or two other groups of ponerines, such as the Amblyoponini and the not so widely distributed genera of Cerapachyinae".
Such equation, however, is little convincing, because the Amblyoponini (about the Cerapachyinae we will speak later) are, as to how they look, not wasp-like, as are the myrmecia's, but scleroderm-like. They are not aggressive, but, on the contrary, peaceful and timid, living not in the open, not on the soil, but concealed, "subterranean", and so forth. In addition, the behavior of adult amblyoponines with respect to the cocoons at that moment when out of them the young ants have to come, is very indicative : Their behavior with respect to this is surprisingly more primitive than what is observed in the remaining ants. In higher ants the instinct of worker individuals to deliver great assistance to the young in leaving their cocoons is so worked out that without this help delivered to the young for them to get out of their cocoons they cannot accomplish this. In the typical case the "workers" open the cocoon from without and the pupa is drawn out of it before the pupal skin is shed, which only later is removed by the "nurses" being present, where then the wings of sexual individuals straighten out.
In Amblyoponini all this is almost straightly opposite to this. The pupal skin is shed by the emerging individual itself already within the cocoon, while the wings, when it is a sexual individual, are spread, and only after this the young insect itself gnaws an opening in the cocoon and comes out. It is further characteristic that the young males leave the cocoon already completely pigmented, and after a few hours are capable of leaving the nest and fly. On the other hand, the young developed females and "workers", although still not yet completely mature, are capable actively to take part in the life of the colony almost directly after their emergence from the cocoons.
Regarding the myrmecia's, this independent emergence from the cocoons is for them, as becomes clear, not typical, and in this respect they come close to the higher ants. To this, first of all points the large number of by far not mature individuals found in their nests in the right season. Further, observation of the behavior of "nurses" in Myrmecia (Promyrmecia) pilosula in respect to their relation to the cocoons in an artificial nest indicates that the dependent emergence from the cocoons is the normal process, at least for the given species.
So we must conclude that truly archaic, the most primitive of all recent ants as to their way of life and behavior, are the Amblyoponini, while the myrmecia's (Myrmecini) are an aberrant, peculiarly deviant group of ponerines. This matches also their morphological relationship.
We must still dwell on one trait in the life-activities of the ponerines which throws a definite light on their origin. This is the so-called "licking of the larvae by the fertile female founding her colony, and later also generally by worker individuals". It consists of the fact that adult ants eagery lick up fat exudates, coming through the delicate skin of the larvae. This licking activity starts almost immediately after the emergence of the larvae and lasts for all of their life. In this the ants, when they lick their larvae, characteristically squeeze and pluck them, especially on the thoracic segments, with the clear intention to assist exudation.
Let us now remember that the fertile female Scleroderma, founding her family, also nibbles at various places of the skin of the paralyzed prey, effecting with this the excretion of small droplets of hemolymph from the wounds inflicted earlier by the sting. She also often licks her larvae, which are going to develop on the body of this prey. From here we must conclude that licking the skin of the larvae is essentially the same act that is applied until now on the body of the prey. So licking the larvae by the ponerines must be seen as a direct indication to an earlier followed course of an ectoparasitic phase of development. But this isn't yet all. There is an indication that licking is just one of the forms of trophallaxis. Therefore we may say that trophallaxis in its most simple form distinguishes itself from mere licking only by the very place of the action, that is, not at the surface of the skin generally, but only at the places of the oral organs of the larvae.
So we come to the conclusion that trophallaxis, having originated in the course of the evolutionary development of ants, has very deep roots. From what is now clear, in the beginning it appears in the form of licking by the female of hemolymph from the body of a large prey, stung by its sting. After that it transformed into the habit of licking by the female of the integument of her developing larvae, and finally, from the moment of the mother offering chewed food to the mouth of the larvae, the beginning was laid down of its typical form -- mutual feeding.
Now we may confidently say that in the line of evolution of the bees (Apoidea) trophallaxis did not at all take place.
Here it is about the absence in bees of mutual exchange of food between adult individuals and the larvae to be reared by them. Feeding by some adult individuals that are more or less satisfied, of other adults in need of food, is also observed in bees. The latter phenomenon essentially differs from the first, and must therefore be distinguished apart as "trophehomy".
On the other hand, of all aculeate hymenoptera it appeared in its clearest form in the high stage of vespine life -- in the true or wing-plaited wasps (Diploptera) and especially in the social wasps (Vespidae). Here, among higher solitary forms (Synagris of the Eumenidae), it was originated from the instinct of chewing ("malaxation") of the prey, being very small and delicate (minute, as compared to the wasp itself, caterpillars), offered in a chewed state by the mother-wasp to the mouth of the larva to be reared by her. Later it attained special development in social wasps, also not connected with the licking of the integument of the larvae. Accordingly, there definitely is no basis for holding that a close relationship between the social Polistes-wasps and the primitive ponerine ants exists, as was supposed by Haskins, because of the phenomenon of typical trophallaxis in the first and mere licking of larvae in the latter.
With respect to trophallaxis in ponerine ants, its stage of development, its forms of appearance, and the breadth of its distribution, the existing data are far from sufficient, partly contradictory, and demand further clarification. Thus, Wheeler reports that Ponerinae, as also some higher ants (of the subfamilies Formicinae and Myrmicinae), feed their larvae with pieces of insects or with whole small insects which they place directly at the ventral side of the larvae next to their mouth organs. Differently from this, the Brazilian ponerine Odontomachus affinis Guér., according to observations of Borgmeier, 1920, feeds its larvae exclusively by throwing up food for them from its mouth. On the contrary, according to data of Haskins, the Australian Myrmecia almost completely do not show the phenomenon of trophallaxis in its typical form, although characteristic "request-gestures" with the fore-legs, that reminds us of the "scrounging" for liquid food in higher ants among each other, are also well developed in myrmecia's, but here they appear principally when adults push the larvae in order to effect in them exudation through their skin.
In addition, there exist indirect indications of the ability of adult individual ponerines of mutual feeding of liquid food. In large colonies of Myrmecia, the well-fed "tsaritsa" with a swollen abdomen resides in the very deep part of the nest, where she, evidently, also receives proper food. But in the autumn-winter season, when rearing of young is halted, and together with this the bringing in of insects [preys] into the nest also stops, it is difficult to understand with what the "tsaritsa" is fed, if not with liquid food from the mouth of "workers".
From the latest observations it became clear that throat-exchange of food takes place regularly between adult individuals, at least in M. regularis, and feeding the larvae with such food takes place also in M. vindex, although this succeeded to be observed only in special conditions. Thus, in holding a colony of M. regularis in artificial nests the ant-workers, having received a sufficient amount of solution of honey in water, regularly went to the heap of larvae, and motionless stood over it without any signs of throwing up food, "if not counting the appearance of drops of nectar on the upper lip". Then the larvae lively stretch out themselves and dipped their head into the drop and emptied it. This observation was later confirmed by adding into the food, that was to be swallowed by the worker ants, a diluted solution of methylene-blue. The color accumulated in the larvae such, that the content of their middle-gut became blue, leaving no doubt that the colored food was obtained from the adults.
In other experiments denying a developing colony of M. regularis sugar, and later offering to one worker, foraging for the colony, a colored solution of honey, a distribution of the content of its crop among other adults could be observed. Here, in the "feeding donor", and not the receiving individual, fast unordered movements of the fore-legs took place. But there was not any "scrounging" for food with the fore-legs from the side of the receivers, so characteristic of the higher ants. The whole process here went its way surprisingly more simple and less stereotypically. The other mentioned species, M. vindex, turned out to be less specialized than M. regularis in many respects.
The obtained results provided authors (Haskins and Whelden, 1954) a basis to hold that trophallaxis, at least in its primitive and generalized form, is not only characteristic to certain Ponerinae, but also to Myrmecinae. Adult ponerines, in addition, eagerly lick the integument of their larvae, their head-capsules, and mouth organs, which is very similar to how this is done in higher ants. From this it follows that trophallaxis is certainly well developed in them. The clear absence of offering food from the mouth, food that was earlier swallowed, in such ponerines as Amblyopone, Stigmatomma, and Proceracium, as the authors held, is rather a result of secondary specialization, than a primitive state.
From this, with respect to ponerines, one might say that although trophallaxis is not for them a unique or characteristic method of feeding, it appears in them fairly usually and principally, in various forms, from simple licking of the larvae to begin with, and all the way to throwing up food by them from the mouth, and mutual feeding. This difference of phenomena stands, it must be assumed, in relation to the general primitive state of the ponerines.
Now we have the question concerning fungal food of the ant-female founding a colony in a cavity closed on all sides. This question until now [1966] is not sufficiently clarified. The very fact of the ability of the "ant-tsaritsa" to live a long time and raise offspring in her isolated chamber without any stock of provisions was so amazing that investigators did their best to indicate the vey ability for her to live and reproduce at the expense of her own [internal] resources.
[. . .]
Ants, in their life-activities, constantly come, in this or that degree, in contact with fungi. As was established, adult ants exclusively take liquid food. Hard particles, on the other hand, ending up in their mouth they do not swallow but gather them in a special infrabuccal part of the oral cavity (in the "lower-mouth purse") in the form of a spherical lump which they later dispose of. Enquiry of these infrabuccal lumps in the most different ants indicated that in almost all cases they contained fungal spores or pieces of mycelium, gathered from the body-surface of the ants themselves or from the walls of their nests [indicating thus that ants are frequently in contact with fungi]. More than that, many ants have the habit to throw these lumps out of the nest on refuse heaps. This "kitchen waste" in certain African ants of the genus Crematogaster (see Figure 9, above ) nesting in cavities of living plants, chiefly consists of precisely those lumps, spitted out of the mouth, that ensure luxuriant growth of fungal threads that are eaten by the ants. We must assume that generally the use of mold, growing on the walls of the chamber and channels of the ants and on their refuses, must be widely distributed in ants. When we now take into account those hypothetical first ants, to which the scleroderms had led us, it is also from this side very probable that there some role is played by possible fungal feeding of the female-ant having been isolated in a milieu suitable for development of fungus threads and spores.
[. . .]
This result [obtained from experiments described by Malyshev, but not reported here on this Website] wholly corresponds to the previous conclusions concerning the presence of additional fungal food in female-ants at the time of their isolated life, and confirms them. We thus see that even the higher ants did not completely leave their typical conditions under which their remote scleroderm-like ancestors that fed themselves, when needed, with fungus threads and spores.
And so the analysis of the habits of recent ants when founding a colony gives us a many-sided confirmation of precisely those positions to which the enquiry of the evolution of parasitic hymenoptera has led us : From a semi-familial ectoparasitic phase, to a familial ectoparasitic one, today still represented by the scleroderms (See Figure 3 in part LVIII ) . This means that the ants, in their phyletic development, had not in any way gone through the solitary phase, not hunting, as the wasps did, for a prey, and not transporting it to their nest, but developed as families [not in the taxonomical sense, but in the sense of mother and children] on large preys paralyzed by them, like the scleroderm does. Further, finding themselves in conditions of prolonged hunger, they fed themselves with fungi and in this way passed over into the typically formicine [antlike] way of life.
In the light of all this, a most deepened study of the scleroderms, it must be assumed, will uncover still more important points clarifying this fundamental transitional stage of so much interest to us in the evolutionary development of the Hymenoptera.
On the other hand, also in primitive ants, as do indicate the latest investigations (Le Masne, 1956), remarkable traits are discovered bringing them close to the scleroderms. Thus it turned out that in the ant Ponera eduardi Forel two forms of females and two forms of males -- the normal (winged) and ergatoid (wingless) form -- are present. The wingless males mate with the very young not yet pigmented workers or even with those that still are in their cocoons. From the, by them fertilized, "large workers" (having large complex eyes and developed ovaries!) again develops a wingless generation.
Some higher ants of the group Attiini (family Myrmicidae), having made use of their original connection with fungi, went their [evolutionary] way toward a special cultivation of fungi, reaching on this path really remarkable results. Such is, for instance, the growing of extensive fungus "gardens" by the leaf-cutter ants (Atta, Acromyrmex), the growing in these gardens of such sorts of fungus that without the ants are not encountered in free nature, and the cultivation on their mycelia of special fruits (bromatia's), which without the direct partaking of the ants do not emerge. As a substrate for the growing of fungi in the mentioned leaf-cutter ants serves a spongy material prepared by them from bitten-off pieces of green leaves, taken from neighboring shrubs or trees. It is interesting that some of the Attiini (Cyphomyrmex, Apterostigma, Myrmicocrypta), evidently being more primitive, take, for the substrate for the growing of fungi, excrements of other insects, especially of caterpillars.
Wheeler assumes that the group Attiini originated in the humid tropics, because almost all more primitive species of Attiini are still confined to the damp tropical forests. But some of them nevertheless penetrated into the dry deserts of Arizona and Mexico. But here they began to dig channels and chambers in the soil to such a depth that they could continue the culture of fungi on a substrate of vegetable material taken from the surroundings. And thus, even in these misbegotten places the ants were in the position to built permanent accommodations and guarantee for themselves normal food.
With respect to other ant families [in the taxonomical sense], having originated from their common amblyopoid ancestor, we may hold that fungal food had to have in this or that degree essential significance also in their evolutionary development. But among recent ants one special group nevertheless distinguishes itself by apparently in its evolutionary course not at all having made use of fungi in some essential or regular way, but really only accidentally so, and not necessarily. These are the tropical army ants, Dorylinae (see Figure 8, above ). The remarkable biological feature of the dorylines consists of the fact that they do not build real nests or ant-hills which is so characteristic of the other independently living ants, but leading instead a nomadic life, carrying with them also the young. For resting and reproduction they remain in temporary stations or bivouacs.
A second important feature of army-ants is polymorphism, reaching in them its maximal development. The female dorylids are wingless by their nature [i.e. they never have wings], the males are winged, although there is an indication of also wingless forms among them looking like "workers" (ergatoids). The morphological features of this or the other sex are here so peculiar that "these sexual forms may constitute, completely and sufficiently, a basis for separating the dorylines as a group of their own, differing from all other ants".
The most primitive dorylines are represented by the genus Eciton, with about 100 species distributed in the tropical regions of America, and some 50 species in the Old World tropics. [see for a species of this genus Figure 6 (middle image), above ]. Thus, Eciton rapax Sm., a still little polymorphic species, wanders in relatively small hordes or troops. The latter nevertheless make up several thousands of individuals which move one after the other in one line and pillage the nests of other ants. In other cases the moving mass of army ants consists of many hundreds of thousands of individuals, on their way destroying all that lives, including snakes and even large animals that are leashed, in a pen, or within some fence. Only certain ants (Atta and Azteca) are able to wage a winning war with them, but the large ant-eater ants, Myrmecophaga, having been specialized to eat ants, kill them.
Interesting is the periodicity appearing in the life-activity of the army ants, substantially having been observed by Schneirla, 1945, 1949, in Panama with respect to Eciton hamatum F. and Eciton burchelli West. When the troop of the mentioned ants, having concluded the trek, halts in a bivouac, they congregate into a ball, like an enormous swarm, in a hollow tree or in some depression underneath a log, leaving in the mass of living bodies channels and chambers. In the stay, after a week the "tsaritsa" begins emptying her abdomen of eggs, and in the course of a few days deposits more than 20 000 of them. After the larvae having emerged and a little bit grown, the troop again goes on the move. After 20 days, when the majority of larvae has concluded feeding and builds cocoons, the ants again halt to rest. In this way, the periods of egg-laying, at least in the mentioned species, set in very regularly, every 35-36 days, and take the whole year [no hibernation, that is]. The dorylines feed their larvae with fairly compact chunks of soft parts of insects, so that the workers must chew the prey and suck out saps from these chunks, before offering them to their larvae. Such food is given to the larvae directly into their mouth as in higher ants, and is thus not placed onto them or next to them, as in ponerines.
About how, precisely, a colony is founded in army ants there are until now [1966] no reports. Wheeler (1933) assumes that they have lost the original independent method of founding colonies, and today multiply by way of "hesmosis", or by splitting up the parental community.
As to the origin of dorylines, the opinions of investigators differ. At the time Emery, taking into account that the female Dorylinae are always wingless, held them to be the most primitive of all existing ants. However, a series of other characteristic features of the dorylines, regarding their unusual polymorphism, the productivity of the "tsaritsa", the density of the colony, the structure of the genitalia, and others, indicate that they undoubtedly have reached a high stage of specialization. Therefore, today they are derived from lower ponerines through a small, still little investigated, group of paleotropic Cerapachyinae, living, as is assumed, a life similar to that of the ponerines. Meanwhile, the Ponerinae, and, as far as is known, the Cerapachyinae, live in permanent ant-nests where they also rear their young, and definitely do not wander with them from place to place. This is a very essential difference. It is difficult to understand how the transition from the stationary life of the ponerines to the nomadic life of the dorylines took place. Some indication really is provided by only the ability, granted by us, of wandering-over with the young of the female Amblyopone, as noted earlier.
Such a deep-seated difference allows us to conclude that the peculiar group represented by the dorylines, which indeed can be contrasted with all remaining ants, did not originate through primitive amblyopones, but independently split off from their common bethyloid (prosclerodermoid) ancestors [common, that is, to the amblyopones and dorylines]. There is, nevertheless, no basis to assume that also in these original conditions the remote ancestors of the dorylines stayed [longer] in the maternal nest on the remains of the prey like the described proformicoid ancestors of the remaining ants. On the contrary, we must assume that the young bethyloid females (ancestors of the dorylines, wingless by their nature) left their maternal nest soon after their emergence from their cocoons. In such a case the remote ancestors of the dorylines did not at all in their phylogenetic history live through a period of starvation, did not work out for themselves "autophagia", went on without degeneration of thoracic muscles, and did not experience a need for fungal food.
Having left, possibly together, the maternal nest, they began to look for new preys. Subsequently, attacking minute insects, they did not transport them, but fed on them at the spot, and fed their larvae with the chewed remains, and, taking the larvae with them, went to new places. And with this had begun the nomadic way of life of the dorylines.
We must assume therefore that the young females of the recent dorylines, founding new colonies, like their ancestors, did not enclose themselves into a first chamber, and did not at all live through a degeneration of thoracic muscles, that so clearly takes place in ponerines, according to Haskins and Enzman, about which we reported earlier.
In the evolutionary development of ants also other remarkable divergations have taken place, for instance with respect to biological and morphological polymorphism, or with respect to the formation of extraordinary forms of "hospitality" and social parasitism leading along one of the lines of evolution to the origin of "solitary ants" (Anergates atratulus Schenck.) [Myrmicinae], having lost the caste of own "workers" and helping themselves with alien "workers" who kill their own "tsaritsa".
Enquiring into these and a series of other lines of evolution in ants is beyond the limits of the present investigation [in Malyshev's book].
With all this we conclude our exposition of the origin and evolution of ants.
Notification : The present Series on the evolution of Hymenoptera will temporarily be interrupted by the insertion of a four-document intermezzo dealing with a further development of our noëtic theory of evolution. After these four documents we will pick up again the exposition of the evolution of Hymenoptera, that is, concluding it with an exposition of the origin and evolution of bees (Apoidea). In all this, still following Malyshev.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part LXa (intermezzo on noëtic theory).
Back to Evolutionary Part XVII
Back to Evolutionary Part XVIII
Back to Evolutionary Part XXII
Back to Evolutionary Part XXIII
Back to Evolutionary Part XXIV
Back to Evolutionary Part XXV-A
Back to Evolutionary Part XXV-B
Back to Evolutionary Part XXV-C
Back to Evolutionary Part XXVI
Back to Evolutionary Part XXVII
Back to Evolutionary Part XXVIII
Back to Evolutionary Part XXVIII-A
Back to Evolutionary Part XXIX
Back to Evolutionary Part XXXI
Back to Evolutionary Part XXXII
Back to Evolutionary Part XXXIII
Back to Evolutionary Part XXXIV
Back to Evolutionary Part XXXV
Back to Evolutionary Part XXXVI
Back to Evolutionary Part XXXVII
Back to Evolutionary Part XXXVIII
Back to Evolutionary Part XXXIX
Back to Evolutionary Part XLII
Back to Evolutionary Part XLIII
Back to Evolutionary Part XLIV
Back to Evolutionary Part XLVI
Back to Evolutionary Part XLVII
Back to Evolutionary Part XLVIII
Back to Evolutionary Part XLIX
Back to Evolutionary Part LIII
Back to Evolutionary Part LVII
Back to Evolutionary Part LVIII