preparation
3-D Crystals
III
REMARK : When the reader feels satisfied with our established theoretical foundation (done in previous documents) that allows (single, non-twinned) Crystals to be promorphologically assessed, he or she can skip all the following, and directly proceed with The Promorphology of Crystals.
Deriving the Complex Motif of Cesium Chloride (CsCl)
Cesium Chloride (CsCl) belongs, like Sodium Chloride (NaCl), to the most symmetric Class of the Isometric Crystal System, namely the Hexakisoctahedric Class, so the point symmetry of CsCl crystals is 4/m 3* 2/m. The CsCl lattice is however different from that of NaCl. While the NaCl lattice is a cubic F lattice (i.e. a cubic face-centered lattice), the CsCl lattice is a cubic P lattice (i.e. a cubic primitive lattice, meaning that the cubic unit cell of such a lattice has nodes only at its corners), and while NaCl consists of two interpenetrating F lattices, shifted with respect to each other by half a body diagonal of the unit cell, CsCl consists of two interpenetrating P lattices, shifted with respect to each other also by half a body diagonal of the cubic unit cell. The Space Group of crystals of Cesium Chloride is, according to me, P 4/m 3* 2/m.
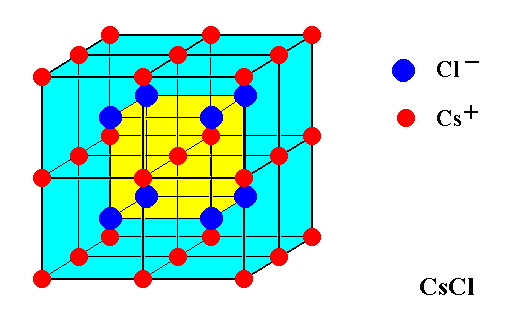
The next Figure illustrates the structure of a CsCl crystal. Eight unit cells are shown.

Figure 1. Structure of a Cesium Chloride crystal.
Eight unit cells are shown. The structure consists of a primitive cubic lattice furnished with Cesium ions (red), interpenetrated by another primitive cubic lattice furnished with Chlorine ions (dark blue). The eight Chlorine ions in the Figure lie at the corners of a cube (yellow) which is placed in the center of the Cesium structure.
The u n i t c e l l of the structure of Cesium Chloride crystals is depicted in the next Figure.
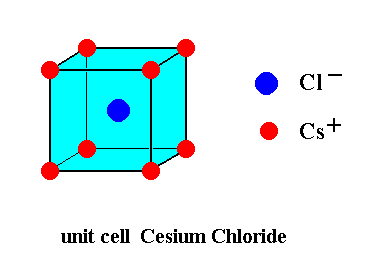
This unit cell can be more precisely depicted, as the next Figure shows. It then is clear that such a unit cell contains 8 x 1/8 = 1 Cesium ions, and one Chlorine ion, i.e. precisely one formula unit (CsCl).
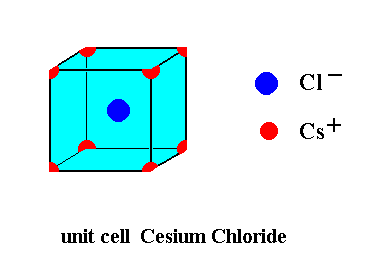
Because both lattices are primitive (i.e. not centered) there are no glide planes. There are also no screw axes present in the structure.
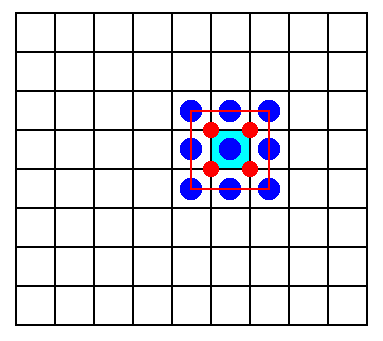
So we must establish the Complex Motif of Cesium Chloride (single, non-twinned) crystals as is depicted in Figure 3. Its symmetry is that of a cube : 4/m 3* 2/m. The number of antimers is evidently eight (each antimer consisting of 1/8 Chlorine ion and 1/8 Cesium ion), so the promorph of such crystals belongs to the Octaedra regularia, where the eight faces of the octahedron represent the eight antimers.
Deriving the Complex Motif of Sphalerite (ZnS)
The structure of Zinc Sulfide, in the form of the mineral Sphalerite, can be thought of as two interpenetrating face-centered cubic lattices, like we saw in the case of NaCl. However this time the lattices are shifted with respect to each other by 1/4 body diagonal of the cubic unit cell (instead of 1/2 diagonal). The point symmetry of crystals of Sphalerite is 4* 3 m. They accordingly belong to the Hexakistetrahedric Crystal Class of the Isometric Crystal System (See our overview of the Crystal Classes and Crystal Systems). The Space Group of Sphalerite is F 4* 3 m. The sphalerite structure is explained in the next Figures.
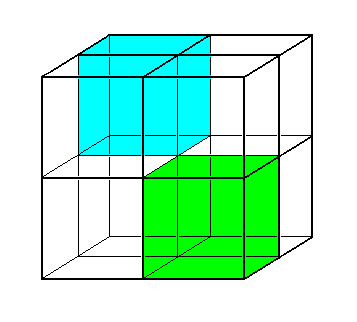
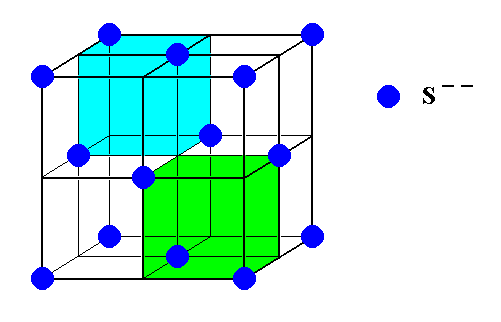
Figure 6. The Sulfur ions (S, blue) arrange themselves in a face-centered cubic lattice ( Also the Zinc atoms (not shown) arrange themselves in such a lattice, which is however shifted 1/4 body diagonal ).
Alternate subcubes of the Sulfur lattice contain a Zinc ion (Zn), making up the ZnS crystal structure. See next Figure.
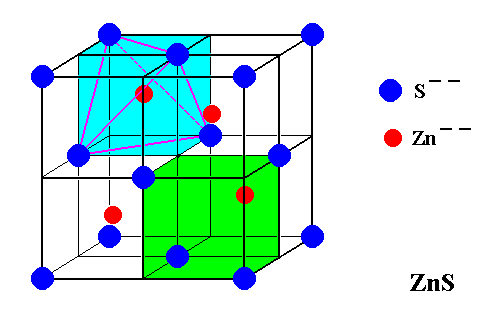
Figure 7. Structure of Sphalerite (ZnS).
Two cubic face-centered lattices, one furnished with Sulfur ions, the other furnished with Zinc ions, interpenetrate, but are shifted with respect to each other by 1/4 body diagonal of the cubic unit cell.
Even though the arrangement of the ions in Sphalerite is like that of Sodium Chloride in consisting of two face-centered cubic structures, there is an important difference from the point of view of the ions. In the Sphalerite structure each atom (ion) has only four nearest neighbors (In NaCl this number is six). If the atoms in Sphalerite were ions of two oppositely charged kinds attracting or repelling one another indiscriminately, they would surely rearrange themselves so that each acquired more oppositely charged neighbors, as is the case in the Sodium Chloride and Cesium Chloride structures. You can infer that the Zinc and Sulfur atoms in Sphalerite are not acting as if they were simply ions. To be sure, the Zinc and Sulfur atoms acquire positive and negative charges, but they decide to stand away from one another at definite angles because they also form (covalent) chemical bonds.
Because in Sphalerite no screw axes and glide planes are present, the only translational symmetry that must be eliminated in order to find the Complex Motif, is the simple translation. The simple translation effects the periodic repetition of a unit cell. So when we eliminate the simple translations we end up with a single unit cell. If there are several unit cell choices possible we must take the one with the highest symmetry (the latter does not relate to the unit cell as empty building block but as a building block furnished with content), because the Complex Motif must have all the symmetry that the crystal has in store.
This unit cell, which, at the same time represents the Complex Motif, is the one that was depicted in Figure 7. Its content is the following :
8 x 1/8 + 6 x 1/2 = 4 Sulfur atoms and 4 Zinc atoms. The symmetry of the Complex Motif is 4* 3 m, i.e. it has the symmetry of a regular tetrahedron. So the promorph of Sphalerite crystals belongs to the Tetraedra regularia (Polyaxonia rhythmica).
Deriving the Complex Motif of Fluorite (CaF2)
The point symmetry of crystals of Calcium Fluoride in the form of the mineral Fluorite is 4/m 3* 2/m, implying that they belong to the Hexakisoctahedric Crystal Class of the Isometric Crystal System. The Space Group is F 4/m 3* 2/m. So the symmetry, the point symmetry as well as the translational symmetry, is identical to that of Halite (NaCl). The lattice type is, however, different : While the Halite lattice can be seen as two interpenetrating cubic face-centered lattices, the Fluorite lattice is an interpenetration of a face-centered cubic Calcium lattice and a primitive (i.e. not centered) cubic Fluorine lattice (the latter is placed in the middle of the former). Here we clearly see that the Space Group alone cannot in all cases fully characterize the s t r u c t u r e, i.e. the l a t t i c e t y p e, and thus cannot all by itself and in all cases determine the inorganic s p e c i e s, i.e. determine to which species (of material being) the given crystal belongs. The chemical composition must also taken into account. And the difference in chemical composition between Halite (NaCl) and Fluorite (CaF2) surely is responsible for their having a different lattice type in spite of the fact that their total symmetry (Point Group and Space Group) is exactly the same.
The next Figures depict the structure of Fluorite.
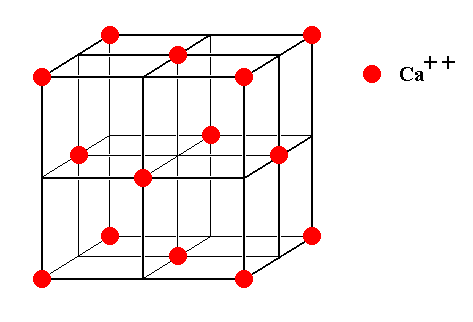
Figure 8. The face-centered Calcium lattice as it is present in crystals of Fluorite.
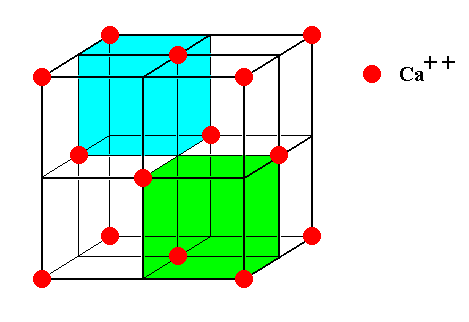
Figure 9. The face-centered Calcium lattice as it is present in crystals of Fluorite. It can be imagined to consist of eight subcubes. Two of them are indicated.
While in Sphalerite only four of the eight subcubes are furnished with an atom, in Fluorite each subcube contains an atom :
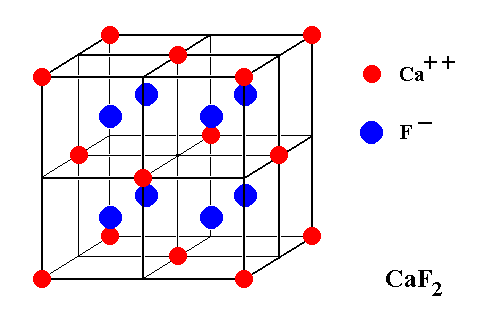
Figure 10. Unit cell of the Fluorite structure. Each subcube of the Calcium lattice contains a Fluorine atom (ion).
One such a subcube is not a unit cell because it is not periodically repeated.
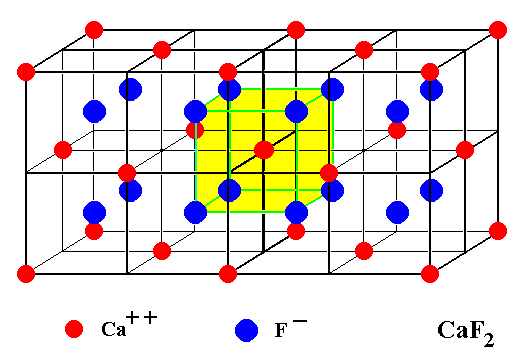
Figure 11. Unit cell of the Fluorite structure extended by one more unit cell. We can see that each Calcium ion is immediately surrounded by eight Fluorine neighbors when the structure is imagined to be extended indefinitely.
As has been said, each Fluorine ion is tetrahedrically surrounded by four Calcium ions, which could suggest the presence of four antimers. But the presence of four congruent antimers (gathered around a point) suggests a tetrahedral promorph, which isn't correct because we know that the translation-free symmetry (point symmetry) of Fluorite crystals is equal to that of a cube (instead of a tetrahedron). So the above established fact that each Calcium ion is immediately surrounded by e i g h t Fluorine ions (Figure 11) indicates that the Complex Motif consists of eight antimers, it has moreover the symmetry of a cube or regular octahedron. All this implies that the promorph of (single non-twinned) crystals of Fluorite belongs to the Octaedra regularia (Polyaxonia rhythmica). The unit cell of Figure 10 is perfectly suitable to represent the Complex Motif. In it the center of the structure is immediately surrounded by eight Fluorine ions.
When a substance is made of many different sorts of atoms, the structure of its crystals will necessarily be more complicated than those that we have considered until now (Magnesium, Copper, Sodium Chloride, Cesium Chloride, Zinc Sulfide and Calcium Fluoride). You cannot avoid this conclusion when you remember that the smallest unit cell found for any crystalline material always contains at least as many atoms (or ions for that matter) as the chemical formula for the substance. The chemist would write (as such a formula) "Cu" and you can find a unit cell for a Copper crystal with only one atom. The chemical formula NaCl for Sodium Chloride and CaF2 for Fluorite tell you at once that unit cells for their crystals must contain at least two atoms and three atoms, repectively.
The unit cell must contain at least one "formula unit" of the crystal substance. If it contains even more, then it must contain some multiple of a formula unit. Looking to the chemical formulae for some other crystals, you can see that the unit cells for most of them must contain a great many atoms. For instance, the formula unit of Alum, KAl(SO4)2 . 12H2O, has fourty-eight atoms, and the smallest unit cell contains four of these formula units. So the unit cell of Alum contains 192 atoms!
In the next document we will determine the Complex Motif of some more crystalline substances.
To continue click HERE to further consolidate the preparation to the Promorphology of 3-D Crystals.
e-mail :

back to retrospect and continuation page
back to Internal Structure of 3-D Crystals
back to The Shapes of 3-D Crystals
back to The Thermodynamics of Crystals
back to Introduction to Promorphology
back to Anaxonia, Homaxonia, Polyaxonia
back to Protaxonia : Monaxonia
back to Stauraxonia heteropola
back to Homostaura anisopola, Heterostaura
back to Autopola oxystaura and orthostaura
back to Allopola (introduction)
back to Allopola amphipleura and zygopleura
back to the Basic Forms of Cells I
back to the Basic Forms of Cells II
back to the Basic Forms of Organs
back to the Basic Forms of Antimers
back to the Basic Forms of Metamers
back to the Basic Forms of Persons
back to the Basic Forms of Colonies
back to the first part of the Preparation to the Promorphology of Crystals
back to the second part of the Preparation to the Promorphology of Crystals
back to the third part of the Preparation to the Promorphology of Crystals
back to the fourth part of the Preparation to the Promorphology of Crystals
back to the fifth part of the Preparation to the Promorphology of Crystals
back to the sixth part of the Preparation to the Promorphology of Crystals
back to the seventh part of the Preparation to the Promorphology of Crystals
back to the eighth part of the Preparation to the Promorphology of Crystals
back to the ninth part of the Preparation to the Promorphology of Crystals
back to the tenth part of the Preparation to the Promorphology of Crystals
back to the eleventh part of the Preparation to the Promorphology of Crystals
back to the twelfth part of the Preparation to the Promorphology of Crystals
back to the thirteenth part of the Preparation to the Promorphology of Crystals
back to the fourteenth part of the Preparation to the Promorphology of Crystals
back to the fifteenth part of the Preparation to the Promorphology of Crystals
back to the sixteenth part of the Preparation to the Promorphology of Crystals
back to the seveneenth part of the Preparation to the Promorphology of Crystals
back to the first part of the Preparation to the Promorphology of 3-D Crystals
back to the second part of the Preparation to the Promorphology of 3-D Crystals