

(Takes a little while loading the images)
e-mail :

Sequel-16 to the Summary and Evaluation (of the documents XVI -- XXVIII concerning the promorphology of complex-shaped two-dimensional crystals).
This document (Part XXIX Sequel-16) further elaborates on the analogy between crystals and organisms.
Sequel to the Preparations (IV) to the Repertoire of the Crystal Analogy
More about the s h a p e s of individual rheocrystals, and crystals in general, especially with respect to the phenomenon of m e t a m o r p h i s m.
In the previous document we spoke about the molethynes as directional forces of crystallization that supersede the surface tension of the growing crystal and in so doing create genuine shape and preventing crystals to become spheres. Indeed, if no other factors interfere, they would become spheres as soon as they give in to surface tension. The surface tension of a crystal is caused by the presence of 'dangling' chemical valences at the crystal boundaries, giving rise to chemically unsatisfied surfaces, which therefore bear an energy cost. This results in further growth (i.e. chemically satisfying the mentioned surfaces) and/or a tendency to reduce the external surface of the crystal, possibly by relocation of its particles. All this also holds for snow crystals. Looking at freshly fallen snow, we see angular crystals in the form of stars, hexagonal plates, 'bullets', etc. It turns out, however, that these forms are not stable, i.e. not only the branched dendritic forms are unstable, but also the plates, hexagonal columns, pyramid-capped hexagonal columns ('bullets'), etc. But this cannot be typical for snow crystals while absent in crystals of other substances. As far as I am able to judge, the instability of angular crystal shapes of natural snow has to do with the fact that when precipitated the temperature of the crystals is, apart from polar regions, normally not far from the melting point of ice. Crystalline minerals such as quarz, the silicates and many others, have high melting points, i.e. high with respect to normal temperatures (which are supposed to be around 200C), namely (melting points of) several hundreds degrees Celcius. So at room temperature the crystals of such minerals are cooled very strongly, they have a temperature of hundreds of degrees below their melting point. And in this temperature state their shapes (which are all angular) seem to be very stable indeed. With natural snow, however, things are different. It is not that ice (as a chemical substance) is special or exceptional in this respect. It presumably is not. But natural snow (apart from polar conditions) normally has to do with temperatures -- as they prevail on this planet -- that are at most some 20 or 30 degrees below the melting point of ice (and not hundreds of degrees). And when, in precipitation, it has reached the ground, the temperature very often is only a few degrees below that melting point. So snow can originate (high in the atmosphere) at relatively low temperatures, but when it has reached the ground its temperature normally has become -- while still subzero -- close to its melting point. And it is in this state that the snow crystals turn out to easily undergo metamorphism (We should compare this to the expected behavior of other mineral crystals having high melting points but are heated up very close to these points). It seems that crystals that have a temperature that is close to -- but still below -- their melting points, eventually, that is, after some time, give in to the tension of their boundaries, i.e. give in to surface tension, resulting in their shapes to become rounded off and approaching the ultimate condition of minimum surface per volume, the sphere.
The metamorphism of crystals we're talking about is one of two possible kinds of metamorphism, namely equitemperature metamorphism (the other one is the so-called temperature-gradient metamorphism). In the following we will concentrate on snow crystals as they undergo metamorphism during their stay in the snow cover (But, as we've said, these phenomena are probably not typical and exclusive for the substance H2O, but are due to prevailing temperatures). So let us quote LACHAPELLE, E., Field Guide to Snow Crystals, 1969, p.15-17 of the 1991 edition (comments between square brackets) :
From the viewpoint of thermodynamics, newly formed snow crystals have very unstable shapes. This is especially true of the intricately branched crystals. A large ratio of surface area to volume means that the surface molecules of the crystal have a large amount of potential energy stemming from intermolecular attraction (large surface free energy) [ It would be better, I think, not to speak of the high amount of potential energy of the surface molecules. They have, it is true, a high potential energy with respect to that of the molecules in the interior of the crystal, but the "high" in the quoted sentence should refer to the "large ratio of surface area to volume", which means that this same volume is able to lower its surface area, effecting in this way a decrease in the total amount of potential energy, and so relieving the system from 'unnecessary' strain. ] The natural tendency of thermodynamic processes (in this case the redistribution of mass and energy through phase changes) is to reduce surface free energy to a minimum. This means that snow crystals will tend to change so that the ratio of surface area to volume will approach a minimum [ In this change no melting is involved ]. The ideal shape to achieve this minimum is a sphere. Thus even the most intricate snow crystals tend in time to become rounded particles of ice [ It would, according to me, be more appropriate to state that "Evidently, then, the most intricate snow crystals (having as such a very large ratio of surface to volume) tend in time to become rounded particles if ice. But, surprisingly, also the most s i m p l e snow crystals -- i.e. snow crystals with no branches at all, having evolved by relative slow growth and expected therefore to have stable shapes -- tend in time to become rounded particles of ice." ] This process is called equitemperature metamorphism, for it proceeds only in bodies of snow which are not far from a uniform temperature. Because it tends to destroy the original shape of snow crystals, it is also known as destructive metamorphism. It is most rapid at temperatures close to the freezing point [ which is identical to the melting point ] and diminishes in intensity as the temperature falls. Below about - 400C it virtually comes to a halt. There is considerable experimental evidence to support the idea that most of the shape changes take place by transfer of water molecules from one part of a crystal to another through the vapor phase (evaporation and subsequent redeposition as a solid) [ Indeed, this is what was meant above by stating ". . . thermodynamic processes (in this case the redistribution of mass and energy through phase changes)" ]. Other processes may also be at work, but this is the dominant one [ This way of deformation of the crystals, i.e. by transfer of their material, also indicates that the crystal lattice is not affected by this type of metamorphism.].
The stages of such metamorphism in a stellar crystal are sketched in the next Figure [ Figure 6 in LACHAPELLE's booklet ].
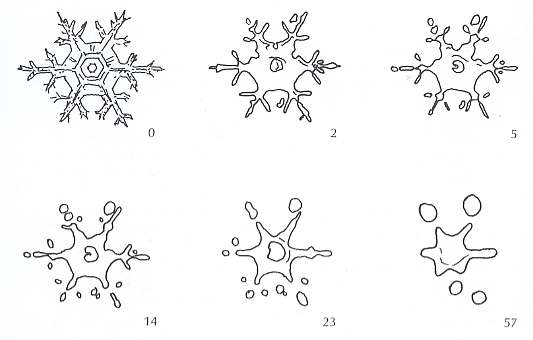
Figure above : The destructive metamorphism of a stellar snow crystal. The numerals give the age of the snow crystal in days.
(After LACHAPELLE, 1969, 1991.)
(continuing quote)
Note [ in the above Figure ] that the branches of the crystal tend to break up during metamorphism, so that the initial product [ "initial" is here meant in the sense that further processes lead -- if the snow does not melt in the summer -- to firnification and finally to glacier ice. With respect to the equitemperature metamorphism the mentioned "intial product" is more or less the final product.] is a number of small ice grains rather than a single large one [ , ] with the same mass as the original crystal. This gives the fine-grained appearance to old snow, which by definition is the end product of equitemperature metamorphism. Reduction in the space each snow crystal occupies causes the snow cover to shrink, or settle. This settlement is the normal accompaniment of metamorphism and is partially responsible for the fact that the snow accumulated on the ground is always much less thick than the total thickness of all the individual layers at the time of their deposition. Lowering of the snow surface through settlement (but not melt) gives external evidence of the metamorphic process taking place within the snow cover.
Equitemperature, or destructive, metamorphism is complete when the snow particles have been reduced to rounded grains (ideally to spheres) of ice.
The next Figures illustrate stages of equitemperature metamorphism.
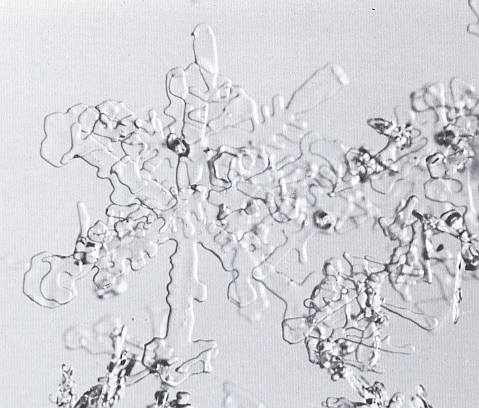
Figure above : Stellar crystals in the first stages of equitemperature metamorphism. The crystals are 40 hours old. (Magnification appr. 35x) (After LACHAPELLE, 1969, 1991.)
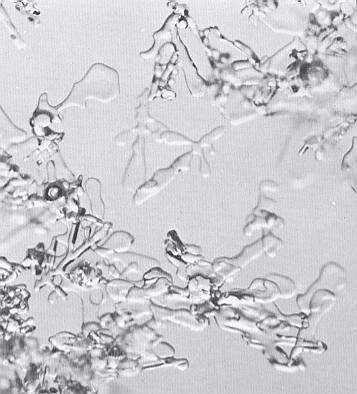
Figure above : The same snow layer represented in the previous Figure, after another 36 hours (in the accompanying text (of LACHAPELLE) it is stated "another twenty-four hours later") of metamorphism. (Magnification appr. 35x) (After LACHAPELLE, 1969, 1991.)
As destruction of the original crystals proceeds, they become completely unrecognizable, although occasional rounded fragments may suggest the last remnants of stellar branches. See next Figure.
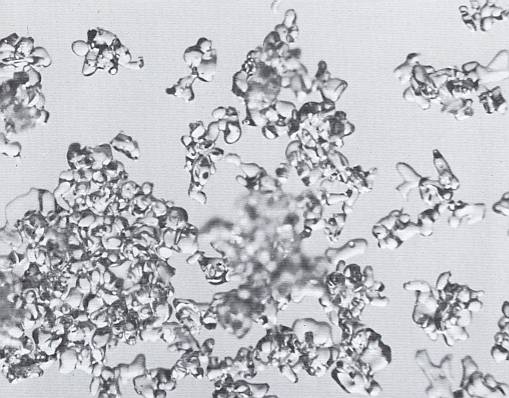
Figure above : Stellar crystals which have lost almost all their identity through equitemperature metamorphism. (Magnification appr. 35x) (After LACHAPELLE, 1969, 1991.)
The next Figure also shows an advanced stage of equitemperature metamorphism.
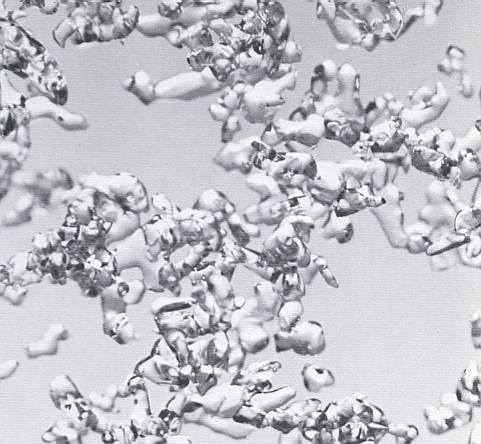
Figure above : Advanced equitemperature, or destructive, metamorphism. (Magnification appr. 35x) (After LACHAPELLE, 1969, 1991.)
Near the melting point, metamorphism proceeds very rapidly. The large, round grains of old snow may begin to appear even before destruction of the original crystals has been completed. An example of this is seen in the next Figure, where large ice spheres have formed at -0.50C in snow that is only twenty-four hours old. This phenomenon seems to be limited to temperatures at or just below the melting point. At lower temperatures, a slower and more uniform metamorphism prevails.
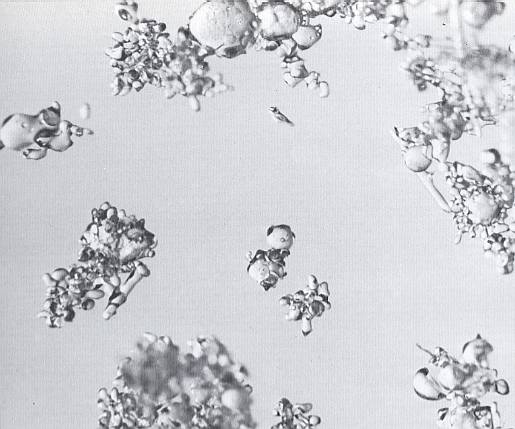
Figure above : Rapid metamorphism and growth of large grains at expense of the small one. Temperature is near the melting point. (Magnification appr. 35x) (After LACHAPELLE, 1969, 1991.)
The product of equitemperature metamorphism is by definition old snow. As produced in the normal winter snow cover at subfreezing temperature, this old snow has a very fine-grained, granular appearance. Close examination of such snow reveals small, rounded crystal grains which often are uniform in size within a given snow layer. The next Figure gives an example of old snow.

Figure above : Fine-grained old snow, 6 weeks old. (Magnification appr. 35x) (After LACHAPELLE, 1969, 1991.)
Qualitative explanation of equitemperature metamorphism
As we stated earlier equitemperature or destructive metamorphism is fastest when the temperature is not too far below the melting point. Maybe we can explain this qualitatively as follows :
When the temperature is relatively high, the equilibrium water-vapor pressure of ice is high, which here means that at equilibrium conditions (equilibrium between the solid and vapor phase) higher pressures of water vapor are possible than at lower temperatures. The value of the vapor pressure corresponding to the higher temperature can actually be realized (be reached) because there are by definition no significant temperature differences in a system undergoing equitemperature metamorphism. No significant temperature differences imply no significant vapor pressure differences and thus no significant flow of vapor from regions of high pressure to those of low pressure. So the high vapor pressure corresponding to higher temperatures can actually be realized, and this in turn implies that the traffic of particles from the solid (ice) into the vapor, and from the vapor into the solid, is 'heavier' than it would be at lower temperatures. And this more intense traffic eases the evaporation of particles from one site of the crystal and their subsequent deposition on another site of that same crystal, resulting in its deformation. And this deformation will be such that the surface to volume ratio becomes as small as possible, because this effects a reduction in surface energy. This process naturally leads to rounded forms with their ideal end product : the sphere. And indeed we see granular rounded ice particles as a final result of equitemperature metamorphism.
In spite of this clear explanation concerning the spontaneous but gradual transformation of angular H2O crystals into spheres (or at least into rounded forms), I still wonder why the end product of the equitemperature metamorphism of, say, a stellar snow crystal, isn't just a hexagonal plate, i.e. a plate having the shape of a hexagon. The only indication as to why also such a hexagonal plate will gradually turn into a more or less circular plate or even into a spherical ice grain, is that -- as has been said -- with natural snow we, in almost all cases have to do with crystals having a temperature not far below their melting point, that is to say, a relative high temperature, effecting much traffice of particles from and to the solid anyway, that sustains the transformation of the crystal's shape. And this is equivalent to saying that at such high temperatures the molethynes of the crystal are weakened and give in to surface tension.
Very low temperatures bring the process of equitemperature metamorphism virtually to a halt. We can see this in the case of cirrus clouds. Cirrus clouds are wispy high-altitude clouds (where, consequently, the temperature is very low indeed) made of 'diamond-dust' snow crystals. Diamond-dust crystals are typically only a few tenths of a millimeter in size. These minute cirrus crystals are not large enough to fall, so they remain suspended in the atmosphere, following prevailing winds. The best place to find copious diamond-dust crystals in the atmosphere (together with the halos they create) and on the ground is at the South Pole. Not only is it always bitterly cold at the Pole, the air is also dry -- ideal conditions for producing small hexagonal prism crystals and spectacular halo displays ( LIBBRECHT, K., The Snowflake, 2003, Chapter 7 ). As has been said, the cirrus crystals remain in the air for a more or less longer period (they can evaporate again or perhaps finally drift to the ground), and the dry air implies slow growth of the crystals, so in principle equitemperature metamorphism could take place provided that there are no strong temperature gradients. But the low temperatures bring with them low vapor pressures implying a weak traffic of particles to and from the ice crystal surface, so these are conditions that are unfavorable for equitemperature metamorphism to take place. And indeed the cirrus crystals turn out to be angular (and thus not rounded) as the next Figure shows.
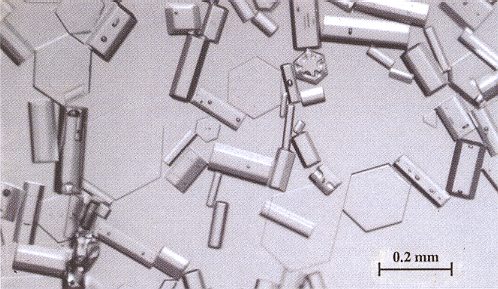
Figure above : Antarctic snow crystals.
These diamond-dust snow crystals fell at the South Pole. They grew slowly in the dry Antarctic air, thus becoming nearly perfect hexagonal prisms and plates. Most are only about 0.2 mm (0.008 inches) in size. Cirrus clouds are made of similar or even smaller ice crystals. These can as such be observed : If the sky is laced with thin cirrus clouds on an otherwise sunny day, you can observe diamond-dust crystals indirectly by the way sunlight is reflected and refracted by the hexagonal ice prisms. Each crystal sparkles in the sunlight as it tumbles about, and the combined effect of millions of sparkles produces an atmospheric halo. The simplest halo is one that occasionally encircles the sun with a 22-degree angle between the sun and any point on the halo. Although a suspended ice prism can deflect sunlight by any angle, a 22-degree deflection angle is especially likely. If you average the deflections of many randomly oriented crystals, a bright halo appears 22 degrees from the sun. The halo's intensity depends on the quality of the cirrus crystals. The most pronounced halos occur when the crystals are nearly perfect hexagonal prisms.
(After LIBBRECHT, 2003.)
Temperature-gradient metamorphism
The second type of metamorphism (but which also is a change of the snow crystals taking place after they have been deposited) is temperature-gradient metamorphism. Let us quote again LACHAPELLE, Field Guide to Snow Crystals, 1969, 1991, pp.17 (comments between square brackets) :
[ This happens] when large differences in temperature exist between adjacent layers of snow. This steep temperature gradient imposes differences in equilibrium water vapor pressure in the interstitial spaces of adjacent snow layers. Vapor then flows from regions of higher pressure (warmer snow) to those with lower vapor pressure (colder snow). Because the vapor is composed of the same water molecules as the solid ice framework, it is not necessary for these molecules to wend their way through the intricate passages among the snow crystals. A "hand-to-hand" transfer process is at work instead. The vapor is deposited as ice on an adjacent and colder crystal, while ice on the other side of the same crystal is removed as vapor and proceeds to the next crystal.
As the snow thus passes through the vapor stage, it is being subjected to temperature-gradient metamorphism. If this process, also called constructive metamorphism, continues, eventually the entire mass of the snow will pass through the vapor stage and be redeposited as new crystals. These crystals have an entirely different character from the original precipitated snow and its subsequent modifications. The tendency of equitemperature metamorphism to form rounded ice grains is reversed, and more complex crystals develop. If the process persists, the new crystals continue to grow and may eventually achieve a much larger size than the original precipitated crystals. The next Figure shows the resulting crystals.

Figure above : Typical crystals of mature depth hoar, as a result of temperature-gradient metamorphism. (Magnification appr. 32x)
( To see such dark pictures better, one should increase the brightness of the monitor if necessary) (After LACHAPELLE, 1969, 1991.)
(continuing quote)
The process of temperature-gradient metamorphism is variable, depending on such factors as altitude and snow structure, but it generally prevails when temperature gradients exceed about 0.10C per centimeter. There is a tendency for larger crystals to form with larger temperature gradients. The regeneration process proceeds most rapidly when the vapor pressure gradient is largest. In addition, the vapor pressure gradient is larger for a given temperature gradient at warmer temperatures than it is at colder ones. As a result of heat from the earth, snow is normally warmer close to the ground than at the surface. Hence the changes are most rapid at the bottom of the snow cover. [...] The newly formed crystals are much more weakly bonded together than are those produced by equitemperature metamorphism. [...] If the process is carried to completion, the snow develops a very fragile structure which will collapse into a cohesionless mass of crystals on slight disturbance. This condition is sometimes called "sugar snow". The scientifically correct term is depth hoar , reflecting the use of hoar as a generic term for all kinds of ice crystals that form by sublimation directly from the vapor. [...](end of quote)
The visible effects of regeneration first appear as angular and faceted crystals among those that retain their original form or have previously been rounded and simplified by destructive metamorphism. As the sublimation and diffusion of water vapor proceeds, the new crystals begin to take shape and the old ones gradually disappear. The new ones assume various shapes -- cups, scrolls, columns -- according to the local conditions affecting their growth, but they all have one feature in common that distinguishes them as depth hoar. All such forms produced by sublimation are characterized by a layered structure that appears externally as a stepped or ribbed surface on certain crystal facets. This is the most reliable identifying feature of depth hoar. There is some tendency for the developing depth hoar crystals to align themselves in faint columnar patterns parallel to the direction of vapor diffusion.
Depth hoar crystals (which are thus the result of a gradual recrystallization process, which one has called temperature-gradient metamorphism), are genuine crystals, i.e. such a crystal is crystallographically a unity, its crystallographic structure is uniform and continuous throughout the crystal.
Resuming quoting LACHAPELLE, p.22 :
[...] Much winter snow terminates with complete equitemperature metamorphism only because the snow is destroyed by melt before firnification [ a process finally ending up in the formation of glacier ice ] can proceed. Depth hoar can exist as an end product of temperature-gradient metamorphism only as long as the necessary temperature gradient persists. Once this is removed, the process reverts to equitemperature metamorphism.
[...] (pp.75)
Depth hoar is the end product of temperature-gradient metamorphism. This snow has been completely regenerated into entirely new structures that bear no visible relation to precipitated snow. A stepped or layered character of the crystal faces is the common identifying feature of depth hoar [ It can also be present, however, in hoarfrost, which is not metamorphosized snow, but the result of sublimation of solid H2O onto either a snow cover or some (other) terrestrial object, and is thus no snow at all. See below.]. The crystals may take many shapes. Cups and scrolls are characteristic, but these actually are a minority. Hexagonal patterns are common, but square corners are often seen. Many crystals, though not true cups, are hollow. The Figure given above and the next Figure show typical assortments of depth hoar crystals.

Figure above : Typical crystals of mature depth hoar, as a result of temperature-gradient metamorphism. (Magnification appr. 32x)
( To see such dark pictures better, one should increase the brightness of the monitor if necessary) (After LACHAPELLE, 1969, 1991.)
(Resuming quote)
These crystals sometimes reach a large size -- up to 10 mm or more in the normal confines of a winter snow cover. Many of the common shapes of depth hoar are visible in these two photographs [ Only a part of each of these original photographs is reproduced here. ], where a sample of snow in each case has been broken apart to display the individual crystals.[...]
The initial kind of snow, its temperature, and especially its temperature gradient, all determine the speed with which depth hoar will form and the ultimate size the crystals will achieve. Very strong gradients at warm temperatures cause depth hoar to form very rapidly [ From all this it is clear that at least in many cases temperature-gradient metamorphism is a process of fast crystal growth comparable to that resulting in branched crystals. ]. The process usually starts in relatively low-density snow. It can proceed only with difficulty when the initial density exceeds 400 kg / m3 . The snow in the next Figure is typical of new snow in which the effects of temperature gradients start to work. It is twenty-four hours old, is composed of stellar fragments (some rimed), has experienced a little equitemperature metamorphism, and has a density of 150 kg / m3 .

Figure above : Stellar fragments 24 hours old. This is the snow used to start the sequence (carried out by LACHAPELLE) of depth hoar formation (see text) shown in the next two Figures (accessible by a LINK). (Magnification appr. 40x)
( To see such dark pictures better, one should increase the brightness of the monitor if necessary) (After LACHAPELLE, 1969, 1991.)
(Resuming quote)
This snow was placed in a cold box under controlled conditions which produced a temperature gradient of 20C / cm, larger than is usually found in nature. Large mature crystals of depth hoar formed within six days.The complete sequence of depth hoar formation is shown in the next two Figures (After LACHAPELLE, 1969, 1991.), of which the first one, depicting the first three stages (a, b, c), can be seen by clicking HERE , while the second one, depicting the last three stages (d, e, f), by clicking HERE (Adjust monitor brightness, and, after inspection, press BACK button, to return to the text, which discusses these two Figures).
(Resuming quote)
At twenty-three hours [ a in first Figure ] , the first stages of temperature-gradient metamorphism have appeared (CM1) [ = first stage of Constructive Metamorphism ] . By forty-eight hours [ b in first Figure ] the first layered crystals of depth hoar are showing (CM2). After seventy-one hours [ c in first Figure ] the partial stage is well advanced and recognizable cup crystals can be seen. By ninety-five hours [ d in second Figure ] metamorphism is complete (CM3) and depth hoar has been formed. The process still continues and the crystals keep growing, ultimately reaching the very large cups shown in [...] [ f in second Figure ] . These photographs are all reproduced to the same scale, so that the magnitude of crystal growth can be clearly seen [ The magnification is approximately 18x ].The large, open depth hoar crystals ("cups" or other hollow forms) tend to form more readily when a temperature gradient is imposed on coarse-grained, older snow. Smaller, more compact crystals are the rule if the recrystallization begins in fine-grained or newer snow. [...]
Once the temperature gradient is removed, equitemperature metamorphism resumes and the crystals start to lose their layered structure. The next Figure depicts large, mature depth hoar crystals which have started to decay in this fashion.

Figure above : Depth hoar crystals slightly altered by equitemperature metamorphism. (Magnification appr. 27x)
(After LACHAPELLE, 1969, 1991.)
(Resuming quote)
Ultimately they will reach the condition shown in the next Figure, where the original depth hoar is barely recognizable.

Figure above : Depth hoar crystals profoundly altered by equitemperature metamorphism. Magnification approximately 40x.
(After LACHAPELLE, 1969, 1991.)
Non-precipitated ice : rime and hoarfrost
In addition to freshly precipitated (or still falling) snow crystals and (already) metamorphosed snow crystals, one other ice-crystal type deserves discussion (in our context of the form potential of ice), namely hoarfrost. While rime (in contrast to hoarfrost) does not show clear crystal form, we will nevertheless briefly discuss it, in order to let the characteristics of hoarfrost to stand out better (Like depth hoar, hoarfrost originates by sublimation directly from water vapor).
Let us quote again LACHAPELLE, E., Field Guide to Snow Crystals, 1969, 1991, pp.22 (comments between square brackets) :
The types of snow described up to this point have either originated in the atmosphere or have been produced by subsequent metamorphism of such crystals. Two other types, which strictly speaking are not snow at all, are formed at the earth's surface. These are rime and hoarfrost. The two are readily confused in nature by an observer and even more readily confused in the literature by authors. Rime is often called hoarfrost and vice versa. The two types of deposited ice crystals have entirely different origins. Once the manner in which they form is clearly understood, it is easy to distinguish one from the other by physical appearance [...].The next two Figures illustrate rime.
Rime [...] [ is ] a deposit of frozen water droplets [ either ] on [ falling ] snow crystals [ that as such become rimed crystals ] [...] [ or ] on terrestrial objects exposed to wind-driven supercooled clouds. The amount of rime so deposited may vary all the way from a light frosting to the enormous accumulations several meters thick which are found on the summits of mountain peaks constantly exposed to winter storms. In each case the rime is built up by freezing of supercooled cloud droplets as they strike the exposed surface [ emphasis mine ]. Depending on wind and weather conditions, rime accretions may take the form of a dense, compact mass, a feathery surface, or even slender, needlelike spikes. Close examination will always reveal a fine-grained, granular structure without any recognizable crystalline pattern [ emphasize mine ]. Rime always grows into the prevailing wind.
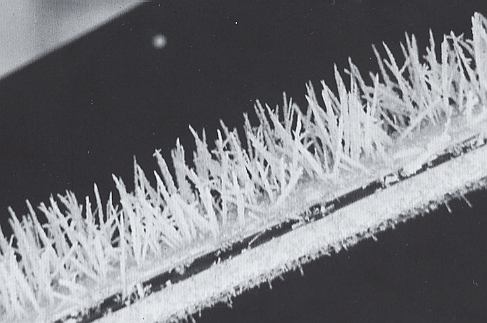
Figure above : Rime spikes formed on a ski pole. Magnification appr. 1.2x
Under certain conditions, which have prevailed here, and which are not clearly understood, the rime particles accumulate in slender needles, or "rime spikes", as the wind-driven supercooled droplets pile up one on top of another. The spikes in this Figure are growing on the windward side of a ski pole that stood outside during a foggy winter night. See also next Figure.
(After LACHAPELLE, 1969.)
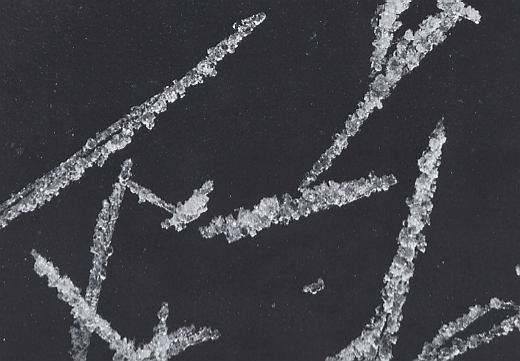
Figure above : Individual rime spikes from the ski pole in the previous Figure. Magnification appr. 9.3x . It is clear that such spikes are not comparable to genuine crystal needles. The latter are each for themselves crystallographically continuous, while rime spikes are not. (increase monitor brightness if necessary)
(After LACHAPELLE, 1969.)
While, from the viewpoint of intrinsic form potential of ice, rime is only of little interest and importance, hoarfrost is definitely important and significant in this respect, although, like depth hoar, the more or less typical shapes of its individual crystals are extrinsic in character. Nevertheless these shapes are definite shapes (in the sense that they are typical) that can be assumed by individual ice crystals and as such express something about ice's form potential, and probably about the form potential of crystals and crystallization in general and so emphasize their theoretical significance (within a crystal analogy) with respect to form-generation in organisms.
(quote resumed, LACHAPELLE, p.23)
Hoarfrost is formed by sublimation of solid ice crystals directly from water vapor in the air [ onto terrestrial objects ] [ emphasis mine ] . It is the ice equivalent of dew. This process has already been mentioned in connection with temperature-gradient metamorphism. It takes place at the snow or other surfaces whenever these surfaces are cooled below the dew-point temperature of subfreezing air. The commonest cause of such cooling is loss of radiant heat on clear, cold nights. Hence hoarfrost is most commonly found following such a night when the humidity is high. Hoarfrost crystals have a definite external structure which usually exhibits the stepped or layered characteristics of crystals formed from vapor. The smooth facets of hoarfrost crystals glitter in sunlight, quite in contrast to the dull, matte surface of rime. A coating of such crystals on the snow surface is called surface hoar.
So although hoarfrost, like depth hoar, originates by a direct transformation of water vapor into solid ice, hoarfrost is not metamorphosed ice. Further, the description by LACHAPELLE of hoarfrost turns out to be such that it -- if I'm not mistaken -- automatically includes that of windowpane frost, not as such discussed by LACHAPELLE, because his booklet only considers outdoor natural ice and snow forms. Windowpane frost ('ice flowers'), although being hoarfrost, is certainly a special kind or variety of the latter, because often very large structures emerge, which, however, remain very flat, closely adhering to the substrate, which typically is glass or the smooth surfaces of (parked) cars. It would be interesting to know more about the fine-scale structure, crystallographic continuity, or discontinuity, and possible twinning features of windowpane frost. Unfortunately no such knowledge is yet available to me.
Close examination of an individual surface hoar crystal (See next Figure (LINK)) shows it to have the layered structure peculiar to all hoar crystals, including depth hoar. Like rime feathers, these crystals start at points and spread out as they grow longer. The narrow end of the crystal in the next Figure (LINK) is the base. Also here the shape of the crystal, although typical for hoar, is extrinsic with respect to that crystal. And also here this shape is already present in the environment in the form of some directedness with respect to water vapor flow. To see such a crystal click HERE . The subscript of this Figure should read :
A single surface hoar crystal. Magnification appr. 14.7x. (After LACHAPELLE, 1969.)
Let us again quote LACHAPELLE, Field Guide to Snow Crystals, 1969, 1991, pp.97 where he further reports about hoarfrost.
Hoar crystals may reach considerable size if growth conditions persist in a quiet environment. A long spell of clear, cold weather will sometimes generate hoar crystals several centimeters in diameter on rocks, vegetation, or snow surfaces adjacent to a good supply of moist air, such as an open stream. Deep-freeze cabinets or cold storage lockers will also generate large hoarfrost crystals if left undisturbed for long periods. The crystal in the next Figure (LINK) [ Figure 63 in LACHAPELLE ] came from the lid of a cold chest in a laboratory. It has a cup shape similar to that often seen in depth hoar.To see this crystal, click HERE . The subscript to this Figure should read :
Hoarfrost crystal from a deep-freeze chest. This cup shape is also commonly seen in depth hoar. Magnification appr. 40x. (After LACHAPELLE, 1969.)
(quote resumed)
Enclosed ice spaces, such as ice tunnels or glacier crevasses, are also breeding places for hoar crystals whenever a temperature gradient persists along the walls [ I assume that "along the walls" here means that at every point of the wall a temperature gradient exists perpendicular to the wall, i.e. from the wall into the crevasse. ]. Very large hoar crystals, sometimes called crevasse hoar, can form if the growth remains undisturbed for months or years. The large, delicate flakes in the next Figure [ Figure 64 in LACHAPELLE ] are crevasse hoar which grew on a 2x2 cm wooden peg in the wall of an ice tunnel. This growth took about eight months to develop.
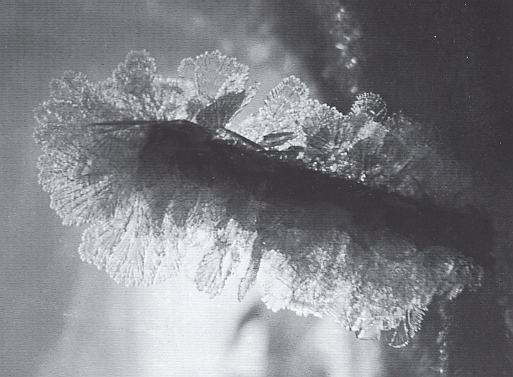
LIBBRECHT, K., The Snowflake, 2003, p.97 reports the following with respect to hoarfrost :
Caves and other enclosed structures sometimes house hoarfrost formations, provided there is a source of water vapor. If the air is still, hoarfrost can build up over days and weeks even when the supersaturation level is low. The most dramatic hoarfrost formations occur in the famed ice caves in Antarctica, where single crystals nearly a meter in size have been reported. These formations likely built up for years in the ever-frozen Antarctic.
From the phenomenon of equitemperature metamorphism we know that the molethynes (directional forces) in ice crystals under natural conditions generally are weak, because these conditions are often such that the temperature of the crystals involved is -- relatively speaking -- not far below the melting point of ice. At such temperatures angular ice crystals seem to be unstable, they gradually conform themselves to surface tension. Nevertheless the molethynes are present and can be detected by inspection of the intrinsic shape of the given crystals.
This concludes our discussion of molethynes and metamorphism in (ice) crystals.
e-mail : 
To continue click HERE for further study of the Theory of Layers, Part XXIX Sequel-17.