

e-mail :  ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
Having, in the pre-previous document, presented an introduction and three Sections on the one-molecular view of the organism (Unimol), and having, in the previous document devoted three more Sections on the unimolecular theory of orgnisms, we now continue with this theory, Unimol, still largely following Oskar Müller, 1959. (See Note of author of website (from pre-previous document) ) (and use the Page-back button of your browser to return to the present text)
Introduction by the author of this website.
"Chemistry" is the investigation of the structure, properties, and affinities (as to reactivity with other compounds or chemical elements) of, first of all, so-called chemical compounds, that is substances consisting of (free) molecules, where a molecule consists of two or more atoms of the same or of different kind. Secondly, of course, it is the investigation of chemical elements as to their affinity to the same or other chemical elements. So chemistry is especially concerned with chemical bonding between atoms or between molecules (resulting in super-molecules). It is concerned with chemical reactions. It is the science of material change, insofar as it is a change of nature of given substances, whereas physics also is about change, but here a change where the substances remain the same in nature while their mutual configuration changes : particles or bodies that move, substances that melt, or evaporate, changes in pressure and temperature, etc. But all processes, whether chemical reactions or physical transformations, proceed, as to their spontaneity, according to the rules of thermodynamics and ultimately to the rules of quantum mechanics.
Chemistry is traditionally divided into so-called "organic chemistry" and "inorganic chemistry". The name "organic chemistry" was derived from the supposed fact that all substances with which it deals are (ultimately) coming from organisms. But when "organic substances" were produced in the laboratory from inorganic substances, it became clear that there is no fundamental distinction between "organic substances" and "inorganic substances". So today the term "organic chemistry" means chemistry that is dealing with carbon compounds (except, perhaps, with substances like calcium carbide (Ca2C, if I'm right), i.e. it is dealing with all substances that are relevant and important in organisms or derived from them. But then, of course also certain metal-ions and, naturally, water, H2O, appear in organic chemistry when this becomes biochemistry, because of their great importance in biological entities. But the typical carbon compounds organic chemistry is dealing with are alcohols, ethers, fats, hydrocarbons, fatty acids, sugars, amino acids, nucleic acids, etc. etc.
All other substances, like metals, sulfides, silicon-compounds (such as in many minerals), atmospheric gasses, etc., are treated of in "inorganic chemistry".
Further, there are intermediate disciplines such as "physical chemistry", studying, for instance, colloidal solutions.
But, generally, chemistry, whether "organic" or "inorganic", is about molecules. And it is now, indeed, a good question to ask what then molecules precisely are. Well, they are chemically bonded atoms ("polynuclear atoms"), but what things in Nature are true molecules? Although molecules may become quite big, one unconsciously presupposes that their size has an upper boundary. But in fact, the industrial production of polymers such as plastics indicates that there is perhaps no such boundary. However, when it is about entities such as individual organisms one hesitates to call them "single molecules", because one knows of the many free molecules that exist in the organismic body. And, as far as I know, no more ot less recent author, except Oskar Müller in 1959, came up with the idea of the organism being a single molecule although embedded in an aqueous serum-like support medium consisting of many free molecules, among them many proteins. And if indeed the very kernel of an organism is a single molecule, then it automatically becomes a subject of chemistry. But then a special subdiscipline of chemistry must be created alongside inorganic and organic chemistry : Organismic Chemistry. And it is this "branch" of chemistry to which the present Section is devoted, following the corresponding (German) text in Oskar Müller's book on Unimol.
The fact that life and chemistry, and thus biological phenomena and chemical processes, are closely connected with each other has become such a self-evident case, that one often has taken the problem as too a simplistic one. This first of all relates to the expectation to obtain almost automatically an explanation of the deep-seated problems of life, and thus of the "essence of life", as a result of intensive observation and clarifying of the "chemical part of life". With this, at least the following should be considered :
The characteristic biochemical processes are not basic constituents ordered to some end, but serving functional mechanisms, which today are analytically discovered. And the energetic metabolism is certainly a characteristic, but not an essential biological phenomenon. It is a specifically selected series of processes which characteristically are found precisely there where life is goung on and simply needs these processes in order to be and to be active. This "to be active" does not consist in the metabolic processes, but as a result of them it is possible ( NOTE 196).
The -- especially psychic -- phemomena of life (bio-phenomena) are not strictly bound up to the established chemical and physical processes, but the latter are accompanying indicators (so to say by-products), often also functionally effective, but with this, however, in most cases not ordered to the bio-phenomenon in question, but being employed by contiguous [subsequent] processes of restitution. It here is about a certain compulsion and unavoidability in which we are used to see necessity, or even, satisfying causality, a presupposition. In order to really apprehend things, it is recommended to assume that the "true" essential event has already been completed at the time when one observes as a residue or trace a chemical or physical "accompanying" process. Desiring to get (gradually) to know, from the total of such observable or observed processes, precisely that which has instigated these processes, that in any case is causally connected with it, is -- even with the presupposition that one is looking in the right direction anyway -- not possible ( NOTE 197).
Also the nature of the processes and their outcome is, in all fundamental significance to the organism, less revealing than one initially believes. Technically remarkable is the neglect [in physiology] of pressure, temperature and strongly deviating pH (degree of acidity) environments, in which, however, one should not forget that the effects of these three factors [pressure, temperature, pH ] in small compartments can be completely mimicked and thus realized without being macroscopically detectable. All is proceeding in one or another -- often leading to the same result -- micro-framework, for instance strong boundary-surface adsorption in place of higher pressure, or the lowering of the reaction energy barrier in place of higher temperature, and other such things. Details as well as the general principles will, of course, allow many conclusions to be drawn as to chemical phylogenetic states.
We cannot with absolute certainty say that all bio-processes are subject to physical or chemical laws familiar to us, but it certainly holds for all clarified details. Part of the bio-processes may, possibly, be based on regularities typical to Life only, but which, in the sense of our categories of knowledge [i.e. the a priori (and perhaps also the a posteriori) conditions of human knowledge], can also be rated as causally working physical or chemical laws.
With a material science of the living we mean the science of the molecular life-carrying, or better, living forms, and thus, in contrast to biochemistry and chemical physiology, not a science of whatever parts or processes, but of the whole. It is a chemical science of structure of the same objects of which botany and zoology treat, where in the latter two sciences external forms and external behavior are described.
If classical chemistry is physics of the normal-atomic [= stoichiometric atomic] self-stable combinations of atoms, then organismic chemistry ( NOTE 198) or simply the organismic, is physics of the life-allotropic atomic [= (something like) weak-stoichiometric atomic] not-self-stable, but maintaining itself through the self-existential function, combinations. The organismic morphology as the functional existence of special matter, then may, in a certain way, be allocated also to chemistry and namely to pure chemistry, and not to physiological chemistry by which we mean something totally different ( NOTE 199), precisely as with, say, crystallography [which also is a morphological chemistry]. The causal connection between matter and form is the necessity of precisely this functional form, i.e. to lead this matter to more or less unlimited existence [We [JB] would rather say : the form is in such a way functional that it can exist in the material order, i.e. that it can in-form matter].
[The molecules of "normal chemistry" are, under appropriate conditions, self-stable thermodynamically and quantum mechanically, and can then exist without more ado, i.e. they do not actively have to behave in certain ways in order for them to exist. They simply can exist because, under the mentioned conditions, they are lowest-energy structures as compared to their free components, and the system formed by their bulk-form is in thermodynamic equilibrium. In contrast to them, we have the organismic molecule, the living mega-molecule : While under given circumstances we must assume that all its internal chemical bondings are lowest-energy substructures, the molecule as a whole, which undoubtedly behaves as a large collection of quasi-individual molecules, and so constituting bulk matter, is thermodynamically far from equilibrium. And as such it is only metastable. This means that it is stable, but a strong enough perturbation may push it into thermodynamic equilibrium, and this implies that it will disintegrate (decay) into stable parts, and then, of course, the organism has perished. The typical structure of the organism can only be maintained by holding it far from thermodynamic equilibrium, so the organism must actively hold itself in this condition, without, however, becoming choked with entropy (which is or entails disorder). So entropy must be exported, dissipated, from the organism (therefore it is called a dissipative structure), and new, usable, energy (and matter) must be imported (feeding). So the organismic living molecule embodies a "strategy" to exist. And this strategy includes the demand for the molecule to be embedded in a special support medium as an extension of the molecule's strategy. And the medium, still belonging to the organism, mediates the living molecule's necessary contact with the extra-organismic environment, the organismic species' ecological niche. And the species-specific activity of the organism in its ecological environment is a further necessary extension of the living molecule's strategy-to-exist.]
A digression (still from the hand of the author of this website) on form, shape, and symmetry, in "organismic chemistry".
In fact the promorphology of organisms contains indications of Unimol : The positive presence of symmetry in bodies (macroscopic solids) does not indicate that these bodies are living, because there exist symmetric inorganic bodies, such as crystals, and because there exist living bodies that are completely asymmetric. Nevertheless, symmetry in organisms is remarkable : while the symmetry of crystals can largely be explained by their (internal) reticular structure, i.e. their strictly periodic structure, the symmetry of organisms (most organisms are symmetric in one way or another) cannot be so explained, because the true organismic structure is is not reticular, not periodic. It is even contra-periodic. And although the symmetries in organisms, as studied by promorphology, are in most cases only approximate symmetries (the symmetry-breaking features may be seen as derived features), they are conspicuous enough and deserve enquiry about how they originate. How can it be that, for example, in vertebrates and insects the left-hand half of the body is, as to many details, the mirror image of the right-hand half? This is an instance of "two-fold symmetry" in the sense that the (only) two antimers (counterparts) of the body are each other's images when reflected in a plane separating these two antimers. And how can it be that every individual starfish develops from such a two-fold state (larva) into a five-fold state (adult), meaning that the body now has five symmetry planes and five antimers (which themselves in turn possess the described two-fold symmetry)? We know it cannot be because of an internal reticular (periodic) structure (because there isn't any in organisms, and, by the way, a reticular structure cannot support a five-fold symmetry). So how is symmetry supported in organisms? Well, if an organismic body were not a true material unity, not materially continuous, but just a collection of smaller and larger free molecules, how can these free molecules be organized such that they result in a relatively stable, constant, repeatable (in next generations), macroscopic symmetric pattern, i.e. result in a symmetric body? And this is where Unimol comes in : If the organism is not a collection of more or less equivalent free molecules, but is a single living mega-molecule (wetted, it is true, by an aqueous support medium that does contain a great many individual, free, more or less small-sized molecules, but a medium not altering the shape of the living mega-molecule when this molecule is considered together with that medium), - then the mentioned symmetries are less puzzling : We have to do with a single molecule (together with the support medium making up the organismic body) that simply has this symmetry (which is almost always, it must be remembered, an approximate symmetry, but a symmetry nonetheless). And this symmetry of the living mega-molecule is not necessarily an expression of a lowest-energy state of the molecule, because, as we know, the organism, and thus also the organismic living mega-molecule, is a "dissipative structure", i.e. a structure being held far from thermodynamic equilibrium (the mega-molecule behaves more or less like a population of free molecules in this respect). It imports energy (and matter) and exports entropy (and matter), and so maintaining its ordered structure. So the fact of the presence of symmetry (such as 2-folded or 5-folded) in many organisms does support the Unimol hypothesis.
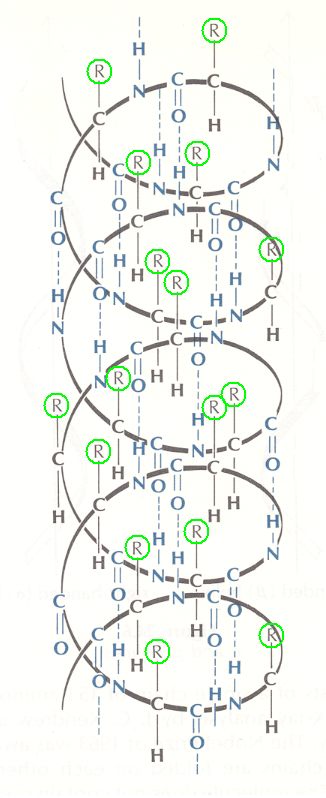
The living mega-molecule is supposed to be protein-like. And a protein has, as its "backbone", its polypeptide chain, i.e. a chain of amino acid residues connected by peptide bonds. The chain can bend and twist at certain places, but not about its peptide bonds (because they resonate between single and double bond). A peptide chain (i.e. any peptide chain, and thus any protein) can also be connected to itself (resulting in loops) or to other peptide chains (resulting in "super-peptides"), but this can be done only by disulfide bonds, hydrogen bonds, or hydrophobic "bonds", meaning that complex and especially branched proteins cannot be the result of branching of peptide chains, i.e. the peptide chain cannot become branched by side-ways directed peptide bonds (at least no such branched peptide chains are ever extracted from organism). So it is safe to assume that also in the protein-like living mega-molecule there are no true peptide side chains. If there are side-chains, and surely there are, they are mediated by hydrogen bonds or disulfide bonds. And these side chains may contain many kinds of chemical groups, and, of course, also stretches of peptide chains (with peptide bonds). A peptide, as a chain of amino acids, can also form cyclic structures, i.e. structures in which the peptide chain is closed into a loop, but, as it seems, only can do so with the help of inserted disulfide bonds (between two adjacent cysteine residues in the same chain) in addition to the usual peptide bonds in the chain. Such are the nona-peptides oxytocin and vasopressin, both secreted by the pituitary gland :
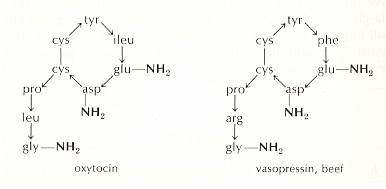
Figure above :
Structure of the nona-peptides oxytocin and vasopressin.
The arrows indicate the direction of attachment from a carboxyl group (COOH) of one amino acid to an amine group (NH2) of the next amino acid (they thus symbolize peptide bonds). In the chain of each peptide two adjacent cysteine residues (i.e. residues of the amino acid cysteine) are connected, not by a peptide bond, but by a disulfide bond (see HERE )
The abbreviations in the chains stand for residues of amino acids : gly = glycine, leu = leucine, pro = proline, cys = cysteine, tyr = tyrosine, ileu = isoleucine, glu = glutamic acid, asp = aspartic acid, arg = arginine, phe = phenylalanine. See for them HERE. The amino groups (NH2) indicated do not belong to the amino-acids-proper but simply belong to the oxytocin and vasopressin molecules.
(After OUELLETTE, 1970)
When thinking of the symmetry in organisms, we might wonder whether the chief polypeptide chain of the living mega-molecule is a sort of material axis (or, perhaps, plane) maintaining the organismic molecule's three-dimensional shape. But this cannot be, because, as had been said, the polypeptide chain can twist and bend at many of its places, and, moreover, if it were hard and stiff, it, the mega-molecule, would have been detected easily. What maintains the three-dimensional shape of most organisms is an internal or external skeleton or integument, which is the product of denaturation and of (subsequent) deposition of inorganic material (as in animals having bones or shells, or are supported by needles).
The chief polypeptide chain of the living mega-molecule may play the role of the material body (main) axis, not as a construction support providing mechanical strength, but as a material symmetry element, that is, a material reflection "plane" or a material "axis" of rotational symmetry, and so materially connecting mutually symmetric molecular parts (body parts) with each other.
So although all this is pure speculation, it is certainly interesting to study organismic promorphology in the context of the Unimol hypothesis. As has been said, Organismic Promorphology can be found in "Second Part of Website" from "Introduction to Promorphology" onwards.
Living substance and its mechanical support.
The living substance [of an organism] is, in all probability, not a true protein as we know them from the support medium. Only its mechanistic-chemical equivalent is, after denaturation, a protein molecule. So the protein collagen, as it is present in skin and bones, and providing external and internal mechanical support in vertebrates, is, according to this view, partly a denaturation product of the living substance, partly still this living substance. And so in vertebrates the non-denaturated part of the skin and of the bones, the non-denaturated state of what we know as collagen, still belongs to the living substance, and thus to the living mega-molecule. And while this non-denaturated collagen, as present in the organism, already expresses the main features of the three-dimensional shape of the mega-molecule, it consolidates it by denaturation of its outer margins, or/and by secretion of non-living matter, or/and by deposition of inorganic material.
While in vertebrates mechanical support of the body and its shape first of all is the internal skeleton (bones) and secondly the (outer) skin, in insects it is only the latter, i.e. the skin, the cuticle, which mechanically supports the body and its shape. And because indeed most animals are insects, we'll dwell a little longer on the insect integument. The following three alineas are taken from RICHARDS and DAVIES, Imms' General Textbook of Entomology, 1977.
The integument of insects consists of the following layers : (i) the cuticle, (ii) the epidermis, and (iii) the basement membrane. The cuticle is the true mechanical supporting layer.
The cuticle is a complex, non-cellular layer secreted largely by the epidermis [the outer cell-layer] and though commonly considered non-living is actually the seat of complex biochemical changes, some at least under enzymatic control. It forms the outermost investment of the insect body and its appendages but is invaginated locally to form endoskeletal structures and also provides the lining of the tracheal system and parts of the alimentary canal and reproductive tract. When newly formed it is flexible and elastic and in many larvae it remains so over much of the body. In most insects, however, the greater part of the cuticle undergoes a process of sklerotization whereby it becomes hardened and darkened to form more or less tough, rigid sclerites separated from each other by membranous zones of unchanged soft cuticle. Such an arrangement combines rigidity with flexibility and in addition to its protective function the cuticle determines the form of the insect [in Unimol we [JB] would say that the living molecule determines its own shape which it then consolidates by forming a more or less rigid cuticle]. The cuticle's relative impermeability to water reduces desiccation and it provides a firm basis for the attachment of muscles. [...]
The cuticle of insects has two major components : The carbohydrate chitin (25-60 percent of dry weight of various cuticles) and a number of proteins [a "carbohydrate" is a substance such as (one or another) sugar or starch. Its general formula is (CxHyOz)n.] In the cuticle, chitin chains are apparently joined to proteins by covalent linkages, proteins involving aspartic acid and histidine (both one of the 20 biological amino acids). The chitin-protein complex is in fact a polydisperse glyco-protein in which rod-like chitin fibres of 2.5 to 6.5 nanometers in diameter are embedded in a protein matrix.
The formation of the cuticle takes place by apical secretion of cuticle substance from the plasm of the epidermic cells, combined with the transformation of plasm into cuticle substance.
The strength (stiffness) of chitin is similar to that of cellulose. Also the molecular structure of chitin and cellulose is similar. See Figure (from WEBER, 1966).
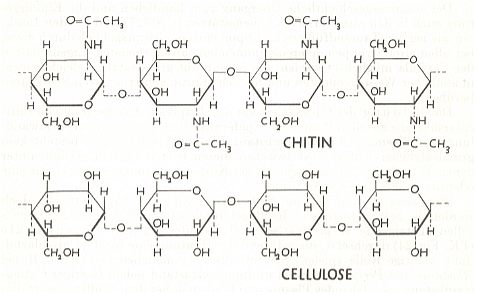
Indeed, it is cellulose that forms the mechanical support structure of plants, i.e. the mechanical support of the living plant substance.
On the other hand, in molluscs (such as snails), as well as in (unicellular) foraminifers, it is the CaCO3 (lime) containing shell that encases the organism-proper, while in (unicellular) radiolaria it is the "skeleton" (consisting of spherical perforated shells and needles made of SiO2 (silica), SrSO4, or other material) that supports the (unicellular) body.
So all this "hardware" encases or mechanically supports, either from within or from without, the living substance. It is either a denaturation product of the living substance or a secretion from that substance, and it often mediates (in insects, molluscs, and foraminifers) between the living substance and the outer environment, or (in vertebrates) holds the body parts in place and keeps up the organism's shape (bones), or stabilizes its external shape (skin, chitin), or both (cellulose in plants).
So according to Unimol, the structure and shape of the living mega-molecule, and thus the structure and shape of the organism, is determined by the molecule's chemical constitution, and becomes, in most cases, consolidated -- mechanically stabilized -- by more or less rigid support elements, meaning that not these elements do determine the three-dimensional structure and shape of the organism, but the living mega-molecule itself. And the latter, with it, especially determines the overall symmetry of the organism (its stereometric basic form or "promorph"). But in vertebrates the internal skeleton, the organism's mechanical support system, seems to determine its symmetry, instead of, as it seems, the living molecule. However, we can be certain that the bones of living vertebrates are not completely dead elements but are imbibed with living matter, such that the bones are living. So also here it is the living matter that determines the symmetry of the organism. And the same can be said of support structures that are secreted by living tissue, such as the skeletons of radiolaria, or the shells of molluscs and foraminifera.
[ Continuing with Müller's text ] Our basic thesis is, as already mentioned, the following : The typical properties of Life are molecular existential functions of definitely ordered ground material. Molecular forms which are a kind of continuation, or better, are a completion of the normal, i.e. non-living lower molecular chemical compounds, for themselves possess properties which we have first come to know in their upgraded effects, namely the organisms of today. Some of these properties give, considered in isolation, in all respects the impression of "mechanical" functions, and we see in them also the consequent phenomena of very definite structures, which [structures] -- just like the non-analyzable [into parts] characteristic life-phenomena -- are in turn made possible and generated by atomic fine-structural properties of the basic material constituents.
If one takes the atomic fine-structural properties of the organogeneous elements (C, O, H, N) first of all as special potencies (because they, as they are in themselves, are unknowable) ( NOTE 200) and let them be part of a complete system of potencies that is also the basis of generation of the simple physical properties, then it surely is self-evident that the totality of atomic potencies cannot be deduced from some randomly chosen inorganic chemical compounds ( NOTE 201). The atomic properties in their totality must rather be estimated from the totality of consequent types [i.e. the complete set of properties (or potencies) of a given species of atom must be assessed from the complete set of all products of which generation this species of atom is capable.]. We don't know what, say, the carbon atom in fact is, but it is not generally inorganic, rather it is pro-inorganic as well as pro-organic, and even pro-organismic. So the question of the origin of life, i.e. how life has originated from the inorganic, is encumbered with a false image [because in the origin of Life the inorganic was neither the initial nor an intermediate state.].
He, or she, who takes the carbon atom (C) to be "animate", because it decisively is involved in the constitution of animate organisms, is not more a dreamer than he, or she, who takes this atom to be inorganic only because it constitutes methane (CH4) or graphite (C). The atomic potencies -- to which we also want to reckon any internal or combinatorically external possibilities of interaction which may show the nature of seemingly working from "without" -- wholly necessarily comprise all consequent combinations coming from the atom, and thus in the organogeneous atoms the inorganic and the organic, as well as the organismic realm including the ability of phylogenetic development. Biologically, it would have been better to have learned to know the organogeneous atoms as constituents of organismic life and not of gases and crystals. Then, certainly the question would not arise how from so few "simple" atoms could ever originate such "complex mysterious forms" ( NOTE 202).
Who, for that matter, wants with certainty to exclude that already the organogeneous atoms -- especially in their bonded states -- do possess a classically methodically not simply demonstrable "mesomeric" [as to intermediate parts] ambivalence with possibilities of transition, for example after "instigation" by life-already or reversely in the case of organismic death (being an intra-atomic denaturation effect). The (to us seemingly unchangeable) continuity of the carbon atom during a cycle that can be investigated and defined, leaves wholly open the possibility of reversible intermediate states [of the carbon atom], and one could conclude from the special qualities of the organismic that here it is about the consequence of a further not demonstrable atomic allomery [atomic versatility] ( NOTE 203).
We have many unshakable physical and chemical experiences. But over and above it we actually don't know what an atom all by itself really is. Almost all definitions boil down to excluding certain things, i.e. saying what an atom is apparently not (therefore being a preferredly negative characteristic). If one wants to derive the potential overall properties of the "free" atoms from those of bonded atoms, then one has in every case properly to take into account the existence of the organismic and its features ( NOTE 204). The most promising future of atomic physics -- which one today views especially as nuclear (fission) physics -- should lie in a true, departing from a broad basis, biophysics.
Adhering to the position of there being a correspondency between reaction-base and reaction-consequent phenomena, we can say that all subsequent diversity of forms is already based in the more elementary particles and systems, and that such capacities of building systems must be attributed to the elementary properties of at least organogeneous matter. Following P. Jordan's amplification theory of organisms, especially emphasizing behavior (s.l.) in the living realm, one could, therefore, say with respect to the statically structural : Microphysically chemical construction-details of the atoms -- also the still potential details -- do not only appear in, but also as macro-forms [these (subsequent) macro-forms, also as macroforms, reflect features of the atomic construction-details]. Or, again, differently formulated : The organismic forms are, as to their substantial and "molecular" and subsequent histological [concerning living tissue] content, amplifications of definite fine-structural properties of a part of the basic matter, but as to their essential functional content [the organismic forms are] amplifications of the life-fundamental conditions of maintenance and improvement tendencies ( NOTE 205), [i.e. as to their essential functional content they are strategies-to-exist.].
Our consideration now should clearly reveal the following : The living state follows [i.e. comes directly after] the chemistry of the non-living [i.e. organic and inorganic chemistry], and together with it forms the whole field of possibilities of molecular combination ( NOTE 206). With this, however, one should not take this asserted consequent connection with classical chemistry in the sense of a real "continuous" ( NOTE 207), i.e. linearly progressing transition, so that, for instance, after the group of the most complex of known chemical compounds -- originating as products (natural substances) from the organismic realm anyway -- there first follows a gap, which today is not yet surveyable and bridged (the missing "links"), and then [follow] the chemical compounds of Life, compounds with highly increased complexity ( NOTE 208). This kind of connection between chemistry and life would neither properly account for the structural construction [of the chemical compounds of Life] nor for the relationships [between chemistry and organisms].
Living matter (substance) is not dead matter + life-principle, but living matter is an especially potent wholeness-matter, which corresponds sister-group-like with, and is physical-chemically equivalent to, "dead" matter, with a more easy and spontaneous transition from life to non-life, than a transition from non-life into life, a transition that is only specifically (template-catalytically) instigated [Here, with "the transition from non-life into life" is meant, not the origin of Life itself, but the transformation, and subsequent incorporation of non-living substance into the living substance of an existing organism.].
If one starts with the stellar matter-formation processes, then one has a kind of kink-free and uninterrupted line of development from nucleons [particles of the atomic nucleus] and elementary particles [such as electrons] up to human beings. But if one simply compares inorganic and organismic substances, and be they so "similar", for instance chicken egg-albumine [a protein] and chicken cell-protoplasm, then a connection line is here as little direct as that beteen insect and vertebrate or between human being and almond tree ( NOTE 209)
The consequent connection (the connection-kinship) does not, in spite of all reality of relationships, lie between living high-molecular substance and the high-molecular substances known to us, but effectively and practically in the act of the origin of Life ( NOTE 210) and, more or less parallel to this, in the present instigational transition of anew-generation of living substance, as well as physically theoretically in the stabilization from demise-threatened "intermediate phases" (better : special-phases) to normal-equivalents. [The "intermediate phases" are short-living interim products, mediate between reactants and products in chemical reactions.]. This stabilization expresses itself in an existential function [ Existenz-eigen-ein-funktion] of a very special imprint, namely that what we call the life-characteristic.
The life-carrying substances known to us -- or at least the most conspicuous and representative part of it -- may succinctly and generalizing be called proteins, but with the limitation that they find themselves in a special, analytically not yet recognizable structural state [referring to tertiary, quaternary, and more, 3-d structure], which exists as long as life is there (and the outher way around) ( NOTE 211). The living substrate contains protein equivalents which may transform into normal protein and which the living organism produces from the same building blocks into which proteins can be disassembled ( NOTE 212).
Proper-to-the-body real protein molecules only exist in the form of serum-like substances of the content of the support medium ( NOTE 213). Apart from this, "protein" is not present in living-matter-proper. So for the chemical content of living matter another expression should be used, for instance "vital-proteins", or "bio-proteins" (to distinguish them from the native proteins [proteins extracted from living beings, and still having preserved their original 3-d structure.] ) or [using] the well over 100 year old -- [word], having, as a result of extensive and partly also abusive application, easily become unmodern (partly also because "un-chemical") -- but still proper word "protoplasm", which one perhaps wants to transform into "proteoplasm", and which [expression] must be liberated from the naive picture (connected with it) that with "protoplasm" (the living substance) it is about a lump of gelatine or slime containing the secrets of life ( NOTE 214).
Because the living substance is, inspite of probable differences, supposed to be protein-like, and thus containing a polypeptide chain, we here shall interrupt the text of Müller (being itself on the structure of living substance) with an intermezzo on protein structure, especially on the ALPHA-helical structure of the protein's polypeptide chain. Considering it in more detail (it was already mentioned in the previous document) will be very instructive to understand the "morphological chemistry" of organisms. For some basics about amino acids and the nature of the peptide bond linking them (i.e. their residues) together into peptides and thus into proteins, see previous document.
Recall that an amino acid (molecule) has the general formula :
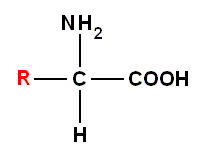
where "H" is hydrogen, "O" is oxygen, "C" is carbon, "N" is nitrogen and "R" is one or another chemical group,
and recall further that amino acids can be bonded to each other (by expelling H2O, such a reaction is called a "condensation") by so-called "peptide bonds". Here an example of the concatenation of two amino acids :
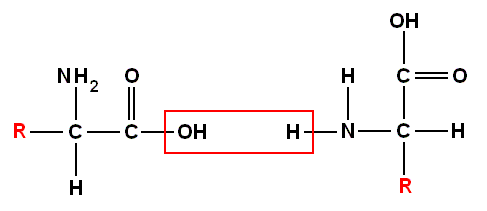
combining to produce :
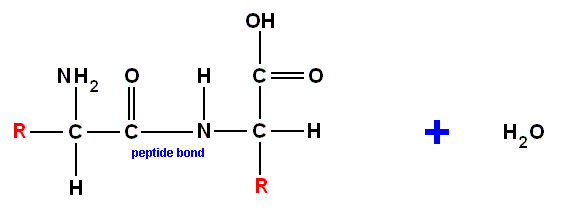
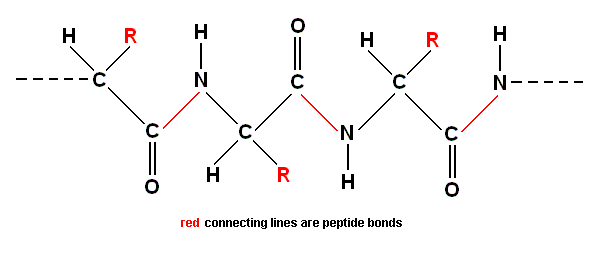
The result of concatenation of a large number of amino acid residues is, to begin with, a zig-zag polypeptide chain :
The C-atoms bonded to R, H, N, and C, are "asymmetric carbon atoms" meaning that to each such an atom four different groups (or atoms) are bonded, allowing for left-hand / right-hand isomers, called enantiomers. The four bonds of the asymmetric C-atom are arranged tetrahedrically.
While the chain cannot rotate around the peptide bonds (which are planar) it can rotate around the bonds on either side of the peptide bond.
The twisting around these bonds can cause the 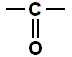 group coming to lie precisely opposite to an
group coming to lie precisely opposite to an  group of the same chain, making possible for hydrogen bonds to form. Here such a hydrogen bond is illustrated :
group of the same chain, making possible for hydrogen bonds to form. Here such a hydrogen bond is illustrated :

Indeed, when all the angles between atoms becoming just right, conforming to best-stability conformation, the polypeptide chain curls up into a spiral stabilized by the hydrogen bonds between CO and HN groups that lie opposite to one another (i.e. between a CO group of one amino acid and a NH group of another amino acid lying a few amino acids further down the chain). The result is that the polypeptide chain takes up the form of an alpha-helix. Such a helix is a right-handed helix as contrasted with a beta-helix which is a left-handed one. For proteins consisting of L-amino acids (which is an enantiomeric form, a stereo-isomer, left-right-hand symmetric with respect to a D-amino acid) the right-handed (or alpha-) helix is more stable than the left-handed helix.
The alpha-helix of the peptide chain can become more or less distorted by the presence of disulfide bonds (i.e. if such bonds are present). The molecule then has to assume a shape consistent with the structural restrictions of both disulfide bonds and the hydrogen bonds. The resultant chain is still roughly helical but with a bent structure that gives the molecule a spherical shape. The regular helix (with a constant pitch) can also be modified by the presence of the amino acid proline which has no NH2 group but instead a NH group, loosing its H in the formation of the peptide bond (the H is -- together with an OH group -- expelled in the form of H2O (water)), so that the protein structure cannot hydrogen bond at the point where the proline residue is present. The helix bends at each point where a proline residue occurs in the polypeptide chain.
Knowing now of these possible distortions of the regular alpha-helix, let us proceed with further enquiring into its precise structure.
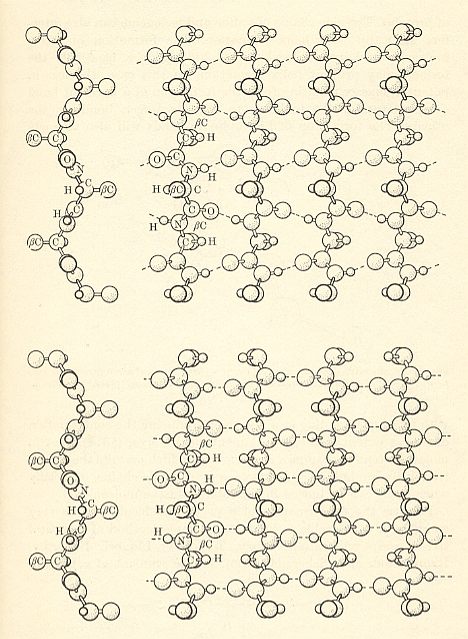
The regular alpha-helix polypeptide structure, as it can be found (as a morphological element) is in many proteins, may schematically be drawn such :
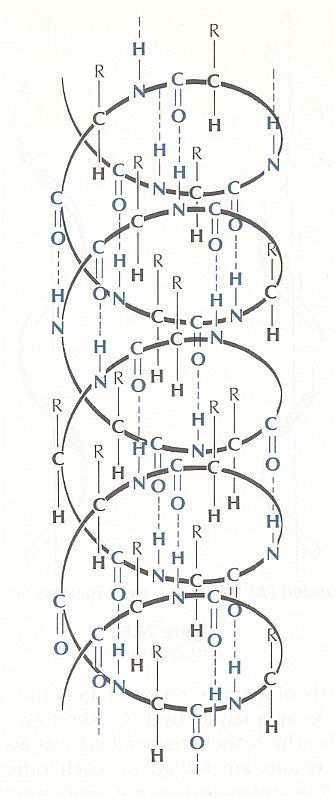
Figure above : Hydrogen bonding (blue) in an alpha-helix.
The structure, here depicted, is, of course, a three-dimensional structure. Hydrogen bonds CO....HN connect subsequent whorls of the helix with one another. So their direction is parallel to the axis of the helix. The asymmetric C-atoms in the chain, each bonded with R, H, N, and C, have their four bonds tetrahedrically arranged. (thus not making angles of 900 with one another). Because the H's of each of these asymmetric C-atoms do not line up with some O, they cannot form hydrogen bonds. The dashed lines in the figure are the hydrogen bonds proper, they are not true valences.
(After OUELLETTE, 1970)
Let us now elaborate on this picture of the alpha-helix.
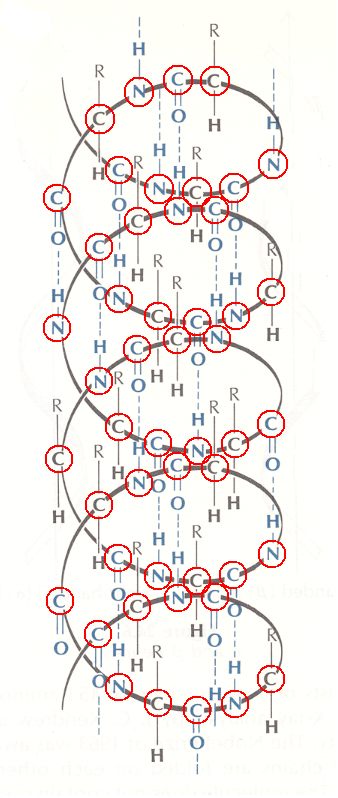
Figure above : Hydrogen bonding (blue) in an alpha-helix.
Atoms forming the continuous series in the polypeptide chain are highlighted.
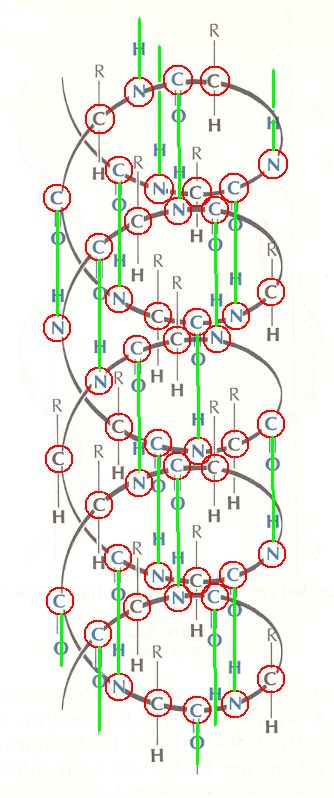
Next we (extra) higlight (green) the hydrogen bonds, connecting double-bonded O with N, through H :
Next we highlight (green) the peptide bonds, i.e. the bonds connecting the amino acid residues. So between every two consecutive peptide bonds there is an amino acid residue (C=O R-C-H NH) :
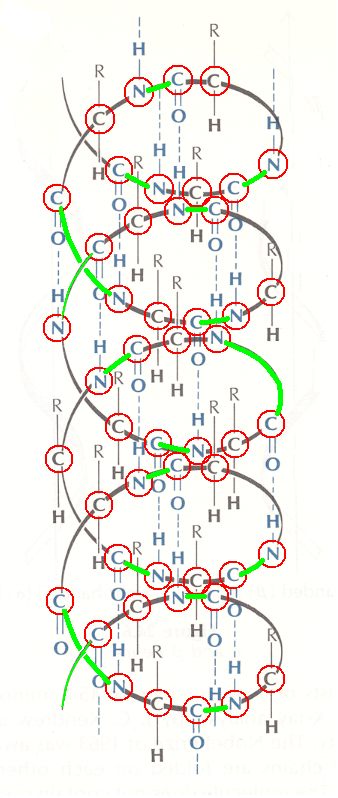
Finally, we highlight the R-groups. The C-atom (of the amino acid) -- carrying the R-group, which group can in principle be any group of bonded atoms, but which in proteins is part of one or the other of the 20 biological amino acids -- in each amino acid connects the C=O group, through this C-atom, with the NH group.
So this is the structure of the regular alpha-helix as it occurs in certain proteins or parts of them.
Having spoken about the alpha-helix in polypeptides, and thus about a structural element in many proteins, it is perhaps instructive to consolidate things with some data from other sources. We shall reproduce here the Section "Polypeptide conformations" from a chemistry text by ELMORE, Peptides and Proteins, 1968.
Polypeptide conformations.
Pauling and his colleagues examined plausible models of polypeptide structure based on considerations of potential energy. They argued that the most stable conformation would have (a) a planar peptide bond, (b) the maximum number of hydrogen bonds of the type

in which [electron] donor atom, [electron] acceptor atom, and hydrogen atom would not deviate by more than 300 from linearity, further, (c) bond angles and lengths similar to those in small molecules, and (d) orientation about C-C and N-C single bonds close to the potential energy minima for rotation about these bonds. The most satisfactory structure, based on the above criteria, is the alpha-helix. Although the helix can have either a right-handed or left-handed sense, the former is energetically preferred.
The main structural features of the alpha-helix can be summarized as follows : (a) Hydrogen bonds between carbonyl-oxygen and peptide-nitrogen atoms occur at intervals such that there are three complete amino acid residues between them :
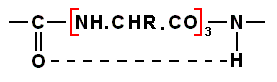
and the hydrogen bonds are almost parallel to the axis of the helix. (b) Five turns of the helix contain eighteen amino acids (i.e. 3.6 residues per turn) and the pitch of the helix is 5.4 Angstrom. (c) Side chains of amino acids protrude from the helix. (d) The hole down the centre of the helix is too narrow to accommodate solvent molecules [so in solution of the polypeptide, the liquid solvent, surrounding the helix cannot penetrate into its hollow interior].
The existence of the right-handed helix in proteins in the solid state has been proved by X-ray diffraction studies, and there is less direct evidence of its presence in solution.
The alpha-helix owes its stability to the formation of intra-molecular hydrogen bonds.
Extended conformations are also feasable if inter-molecular hydrogen bonds are formed. As to the extended conformations, there are two main types, the parallel and antiparallel pleated sheets [here, the elements of the pleated sheets are, as far as I know, not (always) helices but zig-zag polypeptide chains, or distorted helices]. The parallel pleated sheet has all the N-termini [the N-end of an amino acid residue which is, in a peptide, bonded to the C=O end (the C-terminal unit) of the other amino acid residue] oriented in the same direction, while the antiparallel pleated sheet has alternate chains running in opposite directions (see next Figure). Those proteins which exist in extended conformations are usually fibrous and rather insoluble in water.
The foregoing structures represent limiting conformations, since there are several factors which disturb the alpha-helix and give rise to conformations consisting of alternate helical and randomly coiled segments. Thus, although proline (see overview of the 20 biological amino acids) can exist in the alpha-helix, the bulky pyrrolidine ring interferes with helix formation by the amino acid residue which acylates the amino group of the pyrrolidine ring. Thus, proline residues usually mark breaks in the helical structure of proteins. The side chains of valine and isoleucine can also interfere sterically with helix formation. The formation of intra-molecular and inter-molecular disulfide bridges, especially the former, may prevent helix formation. In this case, the loss in potential energy resulting from the formation of a covalent bond compensates for the inability to form hydrogen bonds in the alpha-helix. The formation of hydrophobic bonds will also compete with hydrogen bonding as a means of producing the conformation of lowest potential energy [We have seen earlier that the protein, upon folding, quickly finds the alleged minimum-energy configuration, in fact too quickly in a mere chemical-physical context. Another factor than stability, and in fact another than a physical or chemical one, must somehow guide the folding process. In our view, this factor stems from the Implicate Order]. Protein molecules tend to assume a conformation which permits the formation of hydrophobic bonds between apolar side chains, especially when these can be buried in the interior of the molecule.
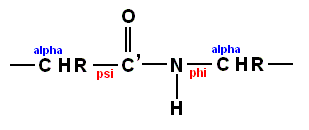
(referred to as a "peptide unit") or if we extend it a bit
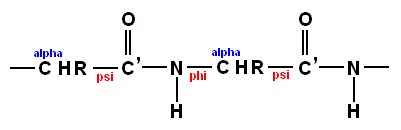
there is usually no rotation about the C'-N bond because the peptide bond is planar. Consequently, it is possible to describe the conformation in terms of the rotations about the Calpha - C' bond (psi) and about the N-Calpha bond (phi). See Figures above and below.
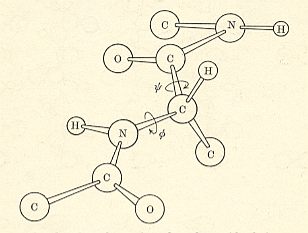
The complete conformation of a protein containing n amino acid residues can thus be described by the parameters
Values of phi and psi for some regular conformations are given in the next table.
| PHI | PSI | |
| Fully extended chain | 00 | 00 |
| Right-handed alpha-helix | 1320 | 1230 |
| Left-handed alpha-helix [= beta-helix] |
2280 | 2370 |
| Parallel pleated sheet | 1130 | 2930 |
| Antiparallel pleated sheet | 1450 | 3250 |
Proteins may be called long peptides, and peptides may be called short proteins. And there are proteins that, in addition to a peptide chain, contain some other chemical groups.
Unconjugated proteins are then classified in the main according to their solubility properties :
Conjugated proteins are classified according to the kind of group they contain :
A modern classification of proteins might be based on structure (e.g. molecular size, amino acid composition, helical content) and function (e.g. skeletal proteins, enzymes, hormones, antibodies).
Of enzymes six main classes are recognized depending on the type of reaction which is catalyzed. The systematic name of an enzyme consists of two parts. The first consists of the substrate or substrates [i.e. the chemical substance or substances which are to be transformed, and whose transformation reactions are catalyzed by the enzyme]. The second part, ending in "-ase" indicates the nature of the reaction catalyzed. For example, N-acetylmuramide glycanohydrolase is the systematic name for lysozyme.
Linkages within and between protein chains (polypeptide chains).

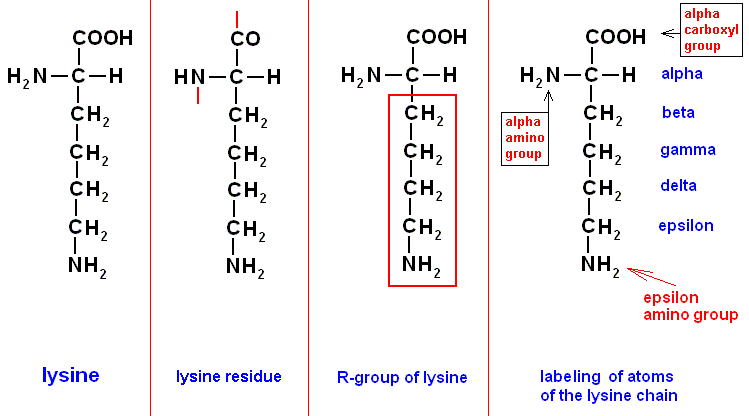
To identify potential linkages it is necessary to indicate reactive groups of the amino acid residues and where in the residue they are attached. For amino acids we label the carbon atom to which the COOH as well as the NH2 group is attached as "alpha-carbon", and, consequently, the mentioned groups attached on this atom are then called the "alpha-COOH group" (alpha-carboxyl group) and the "alpha-NH2 group" (alpha-amino group). The next carbon atoms (along the R-chain of the amino acid) ("R" is the chemical group by which all amino acids differ from one another) are then labeled "beta", "gamma", "delta", "epsilon", etc. And the groups attached to these atoms are indicated by the corresponding prefixes beta-, gamma-, delta, epsilon-, etc. Let us take the amino acid lysine as an example :
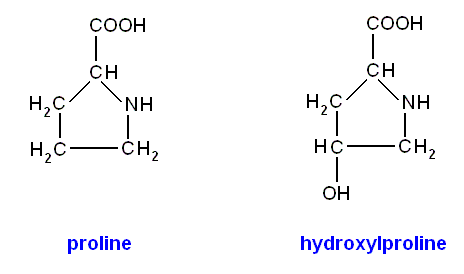
The R-group of the amino acid proline is a ring consisting of four carbon atoms and one nitrogen atom. Because of the closure of the ring the amino-group has lost a H-atom. In the amino acid hydroxy(l)proline (not listed among the 20 amino acids in the above Figure) one hydrogen, (together with another) attached to a gamma carbon atom, is replaced by a hydroxyl-group (OH) :
Now about possible linkages. (In the following discussion one may consult the above Figures). In addition to the peptide bond, there are other intra-molecular linkages, such as disulfide bridges (covalent bonds) and hydrogen bonds which both may be responsible for the formation of loops in the protein chains. Further, we already mentioned the hydrophobic bonds, also determining the conformation of a protein molecule.
Also, between (a) beta- and gamma-carbonyl groups (C=O) of aspartic acid and glutamic acid residues on the one hand, and (b) epsilon-amino groups of lysine residues and guanidinium groups of arginine residues, of the same (or other) polypeptide chain, on the other, there may be formed so-called salt linkages. These latter bonds have a free energy of formation of approximately 10 kcal/mole (while for hydrogen bonds it is about 5 kcal/mole) [i.e. this energy is released upon formation of the bond]. Salt linkages would not be expected to survive in the presence of high concentrations of electrolytes [such as Na+ and Cl - ions]. Since salts do not readily denature proteins [disrupting intra-molecualr non-covalent bonds], it may be concluded that salt linkages do not play an essential role in determining protein conformation.
All these bonds (except peptide bonds), and some others, may also be present inter-molecularly, i.e. connecting several protein molecules (of the same or different species), and thus representing cross-linkages. Of these others we may mention the following (ELMORE, 1968) :
Cross-linkage may also occur through phospho-di-ester linkages involving the hydroxyl group (OH) in the side chain of the amino acid (residue) serine. The next Figure shows two serine residues, each belonging to a different polypeptide chain, being connected by this bond :
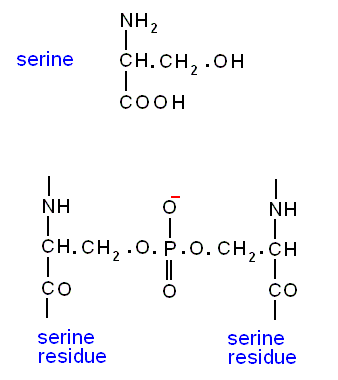
This is also possible with the amino acid threonine. And then, of course also bonds between serine and threonine.
Several unusual types of linkages have been suggested to explain the chemical and physical properties of elastin and collagen. Ester linkages, involving the beta-carboxyl group of aspartic acid and the gamma-carboxyl group of glutamic acid on the one hand, and aliphatic hydroxyl groups of serine and hydroxyproline on the other, may be present.
The epsilon-amino group of lysine may also be involved in cross-linking. In the case of elastin, there is stronger evidence that two unusual amino acids, desmosine and isodesmosine, which are biosynthesized from lysine, are sites for cross-linking. (ELMORE, 1968).
And so we see that there are plenty of opportunities for the formation of a protein-like mega-molecule.
( End of Intermezzo )
( Continuing with Müller's Unimol text on "organismic chemistry" )
Because true (system) protein molecules [i.e. free protein molecules, as they exist in the Unimol support medium] ( NOTE 215) have no other relationship with life than the above mentioned relationship-qua-origin and qua equivalence. They are not filled with whatever vague life or even being "aspiritualized". Despite the fact that they could have been alive mere seconds ago, or becoming alive again soon, they are in this condition [of being true system protein molecules, free molecules] just as non-living as is feldspar [a silica mineral] ( NOTE 216).
For this reason, also the expression" first living protein molecule" can better be replaced by the simpler form of "first living molecule" (whose mechanicistic-chemical equivalent after denaturation was probably a protein molecule).
So one should not imagine the origin of life such that a typical free protein molecule underwent a spontaneous mutation into a living protein molecule. Rather it may have been such that pro-proteins with a certain definite fine-structure came together to form pro-bioproteins and these then were "mutated". We mean a fine-structure which is totally absent in our amino acid and peptide syntheses as also the other way around in polypeptide hydrolysis [decomposition of the peptide chain by chemical insertion of water] (from "living protein"). This fine-structure is always skipped over because [it is] mesomerically [i.e. as intermediate phase] energetically unstable, or [finally] -- of which we are not convinced -- as especially improbable special-structure, practically not appearing anymore for a second time. The mentioned "mutation", perhaps having taken place under the "uteroid" help of nucleic acids or of other materials functionally similar to them, then resulted in a product that actually "felt" the not entirely by itself maintained stability of its condition under the indicative phenomena of delight-unease, and thus possessing for the first time consciousness, a product that attempted to maintain itself, a product that wanted to change its temporary being into timeless persistence, etc. ( NOTE 217). All these practical expressions of life, which in one or another but definite form were spontaneous attributes of the product at the origin of life, can be taken together under the heading of the singular (and only subdivided by us psychically, psychologically and cognition-theoretically-analytically) existential self-function [i.e. (under the heading of] the single overall strategy-plan to materially exist].
Despite the fact that Life may exploit, for its construction, protein substances -- but certainly after it having them chopped up into smaller building blocks ( NOTE 218) -- life can never be seen as the product of protein substances, but always the other way around : Protein as the product of life ( NOTE 219).
The polypeptide-chain-structure is one time referred to by the usual way of writing things down as corresponding to the analytically chemical idea, another time this chain structure is referred to by the form of the synthetic products, and, finally, it is referred to also by the determined structure of the end-products of many [living] bioproteins [meaning that the polypeptide-chain-stucture has been associated, not with living matter, but with analytical ideas, with synthetic products, or with derivatives of living bioproteins] . The polypeptide chain is the stable reduced expression of the interconnected building blocks of proteins. Also in the true (living) bioproteins we, in the end, will encounter something that corresponds to the polypeptide chain, something that also -- at least in many cases -- may transform into it, but something that is, when strictly considered, really something different from this simplified denaturational and gross structure [which is represented by the polypeptide chain] ( NOTE 220).
In living "proteins" one should expect very special bonding relationships, which at least complement the picture of the too clear valence bonding by the also already having become classic picture of resonance bonding, operating just with a kind of constant sum, resonance coupling, etc. But this is still insufficient, and there generally is good reason to see the uniqueness of life being essentially founded in unique connection-, interaction-, and resonance-relationships between the protein constituents ( NOTE 221).
In order to compare living substances with the well-known chemical bodies [molecules], and, to visualize features such as muscle function, membrane formation in "naked" cells, formation of pseudopods, conduction of stimulus, permeability, relationship between stimulus innervation and membrane formation, and the like, the involved bioproteins -- while more or less preserving the gross-stoichiometric proportions -- must possess various structural properties and possibilities of change of structure.
The multiple-valenced members of the organogeneous elements [and thus from them : the elements C, O, and N ] and some non-metals of lower atomic number, do not have diametrically opposed evenly arranged valences, but spatially rigidly angled ones [valences : bonding sites (and directions) for covalent bonding] ( NOTE 223), allowing under already classical conditions for quite special spatial features of chemical bonding. Carbon-chain formation, association of oxygen, hydrogen bridge formation, carbon-nitrogen ampholytes, keto-enol tautomery, various kinds of mesomery [insertion of mediate parts], and angledness of valences, already in non-living protein allow for specific spatial molecular patterns [the pattern of atoms in a molecule] with many possibilities of folding and buckling, allowing, that is, for the assumed protein superstructures [seondary, and, especially, tertiary forms, such as helices, sheets, globules, etc.]. And the asymmetry of substitution leads, in the context of the virtually universal L-form of the protein building blocks [amino acids], to the very important dorso-ventral expression of the three-dimensional protein molecules [Every amino acid molecule has at least one "asymmetric carbon atom", that is, a carbon atom of which its four valences are satisfied with four different chemical atoms or groups. This creates the possibility of left-hand/right hand stereo-isomers, and thus of two so-called enantionmers, of which one is the so-called "D-form", and the other the "L-form". D-form and L-form of a molecule differ like the two hands of one person, they are symmetric with one another but cannot be transformed into each other by any spatial rotation] ( NOTE 224).
While emphasizing that now it is only about models, that is, about chemically systematic model-protein and not about living protoplasm, we like to mention several construction possibilities, for instance :
With the changes of the meta-folding-structure, resulting in overall movements, one has models of organismic primordial motorics. It [the changes in the meta-folding-structure] also may be the primordial form of stimulus conduction and reflex movement, namely as a consequence of [in itself] insignificant physical or chemical changes of the "surroundings" whose determined constant composition may be viewed as element of fixation for normal rest-meta-folding structure. The directing (steering) exploitation of this ability, leading to favorable motion, for instance locomotion, amoeboid enclosing of food, etc, is -- in spite of its seemingly mechanical nature when superficially surveyed -- a fact of coordination, a primitive get-going by spirit. At certain stages and within certain ranges it is ordered to close-range effectiveness (for instance also exogeneous chemotaxis), or, in connection with higher sense organs, especially ears and eyes, it is ordered to long-range effectiveness and high power ( NOTE 227) . In the same way, as found-out-to-be-good (found-out-to-be-favorable), reacting to the same events, stimuli, and effects, we also find the mechanical primordial state of memory of living matter.
Apart from the fact that recent life, as selected progeny from previous optimal performances, stands far from primordial life, the vigorous but fundamentally typical example of differentiation, namely muscle fibers / nerve fibers, renders us, from the perspective of historical development, to be inclined to conclude that something like a primordial living protein with the fundamental features of being alive does not exist anywhere in any indicative or model-like way. In no investigation into substances, i.e. in no chemical analysis whatsoever, the truly life-specific can be found at all, but always only a part, a mechanism, or chemism, which also as such may be expected from our fundamental limits of cognition. Genuine and proper life today resides in super-ordered interconnection (organismic wholeness problem), which is inevitably destroyed in every fine-analytical material enquiry, but which [super-ordered interconnection] in turn is also materializable and comprehensible. Life does not reside somewhere in the organism, but IS the whole organism, because only the whole organism IS living substance. Practice corresponds to this formulation.
Let us, before continuing with Müller's text on Unimol, conclude the present chapter on "organismic chemistry" with the following intermezzo :
In organisms "morphogenetic development" ((individual) development of form) is not the unfolding, explication, e-volution, or unrolling of an existent form, but its creation from something else, from something structurally more simple, from an egg not containing the form in an enfolded state (and then to be unfolded). It is therefore called "epigenetic development" or simply epigenesis.
Epigenetic development of the individual organism from spore or fertilized egg to adult (which then is capable of reproduction) is a very complex and not well-understood goal-oriented process. As a starting point for (future) understanding this process (if that is possible at all) Unimol is an instructing viewpoint, because, if we would adhere to the conventional system-view, namely the developing organism as a system (in the form of a colloid solution or suspension) of physically and chemically interacting free molecules, resulting in them becoming to be organized into spatial and functional patterns, we would have the problem of the absence of mechanical (and chemical) robustness of the resulting organism, the absence of strong and stable material interconnections of all the parts of the developing organism. If we, on the other hand, adhere, from the outset, to Unimol, holding that the organism-proper is one single mega-molecule, holding that the interconnections of its parts consist of true chemical bonds (chiefly covalent bonds (electron sharing)) which are, in the biological domain, the strongest connections between material constituents, this problem vanishes.
And in the Unimol view epigenetic development is the chemical synthesis of the mega-molecule. We must moreover see the mega-molecule to be embedded in, or completely soaked by, its proper aqueous support medium, and the combination "mega-molecule + support medium" is what we normally understand to be an organism. In and through this medium the mega-molecule chemically and morphologically develops epigenetically from a much more simple state, and is maintained by it. For a given organismic species this development and maintenance belongs to the material strategy (for certain immaterial forms) to exist. And the mega-molecule is, like any other molecule (i.e. any non-living molecule), a holistic entity, a true whole, in which 'parts' are qualities of the molecule, or, said better, qualities of the alleged material constituent parts and particles of the molecule are in fact qualities of the molecule, i.e. in the molecule the preserved properties of the once free constituents (atoms) have now become qualities, not of these constituents anymore, but of the molecule, together with the newly generated properties.
In the mentioned individual development of an organism interactions of support medium and mega-molecule take place. In these interactions a particular part of the medium, the DNA, does not change, whereas the rest of the (content of the) medium continually changes, and especially it is the mega-molecule that changes during development and still does so during maintenance. And having (unchangeable) DNA to belong to the Unimol support medium, the (changeable) rest of the medium we will call the "medium-proper". DNA continually changes the medium-proper, and the latter in turn changes the mega-molecule. But the change of the medium-proper and of the mega-molecule is further controlled as to the sequence of changes by feedback : The changed mega-molecule chemically induces changes in the medium-proper, and these changes determine which genes in the DNA are switched on and which genes are switched off. The switching-on of a particular gene or a group of genes is triggered by a particular change in the medium-proper, and this latter change is triggered by the changed state of a particular region of the mega-molecule. And then the newly switched-on gene or group of genes in turn cause -- through RNA ==> enzyme-protein ==> enzyme-induced (and catalyzed) chemical reaction in the medium-proper -- a particular change in the medium-proper, namely the appearance of the reaction product(s) in it, which in turn effects a particular change in a particular region of the mega-molecule. The orchestration of the whole developmental process of an organism must ultimately lie in its DNA, but not without intensive feedback from the mega-molecule. And, further, DNA exclusively produces proteins, in fact produces amino acid sequences, polypeptide chains. The rest, the folding up of these chains and, especially, the formation of organismic shape and structure, is done by the dynamics in the medium-proper and in the mega-molecule, the dynamics triggered by these produced polypeptide chains. So the DNA actually isn't for shapes to develop, but for chemistry, i.e. DNA does not itself construct organismic material shapes and macroscopic structures, but merely triggers their successive construction.
Because of the utter complexity of organismic morphogenesis (epigenesis), in which so many factors interact and conspire to produce an adult from an egg, one cannot even set up a general scheme of events, a scheme entirely consistent with known facts, explaining, i.e. not merely describing, the course of morphogenetic development.
Several attempts to understand individual morphogenetic development of organisms (as for instance in embryos) have been made, for instance by GOODWIN (1994), by STEWART and GOLUBITSKY (1992), by DAWKINS (1987), by SHELDRAKE (1981, 1988), and much earlier, and only purely theoretically and not intended to be complete, by TURING (1952), and certainly also by a host of other earlier authors such as PRZIBRAM (1926), WADDINGTON and many embryologists.
All these attempts are admitted-upon incomplete and hypothetical, but nevertheless professional and interesting. They generally implicitly convey the message that there is no established theory of organismic morphogenesis. Each one of these attempts centers around some favorite theme or feature such as dynamical system and genes, symmetry-breaking (together with dynamical system and genes), genes-only, morphogenetic fields, reaction-diffusion systems and symmetry-breaking, and crystal analogy. All these theories are incomplete and, moreover, are ultimately based on the system-view of a (developing) organism. Nowhere, it seems, the idea of Unimol, as contrasted with the system-view, has been proposed. As far as I know, the idea of Unimol has been proposed and worked out only by Oskar Müller in 1959/60. But also the Unimol view cannot provide a detailed picture of morphogenesis.
And although the system-view presents many difficulties as to the admitted wholeness of an organism, and especially its chemical and mechanical robustness, the Unimol view is not a view that resolves all remaining problems. How, for instance, is the "living-megamolecule-embedded-in-its-support-medium" to be conceived in multicellular organisms? Each cell in such a multicellular organism is conventionally viewed as a sort of living unit of its own. In the organismic body it can develop, migrate, transform, and divide (and then, when the division products remain together, form a living tissue). And especially in multicellular plants cells have rigid membranes (cell walls) resulting in compartimentalization of the body despite the presence of pores and the like in the cell wall. A weaker form of such compartimentalization is the case in all multicellular animals. Further, cells have specialized into skin cells, muscle cells, nerve cells, blood cells, etc., rendering the organismic body also functionally compartimentalized. Therefore, Unimol seems hard to fit in such a picture of multicellular organismic bodies (the morphologically separate cells in an organismic body seem to forbid the [chemical, and therefore the material] continuity of the mega-molecule). But, more or less the same goes for the system-view, it is true, albeit that it still fits better than Unimol does (because in the system-view no such chemical continuity is presupposed).
Where Unimol does fit better is given by the fact that in the (developing) organismic multicellular body there is apparently great and extensive cooperation between the various cells, a cooperation, it seems, for the sake of the stability of the whole organismic body.
So whereas the organism-as-a-single-megamolecule (embedded, it is true, in an aqueous support medium), and thus as such being a true single subject of (natural) development and maintenance (i.e. development and maintenance is development and maintenance of the mega-molecule), renders the fact of cooperation of elements (inside and outside the mega-molecule) more comprehensible and natural, the system-view, on the other hand, -- the organism as a system of free molecules, and only a system of free molecules (at most connected to each other by bonds other than chemical, and thus weak and easily becoming undone -- and also the conventional cellular view (the cells as central biological units, together forming a system) are in great trouble.
So we set out to draw a picture of the developing organism in terms of Unimol, but thereby appreciating the important role of DNA and the medium-proper. Also this emphasizes the wholeness of the living mega-molecule, the organism-proper. It is the very subject that changes, develops, and lives. And despite these changes it retains its SELF, because the successive transformation stages of the mega-molecule are, in contrast to the usual, non-living molecules, ordered to one another. In a system-only we have no true single subject, no true entity, to which all processes in development and maintenance are ordered.
But in spite of these advantages, morphogenesis (epigenesis) can also not be described in detail without problems. So what we [JB] intend to present here (in this intermezzo), namely "organismic morphogenesis in terms of Unimol" is definitely not a scientific theory. It does not explain details of morphogenesis. It cannot take into account all the discovered facts, it even seems to oppose some of them, and it cannot refute conventional theories of morphogenesis.
So what, then, IS it what we want to present here? It can be no more than a philosophical background of organismic morphogenesis, or, perhaps better, no more than an instructive metaphor of morphogenesis in terms of Unimol. It eases thinking in terms of Unimol.
Well, let us then proceed.
To begin with, in a given unicellular organism there is DNA, the nucleic acid molecule possessing (in its active part) a message written with four letters, its nucleotides (ultimately their organic bases). Parts of that DNA may temporarily get unwound in order to "transcribe" the message of such an exposed part to a messenger RNA molecule, which then leaves the cell nucleus and has, in the cytoplasm, its message "translated" into an amino acid sequence, i.e. into a polypeptide chain (a protein), which in most cases is a specific enzyme catalyzing a specific chemical reaction. This DNA is, in the organismic body of a given organismic individual, constant as to its molecular structure or composition, and inherited from the parent. Further, there is a medium, in fact the medium-proper (because the DNA itself also belongs to the Unimol support medium). Both DNA and medium-proper are not alive. And, embedded in the support medium, there is the one single mega-molecule, which IS alive.
In the first stage (we're still talking about unicellular organisms) of morphogenetic development of, for instance a radiolarian, or a single-celled green alga (some species of which may reach macroscopical size), the medium-proper is changed qua chemical content as a result of the production, by a switched-on gene in the DNA, of an enzyme catalyzing a specific chemical reaction between reactants present in the medium-proper. Then the reaction product(s) diffuse(s) through the medium-proper and will eventually reach reactive sites of the mega-molecule. There they will bring about a local change of it resulting in the formation of additional morphological substructures or substitutions in that part of the mega-molecule. This brings about yet another change in the medium-proper, which, again by diffusion, reaches the DNA of the developing organism, and switches off the gene and switches on the next gene of that DNA. This switched-on gene now results in the production of another enzyme (or the production of a constitutive protein like collagen) catalyzing another chemical reaction in the medium-proper. The reaction product diffuses through the medium-proper until it finds a reactive site of the mega-molecule, bringing about some local change of that molecule, i.e. the formation of additional (or substitutional) morphological substructure. This substructure then chemically feeds back to the DNA, resulting in the switching-off of the gene, and the switching-on of the next gene in the organism's DNA, and so on and so on, until the adult organism, the fully-fletched mega-molecule, is completely constructed. From then onwards we have to do, not with growth and development anymore but with the maintenance of the organism by the same mechanism as just described. That in the structure of the DNA of the organism that determines the consecutive order of switching off and switching on of the respective genes (the constituents of the DNA) is the very 'plan' (the genetic blueprint) of the development of the organism, i.e. the development from egg to adult, a development forming the content of the material strategy of the species to which the given organismic individual belongs. However, we must amend this statement about DNA being the "blueprint" of individual development. Although the genome [DNA and all its accessories] is itself a dynamical system as to the functional connections and couplings between individual genes, it is, according to us, the mega-molecule itself that determines the consecutive order of the switching-on and switching-off of genes. The mega-molecule is an holistic entity that is turned toward itself much stronger as in ordinary non-living (micro) molecules. It therefore is able to regenerate lost parts, and to regulate or re-regulate development as it is actually observed in developing embryos. And so any change in/of a part of the mega-molecule is a change of the molecule.
So in this case, namely that of the unicellular organism, "the genes influencing one site (of the mega-molecule) after another" (i.e. the different parts of the organism being differently affected by different genes) is, in our model, represented by diffusion of DNA further by saying that the mega-molecule slides past the DNA molecule and triggers the successive switching-on of particular genes, resulting in a morphological (and here thus also chemical) rearranging or in the formation of (additional) morphological substructures at the respective sites of the mega-molecule, that in passing, 'touches' the DNA :
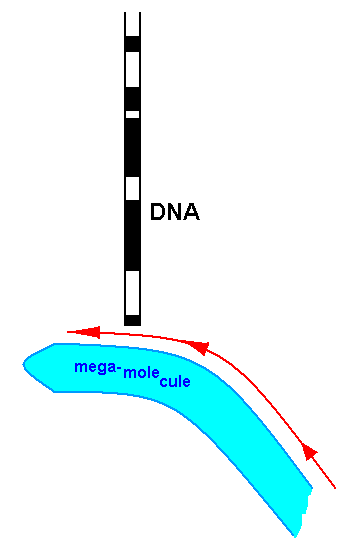
This image (it is not more than a metaphor) can also be applied to the case of multicellular organisms :
Whereas in unicellular organisms the "traveling to parts-to-be-changed" (parts of the mega-molecule) is diffusion, in multicellular organisms it is "the particular cell (or cell group) not-here-but-there" which is affected (i.e. whose cytoplasm is affected) by the switched-on genes of the DNA individual in its nucleus (generally, all cells in a given organismic body have identical DNA). So the "here-and-not-there" (in the mega-molecule) character of particular change of structure of the mega-molecule is realized, not through diffusion (this mainly takes place only intra-cellularly, but often also by active transport through and by the medium-proper), but by the particular change taking place in this-cell-here and not in the other. And also in the present case (multicellular organisms) we can use the image of "the mega-molecule sliding past the DNA molecule", because the DNA content is the same in every body cell of a given multicellular organism. So in fact every site or part of the mega-molecule is spatially close to the DNA (in every cell there is the DNA, and every cell represents some (different) site of the mega-molecule), and we can say that part A of the mega-molecule, as that part is present in a particular cell AA, is at the DNA, and, through the medium-proper, triggers the switching-on of gene A' in that DNA, while part B of the mega-molecule, as that part is present in cell BB, is near the (same [species of] ) DNA, and, through the medium-proper, triggers the switching-on of gene B' of that DNA, etc.
And so in this way different morphological adjustments, substitutions, or additions, take place in the corresponding parts (sites) of the mega-molecule, i.e. at the cells AA and BB of the multicellular organism.
And also here we can give the image (also being not more than a metaphor) of the mega-molecule sliding along the DNA of the organism.
We have emphasized that the process of morphogenetic (epigenetic) development in organisms is not a one-way determination from DNA to the mega-molecule. The latter continually feeds back to DNA, without altering it, but by determining or adjusting the consecutive order of the switching on and off of genes in that DNA, and the developing mega-molecule also may further adjust this sequential order after, for example, damage has taken place in a part of the developing mega-molecule. The genes themselves are not being altered in all of this process, and they, neither their immediate products (when they are enzymes), do not actually construct the morphologies of the organism but chemically trigger such construction. And so again we say : genes are not for shapes but for chemistry. The intra-molecular dynamical system of the mega-molecule combined with the inter-molecular dynamical system formed by the constituents of the medium-proper, forms one single dynamical system, and it is this dynamical system that actually constructs the morphological structures, and not the DNA itself. The DNA is the complete toolbox specifically ordered to the construction and maintenance of the particular mega-molecule. And it is principally the developing mega-molecule that selects the right tools at the right time.
(end of intermezzo on Unimol, Genes, and Morphogenetic Development)
(continuing with Müller's text again . . . )
Let us first give some introductory statements concerning the theory and practice of living substance and try to sketch with some examples the found and also the by us modified situation.
Macromolecular chemistry is the point of departure. After the classical view (for example Staudinger, and others) macro-molecules of the kind of biogeneous proteins -- containing up to 106 atoms, and with a molecular weight of 10 x 106 up to 15 x 106 -- can become micellarly aggregated, resulting in next-level structural units. And also these, forming a transition, as to size and properties, between the chemical molecules and the cellular "proper" elements of life, in turn aggregate to form still higher structural units.
The chemical basic form of the proteins is undoubtedly the linear peptide chain. The practical form of the proteins is -- especially with respect to what we've said in the chapter on "denaturation" [See HERE in previous previous document (part XVg) ] -- a corpuscularly spatial one. Such forms result from layering-by-folding, miscellar aggregation, globular involution, ordered and fixed "entanglement", spiralization, etc. The Pauling protein model shows screws with in turn may be screwed together, resulting in super-spirals. According to Pauling, the existing configuration of a polypeptide chain does not deviate more than several 100 cal / mole from the energetically most stable configuration. So then the C-N-C=O atoms (See part XVh and HERE, for the peptide chain, and read from right to left ) must lie in one plane and there must be a maximal number of H-bridges (hydrogen bonds) ( This we may also assume to be the case in true vital proteins). This results in the screw-like configuration (See the alpha-helix as given in Figure above ). If the above mentioned configurations [layering-by-folding, miscellar aggregation, etc] of practical form would, in the case of living matter, be connected to the same thermodynamic energy-minimas, then this surely is, for reasons of stability, very favorable. But it should not be the only reason. Finally, also a certain inner "rigidity" is preserved which may be important for the life-specific configuration.
The basic framework of protoplasm macromolecular-chemically is imagined to be a net- or meshwork of large fibrous molecules soaked through by an aqueous liquid. We take this image to be useful if one lets the "individual" fibrous molecules to be more or less strongly miscellated [formation of hollow pipes, or of super-helices, as a result of parallel aggregation of fibrous molecules] (they may already primarily have become miscelles by grouping-together of sites-of-synthesis), and, if one lets them form a connection with one another. The spatially continuously ongoing chemical bonding may laterally be realized by bridges. In many biological structures such as muscle and nerve fibres the chemical connection between the primary members, as they spontaneously are "produced" by the nucleotidic sites of synthesis [ DNA-mRNA-ribosomes-tRNA], must eventually end up in head and tail, at least partially so. Of "being soaked through" one may speak as in many tissues and in many organisms. Beyond these it merely is an association [i.e. an analogy, inspired by macroscopic situations].
Actual cytologic observation shows, apart from the familiar objects such as nuclei and mitochondria, especially three structural elements of the basic cytoplasm, namely bilayer membrane, vesicle-like and granular elements. The basophilic granula of the basic cytoplasm (100-200 Angstrom in diameter) do not exist in the form as was up to now assumed. They are not individual grains, but first-cut-offs [surfaces of cutting] of screw-like wound threads, whose coil-diameter is hardly greater than the usual layer thickness. Thickness of fibre = 170-200 Angstrom, diameter of screw = 300-600 Angstrom, screw period = 200-400 Angstrom.
Evaluation of about 300 electron-microscopical shots -- (where it is indicated that cut [in the slicing procedure] screws may look like granular sequences [indeed, if the screw is cut parallel to its longitudinal axis.] ) -- shows the extragranular cytoplasm (root meristem cells of Allium cepa) to be a combination of electron-microscopically almost empty hyaloplasm (mediate, qua dispersion, type) and a substance abundantly distributed in it (dispersion-phase-type), a substance of fibrous screw-like wound structure ("cytonemata"), in which sizes, screw-periods, and other features may be established. The positional distribution showed itself most often as statistically isotropic [having its properties independent of direction within the sample]. The individual cytonemata moreover seemed to have a substructure consisting of fibrous 'seeds' and a somewhat granular mantle. One takes the cytonemata to be nucleid acid-containing building blocks of the basic cytoplasm. The screw-like coil with its 10-fold diameter as compared to that of nucleid acid molecules probably is a functional concentration or compression, but may, however, also be an artefact, having resulted from denaturation contraction, when, for instance, of two chains, connected with each other by transverse bridges, the one more strongly shortens than the other, etc.
In spite of optimistic judgement, one always must leave open all possibilities of non-correspondence between influenced prepared sample and vital condition. Although the spiral or screw-like condition of peptide chains is, so to say, mathematically verified, a prepared sample can show coagulation contraction to which, seen vitally [seen as in the living state], only a tendency in this direction is basic (compare the ability to contract of the spindle fibers [during cell division] ) [i.e. in living matter coagulation contraction is only present in the form of a tendency]. Forms, originating from fixed and pre-manipulated samples, certainly never are "pure" artefacts, but at least true-state-matching compounds between cytoplasmatic content and alien matter, and thus moulds and stainings. Possibility of confusion is attempted to be avoided such that in the same [species of] object different means of fixation are used, and that one pays attention to corresponding structural pictures. Of course one may also attribute things to the prevailing colloid-chemical system view : the fact that one -- as a response to too optimistic a first-time-explanation -- was easily inclined to interpret observed structures to be mere artefacts of fixation. Today one does't question anymore the existence of submicroscopical structures in practically all substrates. One only has strange -- often enough also none -- ideas about the bonding-media [types of bonding] effective in these structures.
Today one is convinced that the earlier taken to be structureless inter-phase chromosome [the condition of the chromosome between two successive cell divisions] retains its structural continuity (in salt solution it was found to be isolated and spiralized). The mitotic [referring to cell division] spindle fibres -- which may be taken to be the most important objects when it is about distinguishing the sequence of events in mitosis (cell division), as a qua bonding completely structured-through system, from a system with colloid-chemical mechanisms of separation [division] -- could also in an undyed and unstained condition be shown and photographed as isolated real vital structural units, not perishable for hours.
The view that the formation of spindles is an intra-cellular colloid-chemical coagulation process, comparable to blood coagulation, can still not yet be totally refuted on the basis of such findings [mentioned above], but because one assumes [the presence of] fixed attaching sites on chromosomes for spindle fibers, and in addition surmises chemical bonding [to be the case] in the fastening (-S-S- ?), one makes somewhat improbable demands to "coagulation" insofar this is something else than a constructively functional condensation or strengthening of that what was qualitatively already present.
If one follows the distribution of RNA, then it is not excluded that in the mitotic prophase not only duplication [of the cell and its content] is introduced, but that also certain after- and new-constructions such as reinforcements are carried out, in which the chemical bonding-to maintains the unequivocal order (and not letting it originate, namely as one had it earlier that spindle fibers form from "nuclear sap", spindle fibers, of whose persistence, despite temporary invisibility, more and more authors are convinced).
Seen unimolecularly, i.e. seen insofar as things are bonded to each other, it is probable that the mitochondria [=subcellular structures (cell organelles) that produce ATP used to transport energy where needed] are not freely, or chemotactically, drifting about in the cell, going there, in it, where they are going to be used, but that they, while bonded, are pulled toward these places. And also it is probable that they, in the case of urgent spontaneous demand, do not burst apart (in order to be quickly emptied), but, as an emergency reaction, are teared apart as a result of contraction and traction forces, because the normal openings in their shell membrane are not sufficient. Comparable things also hold for the intra-cellular chloroplasts [= cell organelles executing photosynthesis], which seem to have a phototactic motility independent of plasm stream.
Here it is perhaps instructive to insert two drawings and two electron micrographs (i.e. images obtained by the electron microscope) depicting some components of the organismic cell : mitochondria, chloroplasts (plastids), endoplasmatic (endoplasmic) reticulum, and the nucleus. The electron micrographs are from the first half of the 1960s and thus comparable with the technique available in 1959, the year Müller's book about Unimol was written. The subscripts and additional information date from 1967, and are taken from JELLINCK, The Cellular Role of Macromolecules.
First, the mitochondria which are important cell organelles :
Figure above : Cutaway drawing of a typical mitochondrion shows the two membrane layers separated by a fluid-filled space called the intrastructure space. The space within the inner membrane is called the interstructure space. The invaginations of the inner membrane are the cristae. The stalkless particles distributed over the outer surface of the outer membrane are involved in various oxidation reactions that supply electrons to the interior of the mitochondrion. The particles extending inward on short stalks from the inner surface of the inner membrane transfer the electrons along a chain of complexes that synthesize molecules of adenosine triphosphate (ATP).
(From GREEN, 1964, in JELLINCK, The Cellular Role of Macromolecules, 1967)
Mitochondria usually are aggregated in areas of the cell in which the demand for energy in the form of ATP is high [ATP, produced in mitochondria, has a high energetic content, and so it can, after having been synthesized, transport energy to places where there is need for it. By becoming ADP (andenosine diphosphate) it releases its energy.]. The origin of much of the protein which is unique to the mitochondrion and does not occur elsewhere in the cell is of considerable interest, and DNA, RNA, and ribosomes [the sites where proteins are synthesized according to an RNA matrix] have been shown to be present in these organelles.
Mitochondria not only are the "power plants" of the cell but also they can take up and extrude water and are involved in the transport and accumulation of certain other substances, in particular calcium, magnesium, and phosphate ions.
Another important cell organelle is the chloroplast. See next Figure.
Figure above : Chloroplast from maize-cell. Chloroplast is the organelle in a plant cell within which photosynthesis takes place. The chlorophyll is contained in the "grana", stacks of membranous sacs called lamellae, seen here in cross section. A maiz-cell chloroplast is enlarged 19000 diameters in this electron micrograph.
(From RABINOWITCH and GOVINDJEE, 1965, in JELLINCK, The Cellular Role of Macromolecules, 1967)
There is now considerable evidence that chloroplasts and mitochondria can arise from pre-existing organelles, and that control mechanisms exist for their differentiation. Furthermore, chloroplasts as well as mitochondria have been shown to contain DNA and RNA.
A further cell organelle is the so-called lysosome.
Lysosomes are now [1967] known to occur in most animal cells, although it remains to be shown that they are present in plants. These subcellular sacs of approximately the same size as mitochondria contain a number of powerful digestive enzymes with pH optima in the acid range. In fact, they function in many ways as the digestive system of the cell.
A very important organelle of the living cell is its nucleus. Only in bacteria and blue-green algae the nucleus is absent. See next Figure.
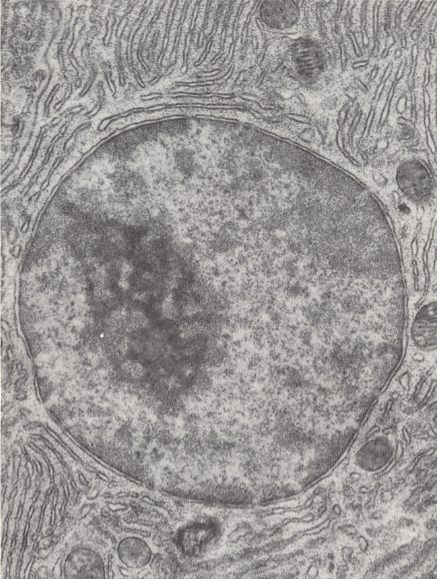
Figure above : Nucleus of the living cell is the large round object in the center of the electron micrograph shown here. The membrane around the nucleus is interrupted by pores through which the nucleus possibly communicates with the surrounding cytoplasm. The smaller round objects in the cytoplasm are mitochondria. The long, thin structures are the endoplasmic reticulum (reticular membrane).
The small dark dots lining the reticulum are ribosomes [where protein (polypeptide chain) synthesis, with RNA as matrix, takes place]. For these ribosomes, see also the slightly enlarged detail from upper part of micrograph :

The structure of the nucleus is particularly important to all biological science, because the chromosomes that it contains are concerned with the storage and also the transmission of hereditary characteristics (genes) of the cell. Furthermore, the nucleus is the main center for the synthesis of DNA [its replication] and of RNA, and for the control of cytoplasmic activities.
Living organisms are not nucleic acid, however, and we must make a real effort to bear this in mind. Though we have spoken chiefly of the gene and the enzyme, it is the organism that lives in the world, that walks and grows and writes books on biology. What is the nature of this complexity that knows so many forms, but fails to hide their brotherhood? What is there about the organism that has made poets sing and scientists despair?
The essence of organism is at once subtle and irresistibly fascinating. The secret is in its name. The organism is an o r g a n i z a t i o n of materials and functions that are dedicated to the preservation of itself and its species. The allure of this concept, however, stems from the intricate system of levels of organization, a pattern which characterizes all living things. Thus, the cell is an organized entity at one level of complexity. It lives in a community of other cells, joining them in certain projects, competing with them for food, and either dying or dividing to form new offspring. Yet these cells may be part of a higher organization, the brain, which is a whole made up of the sum of its parts. Here is a structure on a more complex level of organization, existing and interacting in a community of other organs, not cells. Likewise, the whole man is still higher on the scale of organization, and men talk to other men, not brains or cells. We may also start with the cell and go down the ladder, for within the cell are self-concerned substructures like the nucleus, the particles within the nucleus, and the particles within those particles -- until we reach the level of the molecule and atom. It is this rising table of organization that is characteristic of organism, the elusive hierarchy that makes of thin voices mighty antiphonal choirs.
Particularly interesting in the context of Unimol is the endoplasmic reticulum as it is present in the cytoplasm of organismic cells. Here some information also from JELLINCK, 1967 (not considering the possibility of Unimol).
The endoplasmic reticulum is, in a way, a derivative of the cell wall (the "unit membrane"), and, back in 1967, but, I presume, still today (2011), the general structure of the cell wall is thought to consist of two layers of lipids with their fatty [hydrophobic] tails pointing in, and their water-soluble [hydrophilic] heads pointing out, comprising the middle section of the membrane. This double fat layer lies between two thin sheets of protein (medium gray bands in the Figure). And these sheets in turn are thought to be coated with globular proteins (light gray circles in the Figure) :
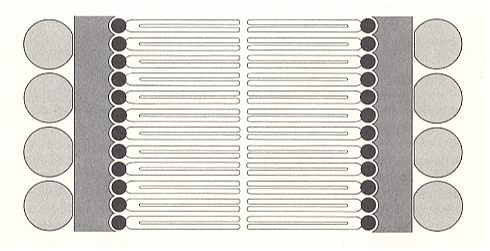
The drawing is after HOKIN and HOKIN, 1965, in JELLINCK, 1967.
After having treated of the structure of the cell membrane, Jellinck tells us where in the cell we can find it while thereby also mentioning the endoplasmic reticulum :
The same kind of membrane is present not only at the cell surfaces of all types of living organisms, but also inside the cells, surrounding the nucleus and various organelles. These internal membranes also form the lining of the system of cytoplasmic canals known as the endoplasmic reticulum, and they are often folded in complicated ways with a tendency to associate in pairs.
As to the formation of the cell membrane in living organisms Jellinck quotes GREEN and HECHTER (1965) :
The formation of a biological membrane may be presumed to be a stepwise process leading from proteins to polyprotein complexes, from complexes to functional subunits and finally to the membrane continuum ... How is this precision assembly problem achieved in accord with a predetermined blueprint? In a membrane system a spontaneous self-assembly process would imply that the proteins to be assembled, once liberated from the site of polysome synthesis [such a site is a string of ribosomes, a polysome, where protein is formed], spontaneously interact in the aqueous environment of the cytoplasm to produce progressively more complex macromolecular structures. The alternative to a self-assembly process is one in which the "built in" specifications of the components to be assembled are insufficient to provide for precise assembly. If external devices for assembly are operative, we should like to know their nature and the mechanism whereby they are controlled by gene action.
The greater or lesser degree of continuity of the endoplasmatic reticulum may be some evidence of unimolecular continuity of at least a large part of the organismic cell, and maybe of the presence of such a continuity throughout the whole organism. Indeed, in Unimol we view the living mega-molecule as central : We describe the multicellular organism as a mega-molecule (embedded in an aqueous medium) onto which the cellular structure is superimposed.
And in so viewing things, we consider the symmetry of the organism (and then also that of the mega-molecule) -- the symmetry as conceived of in promorphology, a symmetry which in organisms nowhere is homogeneous, nowhere precise, but merely approximated, and different at different structural levels of the organism -- as not coming from within, but from without : The symmetry of the mega-molecule, as this symmetry is described in promorphology, is a macroscopical phenomenon, it is an element of tertiary and quaternary structure of the mega-molecule : As in proteins, this structure, and thus the mega-molecule's symmetry, are determined by two interacting factors : stability and function. Indeed, in proteins we see that they spontaneously fold and twist resulting in a three-dimensional shape that fits the protein's biological function.
And so, whether or not Unimol can be verified by observation and experiment, i.e. whether or not the mega-molecule is truly present in organisms, it is nevertheless good to see things from the concept of Unimol and see the cellular structure of the great majority of organisms as something that at least conceptually comes after the one mega-molecule (One can, in a way, see the mega-molecule still being present, at least in plants, where the cytoplasmic content of adjacent cells is connected by thin cytoplasmic threads, the so-called plasmodesmata). Considerations about the general structure and function of organisms must start with the mega-molecule, i.e. must depart from the unimolecular state of the organism-proper. Only then the evident holistic properties of organisms will become more understandable.
(end of Intermezzo on cell organelles)
(continuing with Müller's text again)
With this, one already really is in the domain of surmise and speculation. We only wanted to show in what ways, where it is relevant, one can apply the Unimol view. The many examples [of how to apply Unimol] from cytology may be supplemented by embryological ones [here not only is meant the individual development of the embryo in the egg or womb but also all subsequent development and change of the individual (such as growth, metamorphosis, aging)]. Also these [examples from ontogeny] begin subcellularly. The development from uni- or multigranular amoeboid able-to-divide pro-plastids to somatic chloroplasts is just as much an unimolecular ontogenesis of an intrasymbiotic highly specialized partner, as is the ontogenesis of a living being anyway.
The right from the beginning unimolecularly predisposed structure of an organismic germ, still having its ontogenetic development before it, and thus, an initial molecule, whose structure one should not judge to be too simple, is the leitmotif of a developmental biochemistry dealing with the question in what way chemical transformations lead to visible morphological structures. The familiar common statement that it here is about the construction of species-specific or organ-specific protein, experiences in Unimol an organisms-special orientation [i.e. where "species-specific protein" in fact is going to mean : "living mega-molecule"] and establishes -- at first conceptually, but then also methodically -- a direct relationship between biochemical process and morphological development of the complete organization of every living being, including man.
Now we want to break away from this more or less arbitrarily composed list of initial remarks, and try to present a systematic exposition of how the problem of "organismic life as one-molecule" is taken by us and how we may clear it up further.
The whole unimolecular stimulus-reaction form, as such going back to the origin of life and as such from there onwards existing original form, has, - in the course of phylogenetic development through increase of bulk, and thus also through multiplication, and -- as a result of that, made especially favorable -- differentiating strengthening, - taken that magnitude as is encountered in present-day faunae and florae. The original unimolecular unity, -- not given up in subsequent overall phylogeny, but only having been organismically elaborated on, -- whose principle, - in the form of a true chemically interconnection through bonds ( NOTE 232 (important)) , - today is still realized, -- is at the same time the consequent and true ground of organismic wholeness. It has its, may be one day anatomically demonstrable, physiological-histological expression in the evidently continuous transition between nerve fibers, that is, the fine neuro-fibrilles, and the plasm of body cells (to-be-nervously-stimulated muscle cells, etc.). The anatomical-histological results on neuro-fibrilles in the form of stained samples, still cannot yet decide upon the presence of true continuous chemical bonding, because chemical fine-structure, existing down to single chains of chemical bonding, cannot be made visible neither by treatment with dyes or polarization-optically nor by anything else ( NOTE 233) .
An experimentally verifiable support for Unimol is certainly the extraordinarily high speed of stimulus-conduction of motorial and sensory chief nerves in higher mammals and humans, which speed is of an altogether different order than electrolyte shift in an aqueous medium. Different conduction speeds already all by themselves speak for differently constructed "wires" and not for electrolyte media [an electrolyte is an electrically charged atom or atomic group, such as H+, OH-, NH4+, etc.]. The capacity for conduction of stimuli with its high frequency and quick restitution points to real continuously-going organic wires, and the stimulus conduction can be viewed as an uninterrupted molecular-mesomerous reversible serial repositioning [a "mesomer" may be a part of a molecular chain (or of some other structure) and is, in it, a sort of metamer (consequent part) but such that it functionally mediates between other metamers, so a mesomer is an in-between-part] ( NOTE 234) . If we thus conclude -- conclude from the phenomena of stimulus conduction and further from the experiences of neural damage in neuro-surgery ( NOTE 235) -- electrolytically, nerves can more quickly be fused than by bondingly growing them together -- that the nerve fibres possess genuine chemical bonds, then already only by the fact that all parts of the living organism are penetrated by fine and finest interconnected nerve fibres ( NOTE 236) , the organismic body is made up of a continuous structure, is made up of a complex but still single unimolecular "interlacement" of living substance soaked through by a serum-like medium ( NOTE 237) .
The unimolecular organismic connection is thus symbolized, by reason of especially clarifying relationships, by the nervous interlacement, but we must be convinced that also between nerve-substance and cytoplasm do exist further true bondings ( NOTE 238) .
The fact that the bonding relationship is not present in the partly rather extensive serum-like medium with its many individual substances, and the fact of the trillions of classical small- and macro-molecules, do [both] not oppose the fundamental unimolecular view, is presupposed to be self-evident.
When we now speak, concerning the first living beings as well as all [fossil and] recent organisms, of "molecules", then it is necessary to change the usual physical concept of "molecule" accordingly. First and foremost so as to constant molecular weight ( NOTE 239) and most of the properties made use of in the classical methods of determining molecular weights (also those for macromolecules). This is not significant, not decisive, for the mentioned methods have been worked out with the stoichiometric non-living small-molecules [of classical chemistry] and were sufficient for the determination of their molecular weights. On the other hand, one should maintain the important bonding-criterium for recognizing the molecular boundary : The molecular boundary is always there where the continuous chain of main valence bondings stops. From many data we derive the existence of continuous chemical bonding, but a bonding that may at times, and at a number of interruption sites, [temporarily] open up [all this, during the processes of self-maintenance of the living molecule] ( NOTE 240) , so that at least as a result of the overall nervous relationship, and [as a result of] the omnipresent true bondings-transitions, the entire organism is "soaked through" by one single giant molecule ( NOTE 241) . Living molecules are then such that among them there is only some similarity as to basic structure, not equality, or expressed better : Among individuals of the same species, there is not a precise stoichiometric, but only a functional equality and correspondence ( NOTE 242) . And precisely in this respect, i.e. functionally, they are well defined molecules even in the strict sense of the word [if every chemical bond, also in non-living molecules, is taken to be functionally.].
So the one-molecular view of organisms, Unimol, maintains that within that what is taken to be living substance, i.e. between all points of the organism, there exists a real or at least potential uninterrupted valence-bonding sequence, that we think, schematically idialized, to be realized by the nervous apparatus, which indeed connects all points of the body with one another ( NOTE 243) . As a result, there exists -- together with all, surely numerically preponderant, independent molecular forms [free molecules] of the organismic system containing water, salts, enzymes, etc. -- between every two arbitrary points from head to feet an uninterrupted chain of bonds, so that the molecular boundaries coincide with the body boundaries, and so the whole body representing one single molecule ( NOTE 244) .
The relations of order between organic parts and events ultimately have a physical chemical equivalent in the form of true chemical bonding relationship among the partners, which [partners] thus, because themselves consisting of molecular connections, together make up one single supermolecule in effect coinciding with the organism. So organismic consideration is, -- if it gets rid of the superfluous metaphysical [here indicating "vital forces" and the like], and through its concept not only wants to formulate a fact, but also wants to give it a foundation, -- an Unimol view of life ( NOTE 245) . Unimol doesn't deny, nor overlooks the formally chemical steering as belonging to a broader mechanism, for which there is nothing equivalent, and which [broader mechanism] also cannot be realized by the bonding-relationship alone.
The organismic order may give up the otherwise necessary ranking order such as the stages [levels] of constituents (macromolecules, micelles, cells, tissues, organs, [antimers, metamers] ), because it immediately results from the unimolecular framework, and, what is especially important : remains in it. Or, said differently : the real organismic rank order of constituents is no other than that of table-salt, urea, or sugar molecules.
Seen [things] essentially constructively, there are in the living organism no chemical bodies making it up, but the organism is a (single) chemical body of true molecular nature, albeit not definable by a chemical formula or molecular weight. The living molecule is not an as-if [chemical] compound, not an analogy, but a wholly real stable compound, "mesomeric" or allomeric [a transitional compound or representing the other end of possible compounds] to the currently known [compounds], but so to say static, and not maintained by some mysterious dynamic equilibrium. The expression "metastable compound" [something is metastable when it can resist perturbations below a certain strength of them] makes only sense with respect to reactants and products (of a transformation), not with respect to simple temporal stability, which in life is not a mere apparent one, but a real stability. These unimolecular organismic forms, whose life-specific one-function [i.e. the existential self-function] makes the difference between "to be alive" and "to be dead", each for themselves, also mediate the transition (in cases transposed into the cell fine-structure) between chemical and morphological (biological) structure ( NOTE 246) . The facts of heredity, the "biogenetic basic law", etc., now [in the Unimol view] in many respects appear as consequent states of affairs that cannot be different [The biogenetic basic law states that in ontogeny (individual development) the particular phylogeny [evolutionary sequence] is compactly repeated.] ( NOTE 247) .
The chemical composition of living substances, having largely a protein nature, as well as the features of their atomic constituents, at least do present a one-off great probability and possibility of almost unlimited, also facultatively dissolvable again, bonding, and this bonding apparently is very appropriate [in constituting living matter], whereas it in other types, - such as in hydrocarbons [carbon chains saturated or unsaturated with hydrogen, for example CH3.CH2.CH2.CH3, butane], polysacharides [polymerized sugars], or in artificial polymerization products [such as plastic], - is limited by end-group or kinetic phenomena, or at most being able to generate uniform reticulate endless-aggregates.
Examples of spontaneous or non-spontaneous dissolution of bonds we see :
Examples of establishment of new bonds are given by :
Presupposing that our view is right -- exact contra-demonstrations are lacking -- we may say that in and with the organisms, and already in the smallest of them, truly giant molecular constructs, otherwise unknown, are formed, and we do not doubt that their size is also one of the preconditions for the existence of the life-characteristic. That is, we mean that classically small organic molecules, already by reason of their small size, do not at all admit for the biofunctionally necessary super- and special structures, and therefore can also not be alive ( NOTE 249) .
Because, now, from the point of view of chemistry there are no special objections [to Unimol], we again may recall that also ontogenetic [i.e. as to individual development], neurophysiological, and holistic-philosophical facts do demand a true bonding relationship. Further experimental proof must, for the time being, still give way to extrapolations and logically-constructive thinking, approaching the macro-micro range from the protein-chemical viewpoint as well as from that of histology [study of tissues] ( NOTE 250) .
In medical biochemistry and physiology one, by the way, rightly speaks of mechanical, and not only of chemical protein damage, whose fragments probably enter into function as chemotactically acting "wound hormones", and with it showing a parallel with the chemotactics of sexual attraction, which, in many cases, may also be connected with products of disintegration of "obsolete" special bioproteins, connected, that is, in a physiological or at least ontogenetic sense. To speak of mechanical protein damage ( NOTE 251) is, however, anatomically and chemico-physically only making sense in protein molecules with a magnitude of at least 10000 times that of the other giant molecules ( NOTE 252) . The evident stoichiometric repetitions in protein molecules show, as multiplication phenomena, a parallel with general multiplication in individual development (ontogenesis), namely with progressive metamerization. One may evaluate this as merely an ontogenetic feature or stage, but one may also, especially in discussions about the origin of life, derive legitimacy to think of the often stated "complex organic structure of living matter" as being already present in relatively small closed material entities [later, in evolution, to be repeated, forming a metamerized body], entities having once represented primordial life. Recent living matter in organisms doesn't consist of such small closed entities anymore [meaning that today there are no organisms which are each for themselves such small material (molecular) entities], but is a single large entity, which, if correctly viewed, does not bring with it the usual philosophical objections concerning the materialization of life ( NOTE 253) .
What, precisely, is holding the living world together from within? It is the long-time-known chemical bonding, which is one of the most surprising achievements of several generations of investigators anyhow.
The alternative to our unimolecular view of organisms (Unimol) is the conception that life is not a one-material, homogeneously-material self-ness, but the causally indivisible special order and performance of a (colloid chemical?) multicomponent system, whose intra- and interweaved enormous multiplicity -- of phenomena which each for themselves not only are causal, but also are proven to be so -- does not lend itself to be formula-like "unravelled" and certainly not experimentally so ( NOTE 254) .
The (pure) system view of organisms operates with substrate materials (bioproteins, tissue substance, etc.) and active ingredients (vitamins and hormones with the supplementing bodies). They are, as a result of a multiplicity of chemical reactions, interconnected with one another in an obscure fashion. As a result, a correspondingly obscure chemical determinism, concluded from unshakable regularity, is established, to which every organism is subjected.
In the context of Unimol chemical determinism loses its, seen as life characteristic and therefore just like that respected, incomprehensibility. This incomprehensibility, as it is a consequence of the system view, is at most hard to be saved with the help of an extreme antirealistic view of selection ( NOTE 255) -- because this determinism is by itself nothing actively governing, but a tool of the actually governing.
The (pure) system view is a -- not particularly emphasized but clearly standing in its background -- concoction from the classically colloid chemical system, of which [systems] there exist truly surprising and powerful ones, and from the additive system of empirical data, both [concoctions], first of all in thought, blended together into a unity, which [blending] in organismic life one takes to be successful and verified.
Therefore the system view is a pure mechanism, supplemented by an essence consisting of conjectures, non-illustrative speculations, easy belief, and finally a not-admitted vitalism, and as such all this representing a one-off case of methodical inexactitude. Unimol realizes this essence simply and rationally.
If one takes life, that is, our recent [as it is in recent geologic time] elementary life-form, the cell, as summative living system, then one instantly has to do with all the many difficulties with which science is struggling rather unsuccessfully already for a long time, difficulties, which we can in no way rationally get rid of, and whose description already by itself renders experienced and understandingly experienceable life to become the most obscure phenomenon.
The fact that, in the light of the overwhelming multitude of physically mathematically anticipateable colloid-chemically describable and in thought producible matter-aggregate-systems, only one [such system] is actually realized, namely the known-to-us fairly uniform cellular one, should in a lesser degree convince us that this is a very special system, a unique system as a result of intrinsic necessity and constructive regularity, but, on the contrary, should convince us that it is not a system at all, and so at once relieving us from a host of difficult questions. Not a system at all, at least not in the above sense, but something quite different, which, with completely different internal structure, only superficially resembles a usual (and thus for example a colloid chemical) system. One then would have arrived quickly at the real state of things, or, more modestly expressed : one would have arrived at something much better adapted to reality.
In the living system -- in what follows we mean by this in model fashion "the" cytoplasm of unicellular organisms ( NOTE 256) -- no matter whether one sees it unimolecularly or interactively constructed, we now have the difficulty to view it biologically and at the same time characterize it physically. The temptation for the latter appears to be quite strong, as one may easily experience. Much of it what is said is then [in physically characterizing] correct if not referring to the life system at all, but to certain aspects of it. That [for instance] the living system is subjected to thermodynamics, generally following the entropy rules and also being conform with the Nernst-Planck heat theorem, is self-evident, but because of that it still is not a thermodynamic system to which one may apply the simple basic laws [meaning, it is not j u s t a thermodynamic system obeying the thermodynamic rules]. Most of the conclusions and statements therefore are worthless insofar as more than a particular detail is being characterized [In addition to physically characterizing details, one should biologically view the whole organism].
As system one, taken strictly, indicates a, in one way or another belonging-together, multiplicity or manyfold (from 2 components onwards). Deviating from this, one also characterizes a chemical uniform one-phase body as to be a homogeneous one-component system. As a result of this latter expression we here already have a possibility of transition ( NOTE 257) between (1) Unimol as "homogeneous" one-component system and (2) classically pure system view.
Now one cannot apply the physical concepts of homogeneity (a homogeneous body carries in all of its regions, spots, the same physical quality) and phase (= a region [not necessarily that of a spatial body] within which the substance is homogeneous, and homogeneous bodies are one-phase bodies) to the external living system [meaning that the living system physically is not homogeneous and not a one-phase system], but must, in applying these concepts, see things from the essential (i.e. qua being) and [that is] from the uniformly functional. Already the unequivocal essentiality alone means a "homogeneity" [i.e. an organism not made up of actual, but of virtual parts or particles, rendering the organism homogeneous, i.e. rendering it to be a single being.], and the holistic treatment of the organism can also be viewed as pseudo-homogeneity and with respect to it -- although not completely conform with the chemical and physical habit -- the one-phase state of it as being "legitimate" [in such a holistic treatment] ( NOTE 258) . In discussing problems of living substance, it may under this point of view be taken as a formally one-phase substance, but one should not forget that the experimentally accessible reality somewhat deviates from it. As to physical condition, we must take account of the not strictly one-phaseness of colloid-chemical system-reality ( NOTE 259) .
Does one approach things from this direction and takes the "truly" living system to be a protein-organosol ( NOTE 260) , then one here has, in the strict physical chemical sense a heterogeneous polyphase system (dispersed phase in "dispersion medium"). Strong appositional and impositional hydratation doesn't, it is true, eliminate the continuity, but erases the phase boundaries, and we may assume that in the living substance the external physically chemical phase boundaries have in such high a degree become obscure, that one here has, although not a true homogeneous, but nonetheless practically a one-phase system with a sort of variations as inhomogeneities.
Phase boundaries [think, for example, of the boundary between the ice-state and water-state of H2O, or of precipitate and solution] are precisely characterized by the fact that [when crossing such a boundary] a large number of physical properties simultaneously and "permanently" change. Seen from the standpoint of phase theory, (small)-molecular solutions are homogeneous. The living total-system does possess a sort of spatial layers, but a "normal" phase boundary it only possesses at the external gross denaturation membrane. Is, it might be asked, the intramolecular transition between "exterior" and true "interior" a sort of phase boundary? Not in the physically mechanical sense, but it is [a phase boundary] in terms of the discontinuous change [when crossing the boundary] of many -- we might say, also "all" important, by us so considered, ones, -- properties or groups of properties. Experimentally, this phase boundary cannot in any way be crossed [meaning, experimentally we cannot pass things from non-living to living] ( NOTE 261) .
All dispersive systems ["dispersion" here means "state and degree of scattering of material in systems"] are characterized by the fact that the physically chemical properties and chemical properties screen- or lattice-like periodically change in space [particles, also colloids, distributed in a medium]. In contrast to (1) the in the highest degree dispersive systems (small-molecular solutions, and, as limiting case, even pure small-molecular solvents or media of dispersion themselves) and (2) the coarse-dispersive systems of mechanical suspensions, the living system [mega-molecule + medium] is a kind of inner- and one-dispersive system. If one wants to specify things, then one has at least three completely inter-embedded and mutually penetrating systems (viz., micro-molecular, macro-molecular-colloid, and vital-mega-molecular), in which, however, the concept of penetration does not apply to the proper "vitalized" substance. Here one must confine oneself to the concept of complete embedding. The whole [living] system can -- with certain limitations, which can only be further specified on the basis of precise knowledge of the structure of the living substance -- be taken as an approximated one-phase system [mega-molecule + medium is, approximately, a solution, representing the one phase]. This underlines the special nature of Unimol. Physically hardly applicable seems to be the concept of homogeneity in Unimol, although the concept of heterogeneity applies even worse ( NOTE 262) .
As to the macrophysical living system, and more rightly so, to the specially intra-molecular and intra-atomic ( NOTE 263) system, one can almost only then speak of true state-variables when it is about non-specific system properties. As to the bio-specific participating parts, reaching back to the special constituents, it is best to imagine a mere fluctuation-variability, a kind of inner or short-cut cyclic process, which proceeds at the subquantum level ( NOTE 264) , that is, exclusively in co-action of participating parts from couple- or [each for itself] spread-out quanta ( NOTE 265) .
A system is physically complete or closed, if its state is independent of the events outside it. It is then in every case also a full-static system. Because the living system is a complex system-structure, we can legitimately assume that the criterion of a physically closed system holds in some repects for the living system [mega-molecule + medium], but one in which, on the other hand, a -- perhaps "filtered" or censored -- selection of effects from external processes is necessary. In this sense the living system is partially an open system. But we emphasize : partially and uncharacteristically so, because we take Unimol as the sole super-characteristicum [showing something to be a closed system], the molecule indeed representing the in the highest degree closed form of all things existing. [The molecule is a system of atoms, and, moreover, a closed system].
With a "colloid-chemical" or "physico-chemical", or also "open system-only", we mean : complex interaction between purely molecular closed relatively simple partners. It is the chiefly analytic-physiological view [to see an organism like that]. Within the system-only there is nothing alive, it only lives as a whole. The whole system-only is the space-time aggregate -- the place of transition -- where non-life becomes life.
With "molecular-chemical one-molecule system" we mean : Uniform governing of one single, encompassing, throughout molecularly constructed, piece of matter of biocharacteristic complexity. This is a synthetico-morphologico-biological view. Within the molecular system already life as a whole exists. But not extractable, for because the living molecule is only functioning in its system-medium and living in it, the embedding medium already belongs to the existential conditions ( NOTE 267) . The molecular system [living mega-molecule + medium] is the space-time aggregate, representing life itself.
"One-molecule system" as ontogenetically and architectonically "explained" wholenes view, acknowledges the organism as primary, and views -- ontogenetically functionally and chemico-physiologically -- in its parts secondary , albeit equally important, phenomena. [ We would add : In the molecule as true holistic entity, i.e. as one single being, these parts are, as to their material concretenes, only virtually existing in the molecule. Actually existing in it they only are as qualities, not of these "parts", but of the molecule as a whole.]. The system-only view, on the other hand, would, consequently, view teh plant and animal body as secondary unity [i.e. as derived unity], being made up from the primary parts of the morphological and physiological basic elemments.
The one-molecule view doesn't imply the cancelling of the system [-concept in the domain of the organism], but is a structural completion, [pointing to] an aspect of the living system that explains its coordination. Instead of the system-only, which is enigmatic and contradictory (materialistic mechanism), and [again] instead of the system-only, which is [necessarily] provided with ad hoc factors which then attempt to build-in the metaphysical into the most direct sensorily experienced world (Vitalism [namely by having to assume special directing forces, acting within a mechanical framework] ), there must be a one-molecularly constituted "system-also", which already upon application of the familiar concepts and rules possesses a multiple power of performance, and which lets the metaphysical be effective only there where it is, unopposed, also present in the inorganic world, that is, in the whole world [And so there is no need to suppose the existence of a special non-physical life force, present in organisma, absent in inorganisms].
Evidently, the living organism cannot be succinctly defined without using a number of unexplained concepts. About this we can establish that, for instance, also Driesch's [who was a vitalist, letting organisms, and especially their individual development be endowed with a special vital force, the entelegy] definitions are never false or obsolete. They are based upon one or another something phenomenal, and thus generally say much too little. They are surely in need of extension and completion. We would, for example, say : The living individual organism, characterized by its immanent specific proper existential function, is an essentially unimolecularly constructed matter combinate, which needs to be completed by an aqueous electrolytic medium, in which it is only then fully functional, and whose overall - described as life-characteristic -- functioning is for the sake of securing the temporal existence of precisely this matter combinate. Or, more succinctly expressed : The individual organism is fundamentally (not precisely literally in old-style definition) a single molecule which behaves life-characteristic in order to be able to exist. [ This precisely corresponds to our idea stating that an organism is an embodied strategy-to-exist.]. Life-carrying cell protoplasm is not a colloidal solution ( NOTE 268) but the "suspension" of a single mega-molecule in an aqueous medium similar to it in volume.
In its reduced form (basic) life is an existentionally functional monovalent one-component system with a single (reaction) partner [the aqueous medium], and in this way remaining beneath all, also the most simple models [i.e. it is simpler than even these models of life] with whose help one wants to illustrate important phenomena of life. Thus : not so much, however well intended, inorganic models, simple or complex, but well thought-through reductions of the life-specific to the minimal essential provides the best impression of and insight into the life process.
L. von Bertalanffy introduced the concept of "open system", which was supposed to be in "dynamic ( NOTE 269) equilibrium". This idea surely contains progressive elements, but nevertheless for us it isn't so useful, because life shows itself in analytic research and also in unprejudiced consideration deceptively as a system, and when one attributes to this [system] the feature of "openess", then one still further deviates from that view which we take to be a necessary view. So organismic life is not an open system, but, if the word "system" should occur in it at all, a closed system, indeed in such a degree closed as is possible at all, namely as a result of true chemical bonding relationships between all partners. Only when this is established as being the case, and after having taken account of all its consequences, one may venture again -- but this time from another point of view -- to speak of an open molecular system (or open molecule), namely in complementing opposition to an inorganic small-molecule (inorganic = non-living) which, for it to temporally exist, does not need that what the organismic molecule does need, need in the form of, and complementing the system, a system medium, and which [small-molecule] still is, without "active" (better reactive, adaptively responding) interaction with the "outside world", also internally closed, namely shortcut. The idea of "dynamic equilibrium" may be dropped, because it appears as evident consequence already from the molecule-organismic view ( NOTE 270) .
As to the most difficult question of the origin of life, the question of how true life, with all the fundamental features of life as we know it, is created, it will be most compatible with our experience and powers of reason if we see in the generation of a persistent order of multiplication and increase exclusively the generation of molecular form, and not the generation of a multi-component system, and if we take the multiplication of life not to be a system multiplication - (a system may be generated again [ (re-generated) at several times and places], but not multiply), - but a true molecular increase of substance [copying and then releasing the copies] ( NOTE 271) .
That a free [in the sense of 'closed'] multi-component system is generated spontaneously or could be so generated, is, according to our state of knowledge as also after having exhausted our reasonably speculating imagination, conceivably improbable. But if it [the system] is reduced to a molecular unit, then we can surely, after some prepositions having been satisfied -- imagine its natural generation. So we conclude : The origin of life can only be a spontaneous and, what is, in addition to many other arguments, supported by even the research in psycho-analytical affects, moreover a discontinuous event, resulting in undiminished wholeness (for instance a first living molecule, provided with all the potencies), i.e. the origin of life can only be the generation of an organism. And that is the not-known-to-be-correct conjecture of any creation myth whatsoever.
The holistic order, being produced in every individual and since millions of years in a practically corresponding way, is the result of a uni-molecular unity and can only be like that ( NOTE 272) . The fact that one then at the same time has to do with a system -- without it to be, or to be possible, a system-only -- already ontogenetically is connected with the fact that this em- and circum-bedding solid-liquid colloid chemical system in its aqueous medium with its variable electrolyte content, possesses a uniquely suitable carrier and means of transportation of material hormonal and chemotactical influences and as such being an indispensable practical existential condition.
By reason of the markedly hydrophilous structure of the living substance, a part of the water can be taken [i.e. can be considered as] to be associatively hydratically bonded. And as to the rest, the free water, one could consider that -- individually seen -- the free and hydratically bonded water-molecules constantly exchange state [free/bonded] so that also the aqueous medium, i.e. a representative part of it, formally belongs to the one-molecule [the living mega-molecule] (without, it is true, the aqueous medium being involved in true bonding relationships, which [established true bonding relationships] we do not want [in our theory] to become "loosened" by it). Certainly, this [admitted relationship of the mega-molecule with water] harmonically and consequently mitigates, if not, eliminates a certain intrinsic contradiction between (1) the conceptually isolated one-molecule and (2) exclusively existent cell-"system". Also the difference between watery and dry living substance is erased because also living substance is not free from water ( NOTE 273) .
The sudden[ly appearing] new qualities of organisms [when going from inorganisms to organisms] ( life-specific behavior, which once came into existence) are only comparable [as to their suddenness] with those of the simple [non-living] organic and the inorganic molecular forms, which, as do organismic living molecules, show, relative to their atomic constituents, true and enduring differences [i.e. show suddenly appearing new qualities] ( NOTE 274) . So we mean : The distinction between the living and non-living states does, if one wants to compare anyway, not correspond to [i.e. not reside within] a colloid-chemical or similar system-only, whose effect is a mere additive one, formed -- and increased through certain interactions -- by effects of the [still] free components. But the distinction between the living and the non-living corresponds to [i.e. resides within] a chemically-molecular system, to a new substance [i.e. the description of the qualitative transition from the non-living to the living takes place within the overall description of chemical systems. So the transition is a chemical difference.]. In chemical systems it often happens that the possessed-by-their-own influences [workings, properties] of the free components -- [and then, in fact still not components at all] (properties of the unbonded atoms whose constitutive potencies always allow them to be combined into resulting new quality-types) -- almost completely and irretrievably disappear, and in their place we exclusively see the new properties of the compounds [Earlier, when, following HOENEN, 1947, treating the metaphysics of chemical compounds (See Part XVe and XVf ), we said that some of the properties of the atoms-going-to-make-up-the-molecule are lost or replaced by other properties, and these are now properties of the compound molecule, while the preserved properties of these atoms (residing in their central regions) also become properties, not of these atoms anymore, but of the molecule. In this way the atoms reside, not actually, but virtually in the compound molecule.]. New chemical relationships are new chemical compounds with new bondings. True, highly capable interaction is only to be found in atomic-molecular relationships, for instance in proteins. Interactions between closed self-contained [i.e. individual] molecules, free-swimming floats in a plasmatic-aqueous basic medium, as we have to assume them in systems-only, depend upon little-capable residual forces only.
If one thinks of the atomic partners and constituents of the organismic one-molecules and formally indicates them as "parts", then one may, in this sense, view any molecule (of whatever species or origin) as "system".
But still more characteristic [of a living system] is the typical holistic behavior, completely lacking in the known and imaginable systems-only, but, on the other hand, well known in the (lower) molecular systems. If, then, one also takes into account that the above mentioned two "limiting representatives of the concept of system" are in fact reality types, because true intermediary states do not exist at all, then one -- under avoidance of the unproved assertion : life is some such intermediary type -- will unequivocally classify the life-combination among the molecular phenomena. By expressions such as "one-molecule system", "molecular system", "unimolecular wholeness", "one-molecule view of the organism", "Unimol view of the organism", and other such expressions, the specific feature is sufficiently well expressed. In both parts of the expression, - namely on the one hand : "one-molecule", "molecular", "unimolecular", and "Unimol", and on the other : "system", "wholeness", and "organism", - at the same time the inner and outer order is indicated in a "clarifying" way. By using the short word "molecule" (organism as a molecule) thought will be focussed upon this new possibility.
Ontogenesis [i.e. individual development of an organism] (with its phenomena of regulation complexification, and directedness), regeneration [of body parts and tissues], and transplantation- implantation- and determination [what determines what] experiments, which represent the very difficult-to-interpret cases for the concrete system view [system-only view], are at the same time classically illustrative demonstrations in favor of the one-molecule view (Unimol). So also insect metamorphosis -- especially the processes during the pupal stage with partial destruction of the larval bodily constitution and orientation-anew and growth-anew up to the mature insect -- shows itself to be a true trans-formation [of itself], to be a development-of-and-by-itself, to be a forming itself, and to be a completing itself of a molecularly individual unity, and not [showing itself] to be a rearrangement of a given system or of a system-combination (being already in need for a super-ordination factor [in order to be a system] ) into another system.
All natural and experimental ontogenetic phenomena, which often are [as they are interpreted] not compatible with one another and especially not with current theories, must be understood such that a certain chemically-molecular mega-structure tries to establish itself. And that is at the same time the last fundament of the overall determination.
Spemann's principle of progressive organization is the consequent development of the one-molecule. A system-only is stationarily thinkable, and it also shows voluntary [i.e. spontaneous] re-arrangements, for example from a higher-energy unstable state to a low-energy levelled-out state, but a development of a system, or of something system-like, is not known. But if one then makes the usual assumption of the living state to be a unique state, then this necessarily demands the belief in some "vitalistic" directing agent.
There are neither living systems nor living orders or organizations, but only living true compounds, living molecular substances, that surely normally need the immediate environment in which they had once originated and in which they once lived, need it now as they did then. And therefore these living molecular substances exist in association with that environment, and as such stimulating the view of a living system. These molecular compounds are at the same time living organisms. The Unimol bonding combinations are whole-range-physical individual forms, which functionally existentially must be based on a medium, itself being a system of supporting molecules and other particles, but which [unimol bonding combinations] do not allow themselves to be identified with that medium such that one should have to emphasize the system aspect. To emphasize is always the unimolecular structure only.
The sought-for holistic system-laws of living beings are the chemical laws of molecular formation, here, it is true, in a completing extension. Unimol is already from the very start completed by the system-also view. And when the classical system view is completed by Unimol, then it is automatically transformed into the latter.
System-thinking and system-view have unmistakably strong demonic traits. The living "pure" system [in the sense of system-only] is, it is true, a well civilized view presented and elevated in a modern scientific framework, but nevertheless a relict of a primitive view of Nature and a derived from it way of thinking. The analytic findings in living things do not correspond to an equivalent "matter" (as to chemical bodies, one must think of chemical bondings), but -- be it more or less concealed, that is, not explicitly expressed -- correspond to the old demonisms.
The auxiliary system [the medium] is neither unimportant, nor uncharacteristic, but from it no life impulses come forth, no non-spatial determinant comes forth from it : it is [only] subsequently generated, and maintained by the governing force of a true individally formed and coherent unit-of-being provided with existential urge. Its existence and its existential needs form the central point of departure of all necessary organismic physiological processes. Just as all of these proceed in classical regular fashion, they are also causally evoked. The basic organismic causality which always is ordered to a particular individual, cannot, it is true, be taken in the strict sense of natural law, but only as statistical average behavior, and by comparison among the similar.
Applied to the human species, displaying the most complex and differentiated overall behavior, this would mean that the psychological and historico-cultural experience must oust exact formulation (that is precisely the instructive sense of social science and history). Feeding back the statistical rules of experience onto the individual then gives the impression of apparent acausality and freedom of will. The unimoleculalr view can, within the framework of the known, effectively clarify the problems that pop up here, and is able, here as well as everywhere else, to close wide gaps or at least rendering them less wide. The human thought-categorical description of the phenomenal world, -- which [description], by reason of the ignorance of the potencies of Being, must [unfortunately] replace the [actual] qualitative content of an absolute reality, -- may be substantially simplified [with Unimol]. Already this is a great advantage of the view.
In the next document we continue with the unimolecular view (Unimol) of organisms.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for further study of the Unimol View of organisms, rendering organisms to be homogeneously (metaphysically) classified with all other molecular Substances, Part XVj.
Back to Homepage ( First Part of Website)
Back to Contents (of Sixth Part of Website)
Back to first part of theoretic intermezzo
Back to second part of theoretic intermezzo Back to Part IX
Back to Part XIV, Dipterygia, and conclusion of flight-devices in insects
Back to Part XV, first part of the theory of inorganic nature
Back to Part XVa, second part of the theory of inorganic nature
Back to Part XVb, third part of the theory of inorganic nature
Back to Part XVc, fourth part of the theory of inorganic nature
Back to Part XVd, fifth part of the theory of inorganic nature
Back to Part XVe, sixth part of the theory of inorganic nature
Back to Part XVf, seventh and concluding part of the theory of inorganic nature
Back to Part XVg, Unimol, theory of the organism as one single molecule (first part)
Back to Part XVh, Unimol, theory of the organism as one single molecule (second part)