

e-mail :  ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
Most expressed examples of the type :

Figure 1 : Locusta migratoria, 33-60 mm. European migratory locust. Family Acrididae, Order Orthoptera-Caelifera.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
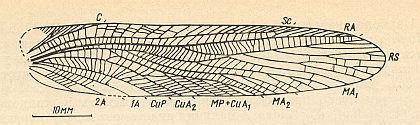
Figure 2 : Oedischia williamsoni Brongn. Forewing. Family Oedischiidae, Order Orthoptera-Ensifera. Upper Carboniferous of France.
(After a photograph in VIGNON, 1929, now in SHAROV, 1968)
Figure 2a : Reconstruction of the Protorthopteron Sthenaropoda fischeri Brongn. Family Sthenaropodidae, Order Protorthoptera. Upper Carboniferous of France. (After PONOMARENKO, in ROHDENDORF and RASNITSYN, 1980)
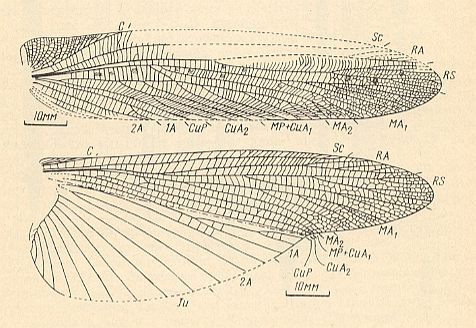
Figure 3 : Jubilaeus beybienkoi Sharov. Forewing and Hindwing. Family Tcholmanvissiidae, Order Orthoptera-Ensifera. Lower Permian, Tsjekarda, Ural, Russia. (After SHAROV, 1968)
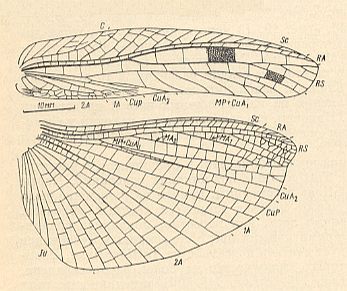
Figure 4 : Drymadusa spectabilis Stein. Forewing and hindwing of female. Family Tettigoniidae, Order Orthoptera-Ensifera. Recent. Archedictyon of forewing (fine meshwork of cells between main veins) only partly drawn. (After SHAROV, 1968)
Description of the type.
Functional features
Both pairs of wings take part in beating synchronously. The forewings may functionally be compared with the anterior firm margin of a costalized wing, while the hindwings play the role of an elastic membrane of the posterior margin. Wing-beat frequency varies within a wide range in the various subtypes. Undoubtedly the wing-beat frequency is low in the representatives of eu-orthopterygia, the true Orthoptera-Saltatoria. Significantly higher is the wing-beat frequency in representatives of homorthopterygia, the cicadas Jassidae. Thus the wing-beat frequency of the minute cicada Typhlocyba is known to equal 123 beats per second. Absolute speed of the representatives of this type is almost not investigated. Undoubtedly a very low speed of flight is characteristic of them. Governability also is very meagre. In fact, the majority of these insects has rectilinear flight, and only in some representatives of the subtype homorthopterygia (see below) governability in flight is observed. Load on a unit of surface-area of the wings is very variable. It is very high in the representatives of eu-orthopterygia. Thus, in the katydid (member of the family Tettigoniidae) load is 0.2100 gr/cm2. In the subtype homorthopterygia the load, undoubtedly, is much lower.
The biological significance of flight to the representatives of this type is rather great -- these insects often fly off, and although the great majority of them is vegetarian, flight to them is nevertheless a chief and powerful factor in their distribution, and has significance in the processes of reproduction.
Differentiations, connections, and representatives of the type.
Straight-wingedness, or orthopterygia, has originated from various sources, and characterizes the acquisition of certain shield properties by the forewings while preserving their important role in the flight-movements. A result of these processes is the development of heteronomy of the wings, also a characteristic feature of this type. A low degree of similarity of the original forms of straight-wingedness determines a marked diversity of this type of wings, which naturally may be subdivided into secondary groups, subtypes.
1. Eu-orthopterygia
The most ancient examples of straight-wingedness (orthopterygia), having originated probably on the basis of first forms of long-wingedness (dolichopterygia ) [perhaps that of the ancient Dictyoneuridae (Palaeodictyoptera) ], are characterized by a rich venation composed of numerous longitudinal veins and cross-veins. Such a venation is possessed by both pairs of wings, especially the hind pair. Also characteristic of the representatives of this subtype are the large sizes of body and wings, being not less than 10 mm, usually up to 25 mm and more. This subtype, true straight-wingedness or [equivalently] eu-orthopterygia is illustrated by the majority of Orthoptera-Saltatoria (Acridioidea, Locustoidea, and others), preying mantises (Mantodea), fossil Protoblattoidea, Paraplecoptera, and Protorthoptera. See Figure 1 and next Figure.
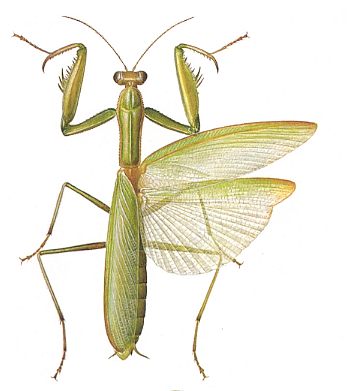
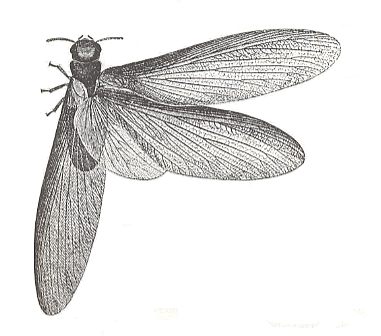
Figure 5 : Mantis religiosa, preying mantis, 40-75 mm. Order Mantodea.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
[The distribution of this and the next subtypes among orthopteroid insects (fossil and recent), as given by ROHDENDORF, 1949, is in need of revision, which we shall carry out here. This is especially relevant to the many orthopteroid paleozoic and particularly mesozoic fossils that have been subsequently described by SHAROV, 1968.]
2. Homorthopterygia
One of the possible variations in the present type consists in, as a rule, a markedly decrease of the venation, which is composed of a moderate number of longitudinal veins and a very small number of cross-veins. The hindwings are [in resting position] folded along just a single fold, turning over the anal region. This subtype is a derivative of neuropterygia and heteropterygia and may be called homorthopterygia. To it do belong many cicadas {Jassidae) [Homoptera] and some bugs [hemiptera] (Veliidae, Gerrididae, Hydrometridae). See next Figures.
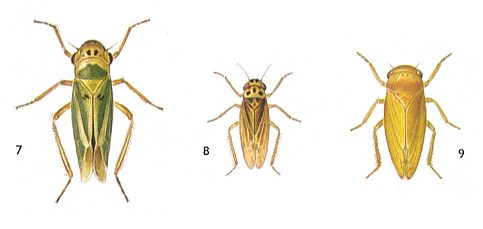
Figure 6 : Jassidae, dwarf-cicadas, Order Homoptera.
7 - Cicadella viridis, 5-9 mm.
8 - Typhlocyba jucunda, 4-4.5 mm.
9 - Jassus lanio, 6.5-8.3 mm.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
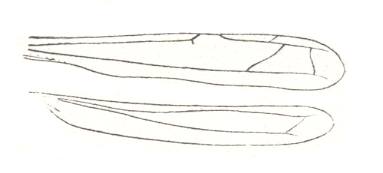
Figure 7 : Wings of the water-bug Hydrometra stagnorum L. Hydrometridae, Hemiptera.
(After HANDLIRSH, in ROHDENDORF, 1949)

Figure 8 : Hydrometra stagnorum, 9-13 mm. Usually short-winged. Hydrometridae, Order Hemiptera. Lives on the surface of water [ponds] close to the shore. (After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
3. Dysorthopterygia
We must distinguish yet another group of orthopteroids in which the forewings show regressive development, shortening and lacking aerodynamic adaptations. This subtype, closely related to eu-orthopterygia, and being partly transitional to elytropterygia (shield-wingedness), includes the crickets (Grylloidea) and the Gryllacridoidea. It may be called regressive straight-wingedness or, equivalently dysorthopterygia. See next Figures.
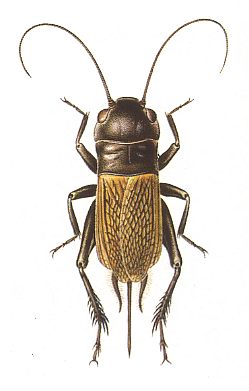
Figure 9 : Gryllus campestris, field-cricket. 20-26 mm. [female] Gryllidae, Order Orthoptera-Ensifera.
Type of wing-apparatus : orthopterygia, subtype gryll-orthopterygia.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
The venation of the forewings of males of the Gryllidae (crickets), although indeed aerodynamically regressive, is nevertheless specialized, not indeed aerodynamically, but to perform a different function -- stridulation. Therefore it may be wise to exclude these wings from the subtype dys-elytropterygia and place them into a new subtype, not recognized by Rohdendorf -- gryll-orthopterygia (see further down ). In fact we, in the Gryllidae, have to do with a comparable phenomenon in beetles : Also there the "forwings" are in an aerodynamic sense degenerated, they are not a component of the flight-apparatus anymore. But they are nevertheless specialized, namely with respect to another, new, function : the protection of the hindwings and the abdomen against mechanical factors when crawling inside narrow spaces of the substrate. This function is also performed by the forewings of the crickets, but at the same time in males they are further transformed such as to be able to generate sounds.
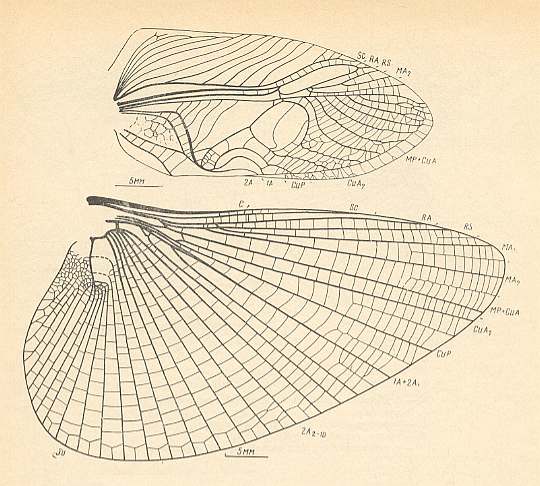
Figure 10 : Brachytrypes portentosus Licht. Forewing (male) and hindwing. Family Gryllidae , Order Orthoptera-Ensifera. Recent. India.
Type of wing-apparatus : orthopterygia, subtype gryll-orthopterygia.
(After SHAROV, 1968)
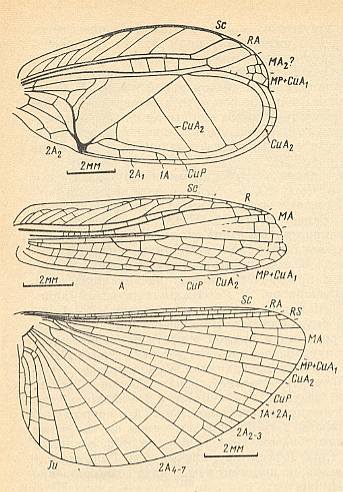
Figure 11 : Family Oecanthidae, Orthoptera-Ensifera. Recent. Showing the heavy transformation of the forewings of the males into stridulatory organs.
Top image : Oecanthus pellucens Scop. Forewing of male.
Middle image : Forewing of female of the same species.
Bottom image : Hindwing of Oecanthus pellucens.
Type of wing-apparatus : orthopterygia, subtype gryll-orthopterygia.
(After SHAROV, 1968)
Rohdendorf also mentioned the Gryllacridoidea having wings belonging to regressive straight-wingedness, dys-orthopterygia. Although for true crickets, Gryllidae, such an assessment -- their belonging to the dys-orthopterygia -- may be understandable insofar as in them true straight-wingedness is destroyed with respect to the forewings (they no longer have an elongated shape, and may not participate in flight-movements as do the forewings of the other representatives of orthopterygia), - for the Gryllacridoidea, or at least for the family Gryllacrididae, this assessment is not directly clear : they have well developed hindwings, and forewings with rich venation. So it would seem that they do not belong to representatives of dys-orthopterygia, but, instead, to representatives of the next subtype, neur-orthopterygia. It is, however, the details of that venation (of the forewings) that show us that at least the forewings have regressive features in their venation, determining them to represent dys-orthopterygia after all. We can see this, when we compare the forewings of Gryllacrididae with those of the Haglidae (themselves representatives of neur-orthopterygia (next subtype)), their apparent ancestors :
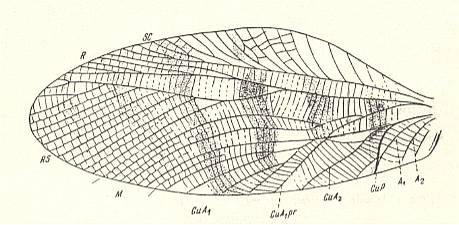
Figure 12 : Aboilus columnatus Mart. Forewing. Subfamily Prophalangopsinae, family Haglidae , Order Orthoptera-Ensifera. Upper Jurassic of Karatau, southern Kazachstan. CuA1 - anterior complex branch of CuA. CuA1pr - its proximal branch. CuA2 - posterior branch of CuA. CuP - posterior Cubitus, transformed into a stridulatory vein. Functional wing-type : orthopterygia, subtype neurorthopterygia .
(After MARTYNOV, 1937, in MARTYNOV, 1938)
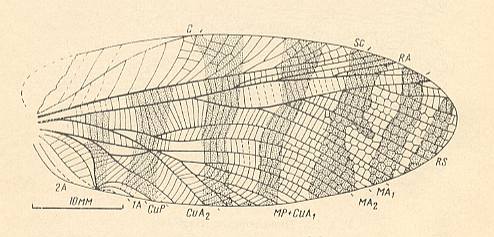
Figure 12a : Aboilus columnatus Mart. Forewing of male. Subfamily Prophalangopsinae, family Haglidae, Order Orthoptera-Ensifera. Upper Jurassic of Karatau, southern Kazachstan. Functional wing-type : orthopterygia, subtype neurorthopterygia . (After MARTYNOV, 1937, in SHAROV, 1968, with additions and changes [made by SHAROV].)
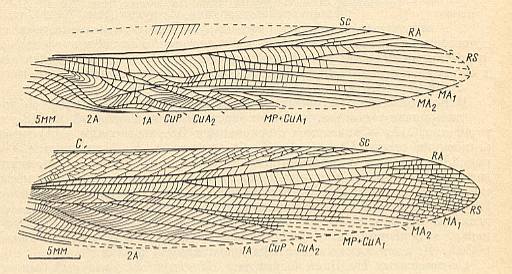
Figure 12b : Triassic Haglidae, subfamily Haglinae.
Top image : Protshorkuphlebia triassica Shar. Forewing of male. Family Haglidae, Order Orthoptera-Ensifera. Lower Triassic of Madigen (Central Asia). Functional wing-type : orthopterygia, subtype neurorthopterygia .
Bottom image : Forewing of female of the same species. Lower Triassic of Madigen (Central Asia).
(After SHAROV, 1968)
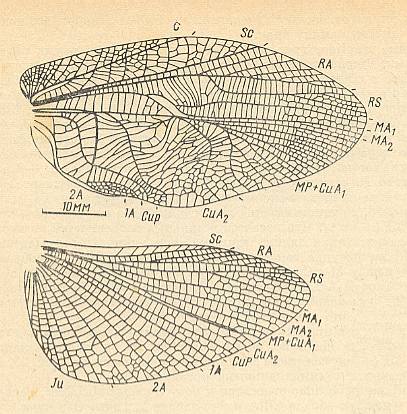
Figure 12c : Prophalangopsis obscura Walk. Forewing and hindwing of male. Subfamily Prophalangopsinae, family Haglidae, Order Orthoptera-Ensifera. Recent. India. Functional wing-type : orthopterygia, subtype neur-orthopterygia or, maybe, subtype dys-orthopterygia.
(After MARTYNOV, 1968)
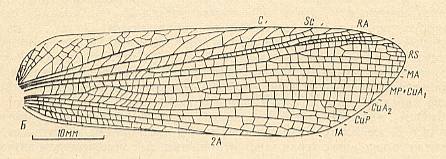
Figure 12d : Hadrogryllacris shalfordi Griff. Forewing. Family Gryllacrididae, Order Orthoptera-Ensifera. Recent. Australia. Functional wing-type : orthopterygia, subtype dys-orthopterygia . (After SHAROV, 1968.)
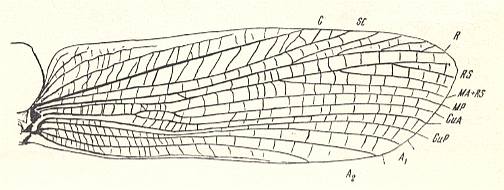
Figure 12e : Paragryllacris marginalis Walk. Forewing. Family Gryllacrididae, Order Orthoptera-Ensifera. [Recent]. Functional wing-type : orthopterygia, subtype dys-orthopterygia . (After MARTYNOV, 1938.)
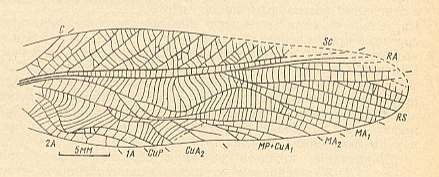
Figure 12f : Hagla gracilis Giebel. Forewing of male. Subfamily Haglinae, family Haglidae, Order Orthoptera-Ensifera. Lower Jurassic of England. Functional wing-type : orthopterygia, subtype neur-orthopterygia . (After ZEUNER, 1939, in SHAROV, 1968.)
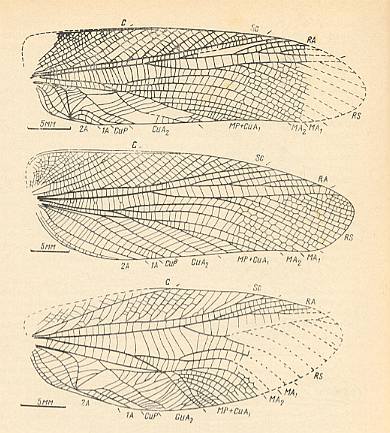
Figure 12g : Cretaceous Haglidae. Orthoptera-Ensifera.
Top image : Parahagla sibirica Shar. Subfamily Haglinae. Forewing of male. Lower Cretaceous of Baisa, Siberia.
Middle image : Forewing of female of the same species. Lower Cretaceous of Baisa, Siberia.
Bottom image : Prophalangopseides vitimicus Shar. Subfamily Prophalangopsinae. Forewing of male. Lower Cretaceous of Baisa, Siberia.
Functional wing-type of all three of them : orthopterygia, subtype neur-orthopterygia . It might be reasonable that we consider the wing of the bottom image (and all morphologically related wings) as belonging to a new subtype that we add to Rohdendorf's four subtypes of orthopterygia : gryll-orthopterygia, to which do belong the wings of all crickets (Gryllidae) and their pre-stages in Haglidae.
In this Figure we see the small pre-costal area as it is in Haglinae, and an extended pre-costal area as it is in Prophalangopsinae.
(After SHAROV, 1968.)
Involving the above, and further Figures, we will translate from the Russian a text-section in MARTYNOV, 1938, p. 110-115, in which he discusses the regressive nature of, especially, the forewings of representatives of the family Gryllacrididae and relatives. Especially interesting is his functional explanation of it, taking into account the more or less concealed way of life adopted by the Gryllacrididae, and the evolution, or de-evolution, of the stridulatory apparatus in the forewings of the males.
In a lecture at a congress of Russian zoologists, anatomists, and histologists, I already in 1923 gave support to the thesis that the wing-venation in Gryllacrididae and Stenopelmatidae undoubtedly carries certain traits of de-differentiation having taken place in their history, resulting in the venation to acquire features of indifference of some kind,- as it were, bearing witness to deep primitiveness, while in fact this phenomenon of reduction is connected with a decrease of work, with weakening of a function. In their ancestors, having lived in the Mesozoic, and perhaps also in the upper Permian, their venation [i.e. in Gryllacrididae and Stenopelmatidae] was much more similar to the venation as it was in primitive Aboilidae (Haglidae), and also to their close relatives, the permian genera of Sthenaropodidae + Oedischiidae. [For Oedischiidae see Figure 2, above]. In some mesozoic ancestors of Gryllacridoidea, or at least in the ancestors of many Stenopelmatidae, a musical apparatus on the forewing of males began to function, albeit still weakly so, to which points the presence of auditory organs or their rudiments on the fore-tibiae in Anostostominae and a part of Henicinae (Stenopelmatidae). The presence of the rudiments of these organs in a number of Stenopelmatidae could not be explained without admitting the existence in their ancestors of one or another stridulatory apparatus in the forewings. Analysis of the wing-venation in Gryllacrididae and Stenopelmatidae from a historico-functional viewpoint led me to conclude that their wing-venation, chiefly that of the forewings, was in earlier times more similar to that in Haglidae, and only afterwards did experience a de-specialization and a seemingly return to the primitive condition.
When we compare the venation of the forewings of such Gryllacrididae that possess a more complete venation (type I of Karny) [Figure 12e] with the venation in Haglodea [such as in Haglidae], then we easily see the following : R and RS are structured in those species of Gryllacrididae completely like in the species of Aboilus (see Figure 12 ). R distally gives off anteriorly precisely such oblique branches and in the same number (5) as in Aboilus. And RS gives off precisely such oblique branches posteriorly as in Aboilus. However, while in Aboilus these branches run, if we may express ourselves in this way, "freely" and strengthen and support the whole distal third of the wing, in [species of] Gryllacris, where a short or long RS is present in them, the branches of the latter run outward in such a compressed series, that they support only a vanishing part of the distal section of the wing, and behind them we find long stretched-out branches of M, Cu, and even the first anal veins. The pre-costal region [i.e. the region lying anteriorly to the Costal vein, i.e. between the Costal vein and the anterior wing-edge proper] is [more or less] small in Aboilus (Figure 12a ) and Hagla (Figure 12f ), as it is small in Sthenaropodidae [i.e. Protorthoptera of the upper Carboniferous], and, usually in Oedischiidae (Figure 2 ). In Gryllacrididae, on the other hand, it became very extensive [especially very long], as in many Tettigoniidae. As I already often indicated earlier, the pectinate type of RS, which we find in orthopteroids (Acridoidea [grass-hoppers, locusts], Haglodea [such as Haglidae, most of them extinct] ), in many Protorthoptera, in Protoblattoidea, and then especially in Neuroptera [lacewings and allies], partly in the larger Homoptera [Cicadas and allies], and, finally, in Palaeodictyoptera [ancient well-flying large insects], that is, in the most diverse groups, from the oldest times having followed lines of evolution independently of each other, the pectinate type of RS, undoubtedly, everywhere was formed parallel and independently and in the same period, as the result of mechanical demands in the development of flight. The distal section of the fore- (and initially also of the hind-)wings is always thinner, more delicate, than the basal section, and at the same time has to perform the fastest and most powerful movements in the air, which might lead to fractures. To better support this membranous distal part and to better oppose against fracture during wing-beat against the air, inevitably there has to originate one or another overall scheme [of veins], and this system we find in the pectinate type of the Radial Sector. The branches of such a Sector, indeed, always occupy the whole distal part of the wing, more or less so depending on the distribution of the veins in the basal part, and on the consistency of the wing or tegmen. Precisely such a type of Radial Sector we also find in the well developed forewings of the genera Aboilus and Hagla, in Acridoidea [short-horned grasshoppers, locusts] and others. In the Gryllacrididae, together with a similar R and a comparable number and way of branching-off of branches of RS, these branches are compressed [i.e. they reduce and occupy a narrower region of the wing-membrane], now involving only a negligible part of the surface of the distal section of the wing, and, clearly, do not at all perform the function of the pectinate RS. The Radial Sector is here in an evident condition of degradation.
Could, in a normally functioning wing, such a type of RS with an extremely compressed series of branches actually be formed? Evidently not, because such a system has no functional meaning. The similarity present in the structure of R and RS as to the number of branches and their relationship to one another clearly indicates that in certain closest ancestors of Gryllacrididae, probably in the Mesozoic, there did exist a pectinate RS of normal structure and size, having performed precisely that function as in Aboilus and relatives and in other insects, but then having undergone reduction and compression. In other species of Gryllacris we find diverse conditions of continued reduction of the normal Radial Sector : its branches are compressed still further, become irregular, and, finally completely disappear as in type V of Karny.
Further, may we assess the structure of the Media (M) in Gryllacrididae to be primitive? Absolutely not. While RS once supported with its branches the whole distal half or third of the wing, so also the branches of the Media inevitably had to run arch-like backwards, taking part in supporting the central part of the wing when beating against the air. Attentively inspecting the structure of the Media in species of Gryllacris with a more complete venation, we easily note that the nature of branching of M and of the connections of its chief branches with RS and CuA is sometimes completely similar to what we see in Haglidae, but further down the branches of M take a completely different course, straight outward. The same we also see in the Cubitus, whereby the nature of the relationships of the branches of M and CuA to each other is more diverse and highly variable. The nature of these connections in the form Gryllacris with a more complete venation (type I and II of Karny) clearly points to the fact that earlier there had to exist in the structure of R as well as of Cu more of similarity with the corresponding structure in more primitive Haglidae (Hagla, Aboilus, Archaboilus) and, partly, in permian representatives of Oedischiidae and Stenaropodidae. Such a type of distribution of these veins was worked out in the process of adapting the forewings of these orthopteroids and their venation to their functioning during flight. [Indeed, in wings belonging to the type of orthopterygia the (partly stiffened) forewings participate in aerodynamic flight movements (together with the (broadened) hindwings), functioning as the anterior margin of a costalized wing.]
In Gryllacrididae, evidently, loss of the earlier normal function of the wings has taken place, which also led to a reduction of the complex RS, and in connection with this also to the loss of the initial distribution of the branches of M, Cu, and the anals. During the reduction of RS the latter veins (M, Cu, and anals) began to stretch out and to become almost parallel, losing their initial individuality connected with a determined function. As a result there was an accumulation of stretched veins which we encounter in the more reduced type V of Karny (at the left of next Figure).
Figure 12h : Left : Venation of forewings of Gryllacris fasciata Walk. [note the intraspecific variability!]. Right : Fore and hindwing of Bugajus coloni Walk. [ I assume that this species belongs at least to the Gryllacridoidea].
[ Functional type of all these wings : orthopterygia, subtype dys-orthopterygia ].
(After KARNY, 1924, in MARTYNOV, 1938.)Here the veins already completely lose their individuality, the venation becomes indifferent and therefore also extremely variable.
In connection with these processes -- we may think -- also took place a strong growth [increase] of the pre-costal area. Initially it was not so large. It was small and even underwent some reduction in a number of mesozoic Haglidae [see for example Figure 12f ]. But in Prophalangopsidae, evidently, it secondarily increased as in Gryllacrididae. [See for the by Martynov mentioned Prophalangopsidae the genus Prophalangopsis Figure 12c above, where it is assumed (Sharov, 1968) to belong to the family Haglidae, but indeed to the subfamily Prophalangopsinae. See also Figure 12g, bottom image. And indeed, also the above mentioned genus Aboilus (see both images), although belonging (according to Sharov, 1968) to the family Haglidae belongs, within the latter, to the same subfamily Prophalangopsinae.].
While discussing these things we inevitably must concede that the flight-function in Gryllacrididae already long ago underwent a marked weakening. This precisely corresponds to reality.
All Gryllacrididae live a more or less hidden way of life, hiding in cavities, in dense foliage, etc., and usually seldom take flight, and others [i.e. other members of the mentioned family] either do not fly at all, or become wingless. This way of life was typical to them evidently for already a long time. The flight-function in Gryllacrididae thus had significantly decreased, changed, or was completely lost, and in connection with this the wing-venation, initially, evidently, adapted the flight-function and having been very similar to the venation in primitive Haglidae, and also to that in Sthenaropodidae and Oedischiidae, in further evolution had to degrade, despecialize, and reduce. And as in every organ being in the process of reduction, also the wing-venation became very variable. The longitudinal branches of the stretched-out M, Cu, and anals lost their former functional significance and became functionally indifferent. In connection with this, in their distribution there appeared some sort of secondary homonomy [among these branches], and as a result of this : also variability. The venation began to resemble more that of cockroaches, but this is not at all a return to primitiveness, not something contradicting Dollo's Law [of non-reversible evolution], but a degrading as a result of the loss of function.
Stenopelmatidae, Raphidiophoridae, and Schizodactylidae had become already 'fully-fledged' cave-dwellers, having worked out a number of adaptations for such a life. Among Stenopelmatidae we still can find a few winged, albeit not flying, species, but the majority has completely lost the wings. Inspecting such relict wings of Stenopelmatidae, we, naturally, find in them traits of degradation and reduction of the initial wing-venation of their ancestors. The normal pectinate RS here, evidently, existed, but in recent forms, although as a result of hereditary inertia still having preserved its branches, its whole system nevertheless underwent extreme compression and the branches do not of course have their former functional significance anymore. The remaining veins were strongly stretched out and became almost parallel. The hindwings are markedly broadened as in Gryllacrididae, but also carry features of degradation. The wing-venation in such forms, naturally, had become very variable.
In connection with the early transition to a concealed, nocturnal, and more so, cave-dwelling way of life, we bring in also the monotonous greyish-brown or grey color of the forewings in Gryllacrididae and Stenopelmatidae.
So, although in certain respects Stenopelmatidae and Gryllacrididae do show traits of a high degree of primitivity, the venation of their wings is strongly degenerated, and therefore is merely pseudo-primitive. In the majority of forms the wings already had lost their initial function and are present only as a result of heredity, as relict structures, being in a condition of reduction. On answering the question of how these wings did look like in earlier times we may base ourselves on the in some of them preserved complete number of branches of RS and R, and on the configuration of the basal part of the Bicubitus. Reconstructing that form and those sizes, which necessarily these structures must have possessed when they were functioning normally in the flying wing, we come to the conclusion that the wing-venation, chiefly that of the forewings, initially only little differed from that in Aboilus and in other more primitive Haglidae. And because in the males of Haglidae CuP had already become a stridulatory vein, and in connection with this in them was worked out also the tympanic auditory organ in the fore-tibiae, it is legitimate to ask the question - did or did not the ancestors of Stenopelmatidae and Gryllacrididae possess a stridulation device similar to that in Haglidae? Taking into account the presence of rudiments of such tympanic organs on the fore-tibiae in Stenopelmatidae, it is natural to conclude that such a musical device indeed was already worked out in the ancestors, when not in all, in a part of the Stenopelmatidae. However, among them, evidently, there are also other methods to produce sounds.
In Gryllacrididae remains of tibial auditory organs apparently are nowhere to be found, and therefore to conclude about the existence in their ancestors of a stridulatory device of the type Aboilus would be risky. In some forms stridulatory organs do exist but are different, namely placed on the first and second segments of the abdomen, as for instance in Pneumoridae and Tmethidae from the Acridoidea [= short-horned grasshoppers]. However, if even we concede that the ancestors of the Gryllacrididae did not possess a stridulation device of the type Haglidae, then nevertheless we should recognize that the distribution of the veins in the cubital and anal regions here was formerly all in all similar to the distribution of them in the males of Haglidae, only without CuP curving backwards and without its transformation into a stridulatory vein.
In permian Sthenaropodidae and Oedischiidae the whole structure of the future musical apparatus of the type Haglidae in fact was basically already formed, and only a few things were lacking, namely the bend of CuP and its transformation into a stridulatory vein, and then some other partial changes in the cubital region, in order to get the musical device of the type as we see it in the more primitive Haglidae (Hagla, Aboilus).
Sometimes it is said that in Gryllacrididae and Stenopelmatidae "the anal veins became many". This is not true : Of anal veins there are here as much of them as in Haglidae and other Saltatoria, i.e. two. Only A2 in them branches, as a rule, into two or even three, which is also seen in crickets.
All the time we said that in the males of Haglodea the venation of the cubito-anal region was a direct little-changed inherited distribution of veins in this region [as it was] in permian Stenaropodidae and Oedischiidae. Such was the venation in males. How did it look like in females of Haglodea? To me now is also known the venation of females of Aboilidae. It will be described and drawn somewhere else. Nevertheless we already now may say that it was very different from that of the males. The anal veins and their branches here ran outwardly, only weakly bending, as do the branches of the Cubitus, and these as well as those were markedly stretched out. Generally, the course of the anal and cubital veins in them reminds us of partly the crickets, partly the Gryllacrididae. Because the distribution of the veins in the cubital and anal regions in the paleozoic Stenaropodidae and Oedischiidae already was almost completely similar to the distribution in the males of Haglodea, and was, consequently, already highly differentiated, we may hold that the venation in the females was formed as a result of regression, de-differentiation. The parallel course and a certain homonomy of the veins in them is thus purely secondary [i.e. derived, not original], as it is also in the males and females in Gryllacridoidea.
So, the wing-venation in Gryllacrididae and Stenopelmatidae was worked out, according to us, by way of degradation from the venation of the type as we see it in the more primitive Haglidae or their ancestors, as a reult of the transition of this whole group to a more concealed way of life. However, they could not have been originated from the mesozoic Haglodea known to us, the more so because Gryllacrididae, and, probably, also many Stenopelmatidae and Raphidiophoridae never, apparently, had possessed a stridulatory device of the type as we see it in Aboilus [which belongs to the Haglodea (namely to the subfamily Prophalangopsinae of the family Haglidae) ]. Therefore, one should not derive the Gryllacrididae from true Haglidae of the Mesozoic but from earlier common ancestors of them, having lived in permian times. In our phylogenetic scheme we unified the stem of Gryllacridoidea at the base with the stem of Haglodea in order to indicate their close relationship and origination from common ancestors. The stem of Haglodea developed progressively, acquired a musical device and auditory organs, and reached their peak already in the early Mesozoic. For the greater part these were large forms with long antennae [long-horned grasshoppers], with an ovipositor of the type as we see it in katydids (Tettigoniidae) and with grey-brown transverse bands on their forewings indicating that they were diurnal forms. The presence of transverse grey-brown bands may also, perhaps, have a biological meaning, namely that of camouflage for these insects, living among the foliage of the plants of those times, i.e. sago- and mesozoic pine. However, at the end of the Mesozoic, and, maybe, at the beginning of the Teriary, this whole large group of luxuriant forms quickly and radically becomes extinct, leaving for the present only two forms -- Prophalangopsis and Cyphoderris, of which the first is already utterly rare (India, up to now known by only a single specimen).
The chief cause of the quick extinction of this group, as well as of that of a number of other insects, were, undoubtedly, climatic changes and the quick extinction, especially in the Northern hemisphere, of mesozoic types of plants when, in their place, quickly developed and expanded the flowering-plants (Angiospermae). The Gryllacridoidea are genetically very close to the Haglodea and initially, in the upper permian or in the lower Mesozoic, their immediate ancestors, probably, were very similar to Haglodea, but further in evolution strongly deviated from them, which can be explained only by the transition of them to a concealed way of life and the weakening, and then also the loss in some of them, of the ability to fly.
In contrast to Haglidae, however, Gryllacrididae, Stenopelmatidae, and closely related to them families, did not go extinct, but live also today by a significant number of species. Their survival, I am inclined to clarify by the concealed, in many of them even cave-dwelling, way of life, acquired, evidently, long ago, as well as by their feeding habit, -- they are taken to be predatory, and certainly not vegetarian. The latter makes them less dependent on the nature of the flora and its replacement, and the former -- their concealed way of life -- as it were led them away from the open arena of the struggle of life and allowed them in far-off biotopes to escape competition with new forms, -- I think of short-horned grasshoppers (Acridiodea) and true katydids (Tettigoniodea), appearing at the scene together with the appearance and expansion of the angiosperm flora.
Tertiary fossil Gryllacrididae are very little known, but nevertheless important is the fact that in tertiary times they surely existed. Undoubtedly also Stenopelmatidae and Raphidiophoridae did exist, but were not preserved [as fossils] as a result of their cave-dwelling way of life. Gryllacridoidea are not known from the Mesozoic. I think that in the lower Mesozoic precisely also took place the process of their formation from their ancestors that were similar to primitive Haglidae.
The majority of authors holds that Prophalangopsidae (and Haglidae) [later -- SHAROV, 1968 -- they, Prophalangopsidae, were seen as just a subfamily of Haglidae] and Gryllacridoidea are more closely related to Tettigoniodea than to Gryllodea, but Karny included them into "Gryllodea". Zeuner derived from stenopelmatoid ancestors on the one hand the Gryllodea + Tettigoniodea, and the Acridiodea and Tridactylidae on the other.( End of quote from MARTYNOV, 1938 )
(Continuing the description of subtypes of orthopterygia)
In the subtype dys-orthopterygia we may also place recent Phasmatodea with still large forewings, the venation of which is in a degenerative state :
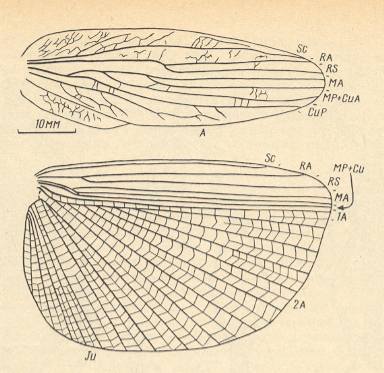
Figure 12i : Wing-venation of Ctenomorpha titan. Fore- and hindwing. Phasmatodea. Recent.
(After BRONGIARD, 1893, in SHAROV, 1968)
and also the wings of the family Schizodactylidae of the Orthoptera-Ensifera :
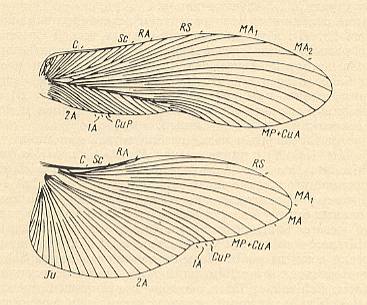
Figure 12j : Wing-venation of fore- and hindwings of Schizodactylus monstrosus (Drury). Schizodactylidae, Orthoptera-Ensifera. Recent. India. (After RAGGE, 1955, in SHAROV, 1968)
Habitually, and also as a result of the sharp fold-down of the forewings in their proximal half along the Radius, the representatives of the family Schizodactylidae remind us of Gryllidae (Crickets), but the 4-segmented tarsus, the absense of an ovipositor, and the unique method of curling up of the apices of the fore- and hindwings into a spiral at rest, are not characteristic of Gryllidae. These insects are able to burrow into the soil, spending the daylight hours in the holes they have dug and seeking their prey at night.
Apparently, the venation of the forewings is flight-aerodynamically regressive, but the forewings are more or less progressive as to their shielding function.
4. Neurorthopterygia
The fourth subtype of straight-wingedness -- an immediate derivative of neuropterygia (neuro-pterygia) -- is characterized by the moderately developed little-specialized venation of the weakly stiffened forewings, and by the broad fan-shaped hindwings provided with usually many folds, and may be called neurorthopterygia (neur-ortho-pterygia). To it belong the stone-flies (Plecoptera) and some extinct groups such as Protoperlaria :
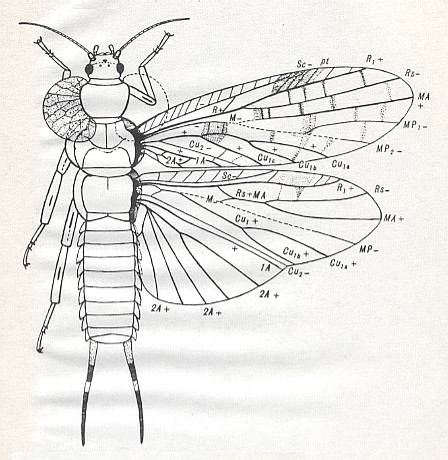
Figure 13 : Lemmatophora typica Sell. Lemmatophoridae, Order Paraplecoptera (Protoperlaria). Lower Permian of Kansas (USA).
(After TILLYARD, 1928)
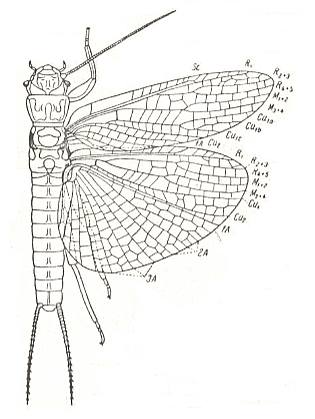
Figure 14 : Eusthenia spectabilis Woodw. Eustheniidae, Order Plecoptera (stone-flies). Recent. Australia. Length of forewing 23 mm.
(After TILLYARD, in ROHDENDORF, 1949)
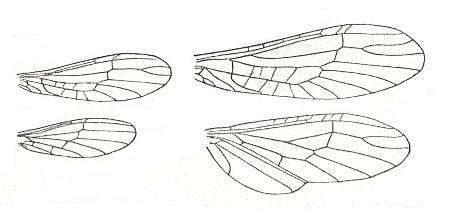
Figure 15 : Wings of Plecoptera, Perlidae.
Left - Chloroperla cydippe Newm. North America. Wingspan 12-16 mm. Recent.
Right - Alloperla borealis Banks. North America. Wingspan 20-29 mm.
(After NEEDHAM, in ROHDENDORF, 1949)

Figure 16 : Plecoptera (stone-flies).
5 - Nemoura cinerea. 4.0-6.8 mm. Length of forewings 5.0-7.9 mm. Recent. Family Nemouridae.
6 - Perla bipunctata. 15-25 mm. Recent. Family Perlidae.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
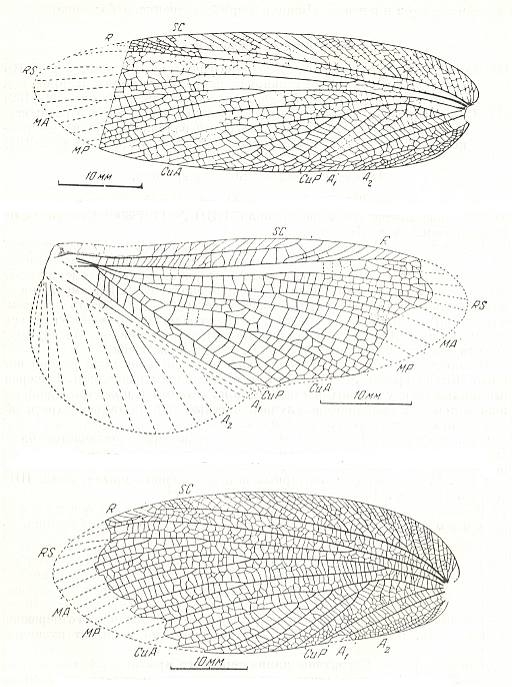
Figure 17 : Paraplecoptera from the Lower Permian of Kuznets Basin, Siberia.
Top image : Archidelia elongata Shar. Ideliidae. Order Paraplecoptera. Forewing. Combined drawing from positive and negative impression. The forewing in the genus Archidelia is compact, coriaceous.
Middle image : Archidelia elongata Shar. Ideliidae. Order Paraplecoptera. Hindwing. Combined drawing from positive and negative impression.
Bottom image : Archidelia ovata Shar. Ideliidae. Order Paraplecoptera. Forewing.
(After SHAROV, in ROHDENDORF et al, 1961)
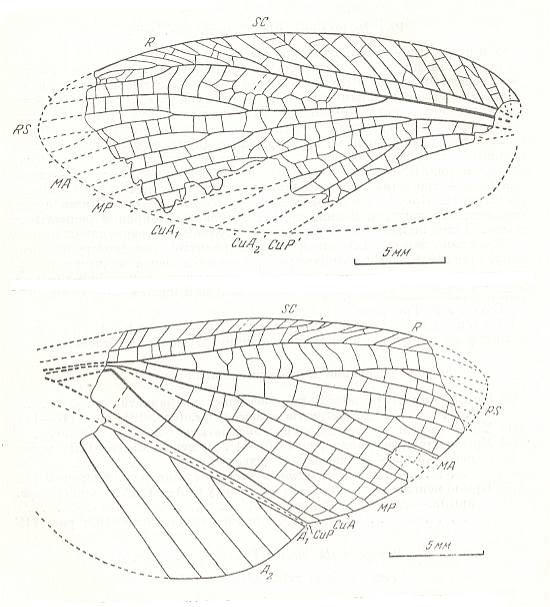
Figure 18 : Paraplecoptera from the Lower Permian of Kuznets Basin, Siberia.
Top image : Parapermula siberica Shar. Liomopteridae. Order Paraplecoptera. Forewing.
In the family Liomopteridae the forewings are membraneous (Rohdendorf et al., 1961).
Bottom image : Parapermula siberica Shar. Liomopteridae. Order Paraplecoptera. Hindwing.
(After SHAROV, in ROHDENDORF et al, 1961)
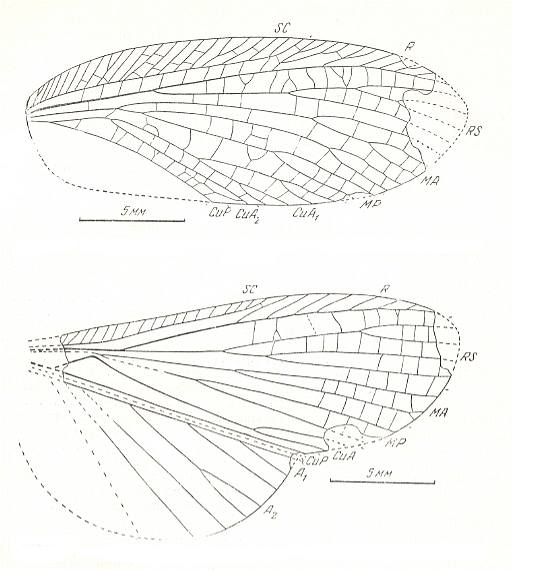
Figure 19 : Paraplecoptera from the Lower Permian of Kuznets Basin, Siberia.
Top image : Kaltanella lata Shar. Liomopteridae. Order Paraplecoptera. Forewing.
In the family Liomopteridae the forewings are membraneous (Rohdendorf et al., 1961).
Bottom image : Kaltanella lata Shar. Liomopteridae. Order Paraplecoptera. Hindwing.
(After SHAROV, in ROHDENDORF et al, 1961)
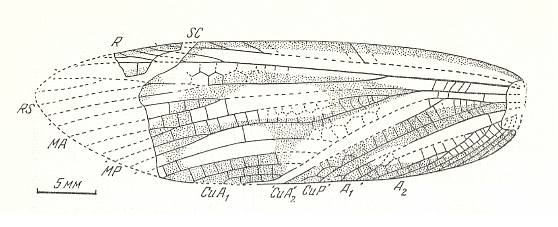
Figure 20 : Paraplecopteron from the Lower Permian of Kuznets Basin, Siberia.
Ornaticosta amoena Shar. Liomopteridae. Order Paraplecoptera. Forewing.
The forewings in the family Liomopteridae are membraneous (Rohdendorf et al., 1961).
(After SHAROV, in ROHDENDORF et al, 1961)
In the generic description (p. 197) it is said : Anterior margin of costal field [of forewing] darkly pigmented in the form of a compact band. More weakly pigmented dark spots are present on the remaining surface of the wing.

Figure 20b : Probnis speciosa Sell. Family Probnisidae, Order Paraplecoptera. Lower Permian of Kansas (USA). Restoration by Carpenter. The forewing of Probnisidae is slightly granular in appearance, coriaceous, but not elytriform.
Forewing : length, 9-14 mm. Width, 3-4 mm. Hindwing shorter than fore wing, 7-12 mm. in length.
(After CARPENTER, 1943)
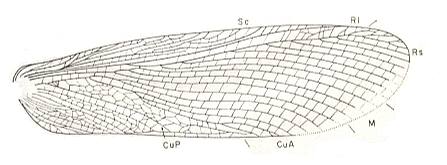
Figure 20c : Wing of Homocladus grandis Carp. Forewing. Length of forewing 43 mm., width 11 mm. Family Strephocladidae, Order Paraplecoptera. Lower Permian of Kansas (USA). Forewings of Strephocladidae are coriaceous.
(After CARPENTER, 1966)
In the neur-orthopterygian subtype of straight-wingedness we may also place the termite Mastotermes [while all the other winged termites belong to the long-winged type (dolichopterygia) ] :
To these four subtypes [1., 2., 3, and 4.] of orthopterygia we [JB] add two more subtypes, viz., gryll-orthopterygia and titan-orthopterygia, where the first is based on the far-reaching transformation of the forewings in males in connection with the formation of stridulatory areas and veins, and the second is based on newly discovered orthopteroid fossils (Titanoptera, SHAROV, 1968).
5. Gryllorthopterygia
Chiefly in males of the family Gryllidae (Orthoptera-saltatoria, division long-horned grasshoppers (Ensifera)) the cubito-anal area -- as foreshadowed by the mesozoic Haglidae -- of the forewing has undergone a drastic transformation in connection with the developent of a stridulatory device made up by a special configuration and nature of the veins in that area. Eventually this specialization may involve almost the whole forewing, as for instance in Oecanthidae, see Figure 11, above. Such forewings are not expected to perform a normal flight-function, but are specialized in connection with another function -- stridualation. And moreover, only the males do have such forewings. We think these wings do represent a special subtype of orthopterygia, not distinguished by Rohdendorf. We will call it gryllorthopterygia (gryll-ortho-pterygia). For this type of wings see also Figure 10 and the next Figures.
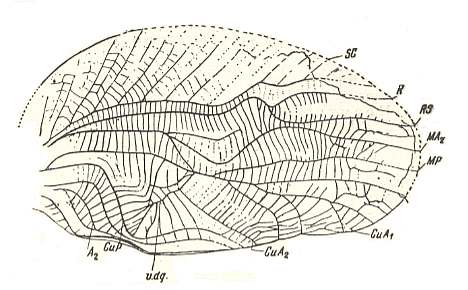
Figure 21 : Isfaroptera grylliformis Mart., male. Forewing.
v.dg. - rudiment of diagonal vein of the crickets. Lower Jurassic of Shurab (Central Asia).
Functional wing-type : orthopterygia, subtype gryll-orthopterygia.
(After MARTYNOV, 1937, in MARTYNOV 1938)
The next Figure depicts the same specimen as in Figure 21, but now after renewed examination of the fossil by Sharov, 1968. There the latter writes the following : " The anterior branch of MA [Media anterior], in Isfaroptera, drawn by A. V. Martynov, 1937, fig. 28 in the form of a short branchlet, ending blind, in fact is a fold of the membrane of the wing, originated during the process of fossilization."
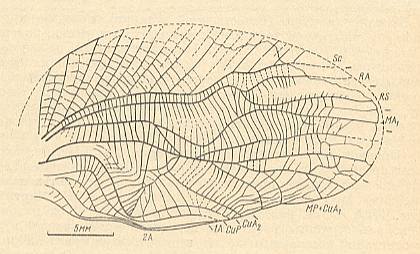
Figure 21a : Isfaroptera grylliformis Mart., male. Forewing. Lower Jurassic of Shurab (Central Asia). Family Haglidae, subfamily Isfaropterinae.
Functional wing-type : orthopterygia, subtype gryll-orthopterygia.
(After SHAROV, 1968)
For wings, not belonging to the subtype gryll-orthopterygia, but being in the process of developing gryll-orthopterygia, see Figure 12a, Figure 12c, Figure 12f, and Figure 12g, bottom image.
And for yet two more fully-fledged representatives of gryll-orthopterygia, see next Figure :
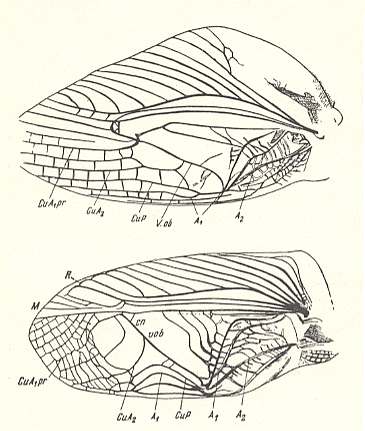
Figure 22 : Top image : Forewing of Gryllotalpa vulgaris, Family Gryllotalpidae (Mole-crickets). Recent.
Bottom image : Forewing of Gryllus campestris [male] [for female see Figure 9, above ]. Family Gryllidae. Recent.
V.ob. - diagonal vein. CuA1pr. - proximal branch of CuA2.
Functional wing-type : orthopterygia, subtype gryll-orthopterygia.
(After MARTYNOV, 1938)
So gryll-orthopterygia is not only dominant (males) in the family Gryllidae (crickets) and some Haglidae, but also is found in the family Gryllotalpidae (mole-crickets).
6. Titanorthopterygia
Fossils have been found of giant orthopteroids -- Titanoptera -- which possess wings with such a peculiar, and in some way highly specialized, venation, that they deserve to represent a sixth subtype of orthopterygia : titanorthopterygia. Here some examples.
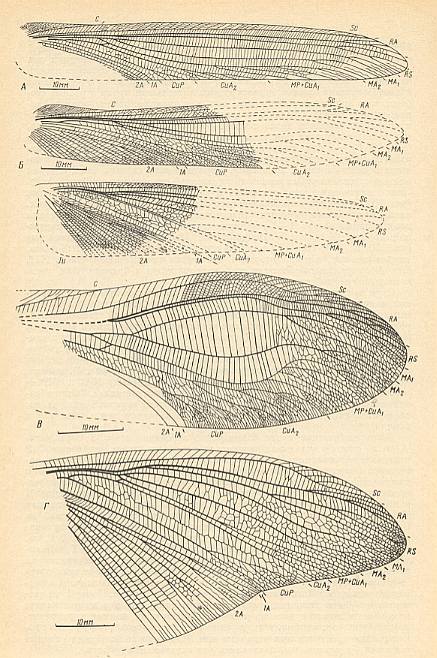
Figure 23 : Titanoptera of the family Mesotitanidae from the lower Triassic of Madigen (Central Asia).
From top to bottom :
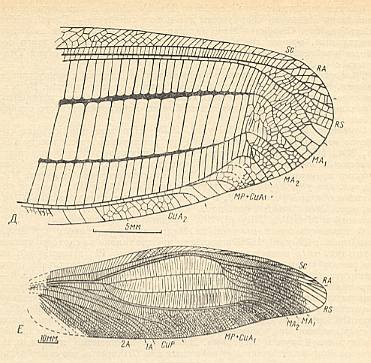
Figure 24 : Triassic Titanoptera of the family Mesotitanidae.
Top imge : Ultratitan superior Shar. Forewing. Measure bar 5 mm. Lower Triassic of Madigen (Central Asia). Extreme specialization of titan-orthopterygian venation.
Bottom image : Mesotitan scullyi Till. Forewing. Middle Triassic of Australia. (Drawing based on a photograph of the holotype). Measure bar 10 mm, letting the wing be 140 mm long. Fully-fledged titan-orthopterygia.
(After SHAROV, 1968)
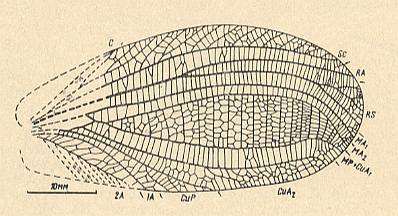
Figure 25 : Ootitan curtis Shar. Family Gigatitanidae. Forewing. Lower Triassic of Madigen (Central Asia). Measure bar 10 mm. Titan-orthopterygia.
(After SHAROV, 1968)
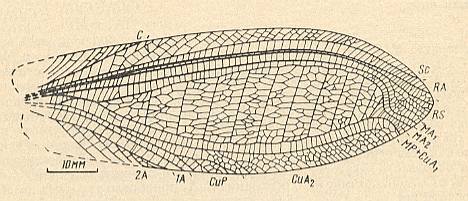
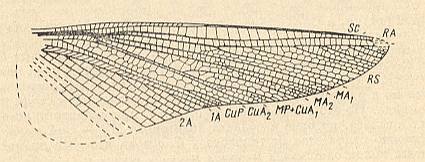
Figure 26 : Nanotitan extentus Shar. Family Gigatitanidae. Forewing and hindwing. Lower Triassic of Madigen (Central Asia). Measure bar (for both wings) 10 mm. Titan-orthopterygia.
(After SHAROV, 1968)
[The ensuing exposition of the Titanoptera is based on SHAROV, 1968]
Family Mesotitanidae Tillyard, 1925. The most primitive family of the Order Titanoptera. There are three broadened fields between RS, MA1, MA2, MP + CuA1. Cross-veins in them simple [i.e. not branched], positioned alternately convex and concave. In the fore- and hindwings RS branches off [from R] at the beginning of the proximal third of the wing. MA forks not far from the beginning of MP, forming, as in Othoptera, two long branches -- MA1 and MA2. There is a short free section of MP. To the family do belong the genera Prototitan, Mesotitanodes, Ultratitan (lower Triassic of Fergana [Central Asia]), and Mesotitan (middle Triassic of Australia).
In the lower Triassic deposits of Madigen representatives of the family Mesotitanidae, formerly known only from the middle Triassic of Australia, although (in Madigen) not in large numbers (all in all found 13 specimens), but already diverse, belong to three genera. Of representatives of the most primitive genus and species -- Prototitan primitivus -- only the forewings are preserved, see Figure 23, top image. They are markedly elongate, in the proximal half of the wing with almost parallel anterior and posterior margins. As to the nature of the venation P. primitivus is still very close to Orthoptera, and when there were not known other, more transformed Titanoptera, this species, possibly, would have been interpreted as a representative of one or another aberrant group of Orthoptera. The most substantial features by which Prototitan differs from Orthoptera known to us consist of RS beginning near the wing-base, running very closely along R while forking only at its end, and also [differs from Orthoptera] by a certain broadening of the fields between RS, MA1, MA2, and MP + CuA1, wherby the cross-veins in all three fields already do alternate, having then a convex, then a concave position [in the membrane]. Also attracting attention is the formation of short, clearly secondary [= derived] branches of CuP. A1, however, does not fork. In Prototitan similis ( Figure 23, second image ) together with the forewings in one and the same specimen also the hindwings are preserved. In the hindwings 2A is simple, not yet carrying a pectinate series of branches characteristic of other Titanoptera. CuP, apparently, also simple.
Testified by the configuration of the forewings and the nature of the venation P. primitivus stands nearby primitive representatives of the family Tcholmanvissiidae ( Figure 3 ). There is also similarity in the nature of the cross-veins, and also in the distribution of the simple cross-veins between the longitudinal veins. These features support the assumption of the existence of phylogenetic connections of Titanoptera with the family Tcholmanvissidae. From them Titanoptera inherited also the 5-segmented tarsi with arolium. Apparently Tcholmanvissidae, like the recent Tettigoniidae [katydids], were plant-feeding as well as predatory insects. Some of their descendants (Caelifera [= short-horned grasshoppers] and Phasmatodea [= stick- and leaf-insects] ) became exclusively vegetarian, while others -- Titanoptera -- were specialized as predatory insects.
Although in Mesotitanidae the perfection of the "disc" [the whole of the broadened fields], which, evidently, really was the sound-producing organ, went farthest, the basic venational features in this family remained more primitive, more close to the original type characteristic of Orthoptera, than in the two other families of this Order. We must also note that the action-principle of the sound-producing apparatus in Titanoptera was based on rubbing the whole wing against the other while the wings are lying horizontally on the insect's back, and in this respect it differs from the action-principle in Tettigoniidae and Gryllidae, in which stridulation is realized by rubbing only the basal section of the wings [these sections] lying one on top of the other. In Titanoptera the wing-bases were not involved in the formation of the stridulatory apparatus. In wings lying flatly on the back only the central part of the wing could be the zone of stridulation.
(General remarks)
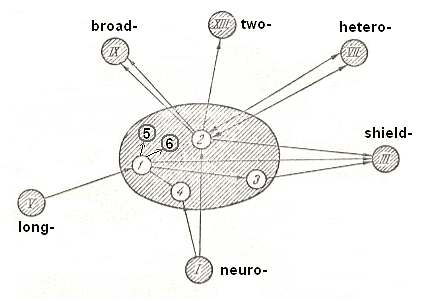
Figure 27 : Relationships with other types and structure of straight-wingedness (orthopterygia).
1 - subtype eu-orthopterygia.
2 - subtype hom-orthopterygia.
3 - subtype dys-orthopterygia.
4 - subtype neur-orthopterygia.
5 - subtype gryll-orthopterygia.
6 - subtype titan-orthopterygia.
(After ROHDENDORF, 1949, but having added two subtypes.)
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for further study of the types of flight-devices in insects, Part VI, Dolichopterygia.