

e-mail :  ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
Most expressed examples (Coleoptera) of the type :
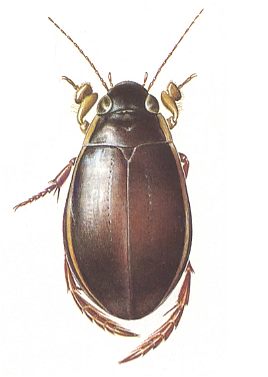
Figure 1 : Cybister lateralimarginalis, 30-32 mm. Aquatic beetle, recent. Family Dytiscidae, Order Coleoptera.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
In the Order Coleoptera (beetles) the forewings have been transformed into stiff shields. Such a shield or cover we will call elytron (pl. elytra), to distinguish it from a tegmen (pl. tegmina) - the stiffened forewing in cockroaches, grasshoppers, bugs, ect. The hindwings, if present, are always membranous with clear venation. So when we speak of the "wings" of beetles we mean the hindwings.
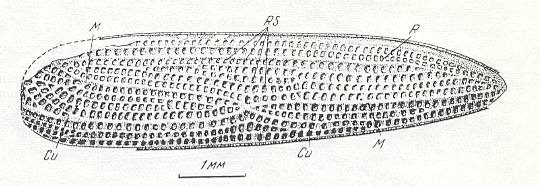
Figure 2 : Right elyrtron of Asiocoleus novojilovi Rohd., Length of elytron 7.4 mm., width 1.6 mm. Family Asiocoleidae, Order Coleoptera. Lower Permian of Basin of Kuznets, Siberia. (After ROHDENDORF et al., 1961)
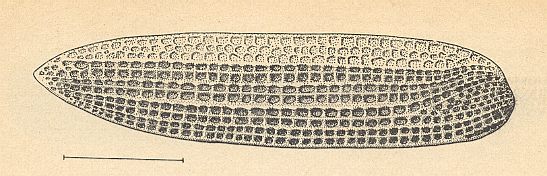
Figure 3 : Elyrtron of Cytocupoides elongatus Ponom., Length of elytron 4 mm. Family Permocupedidae, Order Coleoptera.
Upper? Permian, Basin of Kuznets, Siberia. (After PONOMARENKO, 1969)
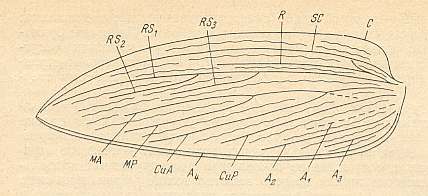
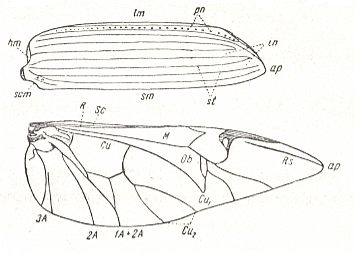
Figure 4 : Schematic drawing of the venation of the elytron, considered to be original for the beetles of the family Tshekardocoleidae (lower permian). Continuous lines indicate chief veins. Wavy lines indicate intercalated veins. Dashed lines indicate assumed chief veins not found in known beetles. (After PONOMARENKO, 1969)
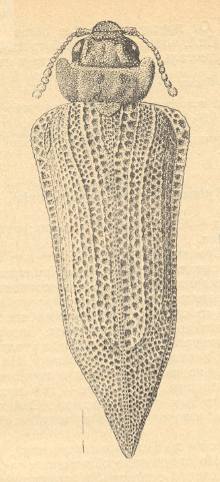
Figure 5 : Sylvacoleus sharovi Ponom., Tshekardocoleidae. Coleoptera. Reconstruction. Lower Permian of the Ural, Russia.
(After PONOMARENKO, 1969)
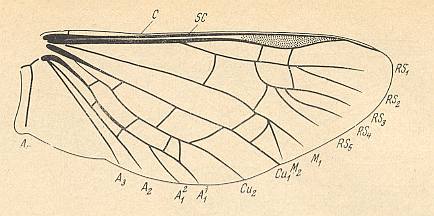
Figure 6 : Scheme, drawing all veins found in the wings -- the hindwings of course -- of beetles (Coleoptera).
(After PONOMARENKO, 1969)
Description of the type
The elytra in Tshekardocoleidae (lower Permian) are markedly longer than the abdomen. In the majority of Archostemata they do not run beyond its apex. In Micromalthus they by far do not reach it. Many beetles-archostemata possess strongly broadened epipleura laterally, and the elytra of many archostemata are of a meshwork structure, and [having] a venation consisting of chief and intercalated veins. The chief veins usually are markedly thicker than the intercalated veins, but in Taldycupedidae [upper Permian and Triassic] they are of similar structure. The intercalary veins and cross-veins originate from the archedictyon [an ancient original dense network of very small cells between the main veins]. The original configuration of the cross-veins was irregular, and the intercalary veins followed a zig-zag course, and after this the cross-veins ran through the regular interspaces perpendicular to the longitudinal veins, and the intercalary veins became straight veins similar to the chief veins. The largest number of chief veins and their branching is found in the elytra of Tshekardocoleidae. Here we may count up to 14 chief veins (see Figure 4, above ) : Subcosta (SC), forming the line of bending of the epipleura, Radius (R), usually with a short forwardly curved branch, up to three branches of the Radial Sector (RS1, RS2, RS3), Media (M), two Cubital veins (CuA, CuP), and three Anal veins (A2, A3, A4) [A1 having been lost, see below]. M and CuA may begin with a common stem.
In the course of evolution the number of veins became reduced and became to run parallel to the [longitudinal] axis of the elytron, such that in Cupes were preserved only three complete chief veins -- M, CuA, A2, and a shortened A3. A1 is not, in the form of a clearly expressed chief vein, found in any beetle, but in the majority of Tshekardocoleidae the field between the veins taken by us as CuP and A2, proximally is markedly broadened, having close to the base of the elytron 4-5 rows of cells. Such a form is usually possessed by a field in which a chief vein has been reduced. Therefore, the first clearly expressed anal vein is taken by us to be the second, and not the first. A3 is in all known beetles strongly shortened, A4 forms a seam edge, and cells behind it may be found only in the most primitive Tshekardocoleidae. Beetles of the family Ademosynidae (Permian-Cretaceous) had elytra with 8-10 punctuated grooves. The elytra of the majority of schizophoroid archostemates are smooth on their upper side, with many preserved traces of cells on the underside. In many, apparently aquatic forms the elytra had on their underside at the external edge a projection. This projection hooked, when the elytra were closed, with a corresponding projection on the dorsal part of the metepimeron, forming a lock, preventing the elytra from being lifted up by the air in the subelytral cavity. In fossil remains of beetles this projection appears to look like a depression, and it was described as the "split" (Rohdendorf et al., 1961).
(End of quote from PONOMARENKO)
Functional features
Differentiations, connections, and representatives of the type.
Shield-wingedness is a widely distributed and differentiated type of flight-device. The sources of the formation of shield-wingedness are rather diverse. The basic type out of which shield-wingedness began to form, undoubtedly was blattopterygia. Another important source consisted of straight-wingedness (orthopterygia) and neuropterygia. And also, undoubtedly, peculiar forms of shield-wingedness originated on the basis of regressive development of heteropterygia. In all these cases the stiffening and shortening of the forewings, elytra, determined the appearance of features of shield-wingedness (elytropterygia). In addition, the different sources of the formation of this type, together with further differentiation, has determined its diversity.
Subtypes of elytropterygia
1. Orthelytropterygia
Most primitive are derivatives of orthopterygia, in which the forewings were strongly shortened, while the hindwings had not yet acquired markedly expressed costalization, preserving the fan-like shape and very rich venation. Such a kind of structure may be distinguished as a special subtype - elytropterygia-of-orthoptera or [equivalently] orthelytropterygia (orth-elytro-pterygia), to which belong winged stick insects (Phasmatoidea)and some Orthoptera-Saltatoria, for example Tetrigidae and Tridactylidae. See next Figures.
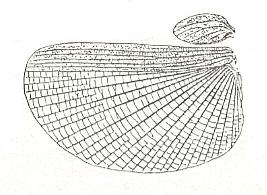
Figure 11 : Wings (elytron and wing) of Xeroderus kirbyi Gray., Bacteriidae (Phasmatoidea [Phasmatodea, Phasmida] ), Australia. Length of wing 65 mm. (After HANDLIRSCH, in ROHDENDORF, 1949)
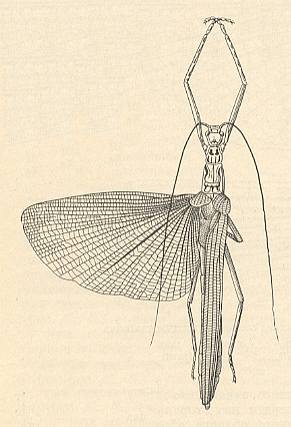
Figure 11a : Calvisia atrosignata Stal. Phasmatodea. Recent. (After BRUNNER, 1893 in SHAROV, 1968)
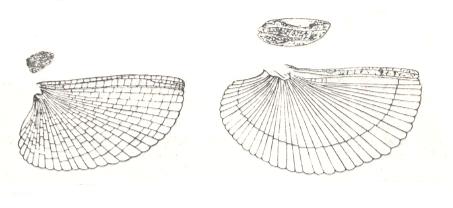
Figure 12 : Wings of orthopteroids with a shortened anterior pair.
Left image : Tetrix sp., Tetrigidae, holarctic. Elytron and wing. Length of latter 10-13 mm.
Right image : Tridactylus variegatus Latr., Tridactylidae, Europe.
(After HANDLIRSCH, in ROHDENDORF, 1949)
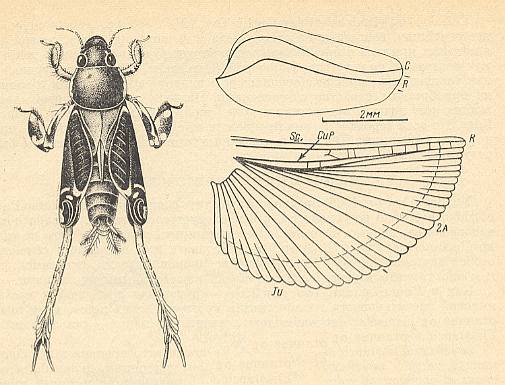
Figure 13 :
Left image : Tridactylus variegatus Latr., Tridactylidae. Recent. (After BJEI-BYENKO, 1964).
Right image : Fore and hindwing of the same species. (After SHAROV).
(In SHAROV, 1968)
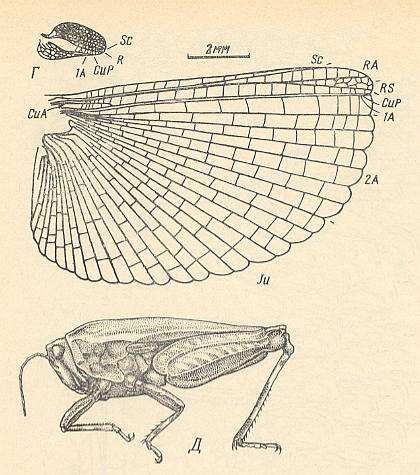
Figure 14 :
Upper image : Tetrix depressa Bris., Tetrigidae. Recent. Fore- and hindwing. (After SHAROV)
Bottom image : Tetrix nutans Hag., General view of the insect. Recent. (After BJEI-BYENKO and MISCHENKO, 1951)
(In SHAROV, 1968)
2. Protelytropterygia
A second, also little-specialized example of shield-wingedness is represented by derivatives of blattopterygia, in which the forewings already have acquired the form of true stiff elytra, having preserved [in fact, "already having"], it is true, a reduced venation, while the hindwings had worked out the ability to fold along a transverse seam, and preserving a still fairly rich venation. This subtype, ancient elytropterygia, or [equivalently] protelytropterygia (prot-elytro-pterygia), is illustrated by the cockroaches of the family Diplopteridae, and the fossil permian Protelytroptera. See next Figures.
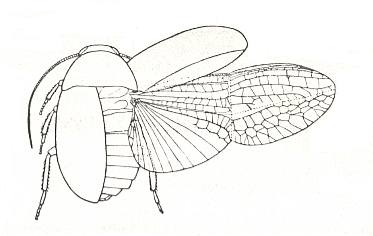
Figure 15 : Diploptera dytiscoides Serv., Diplopteridae, Order Blattaria, Australia. Body-length 13.5 mm.
(After TILLYARD, in ROHDENDORF, 1949)
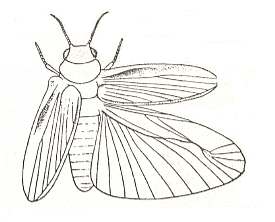
Figure 16 : Protelytron permianum Till., Protelytridae, Order Protelytroptera. Permian of America. Reconstruction.
(After CARPENTER, in ROHDENDORF, 1949)
3. Hemelytropterygia
Special forms the type shield-wingedness has in the groups being derivatives of neuro- and orthopterygia and also of heteropterygia, in which the forewings were strongly stiffened [but not so strongly as in eu-elytroptererygia], and the hindwings acquired a broadened shape, provided with longitudinal veins, configured radially, but in significantly lower numbers than in true orthopteroids. To this subtype, hemelytropterygia (hemi-elytro-pterygia), do belong [first of all] some cicadas (Cercopidae [next two Figures],

Figure 17 : Cercopis vulnerata, Cercopidae, Order Homoptera. Recent. 9.5-11 mm.
(After SEVERA, in Thieme's insektengids voor West- en Midden-Europa, 1977)
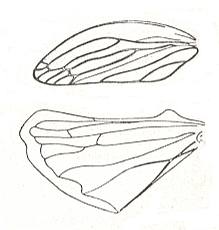
Figure 18 : Wings of Aphrophora maritima Mats., Cercopidae, Order Homoptera. Recent. Europe.
(After BECKER-MIGDISOVA, in ROHDENDORF, 1949)
. . . and the fossil Scytinopteridae (next three Figures),
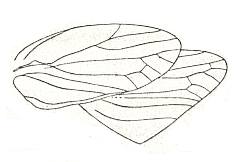
Figure 19 : Wings of Scytinoptera reducta Mart., Scytinopteridae, Order Homoptera. Permian of the Archangelsk region, North-western Russia. (After BECKER-MIGDISOVA, in ROHDENDORF, 1949)
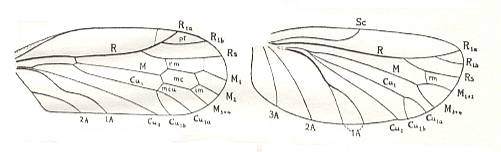
Figure 19a :
Left image : Permojassus australis Till. Family Scytinopteridae, Order Homoptera. Upper Permian of Warner's Bay. New South Wales, Australia. Tegmen. Length 6 mm. Width 2.5 mm.
Right image : Permojassus australis Till. Family Scytinopteridae, Order Homoptera. Upper Permian of Warner's Bay. New South Wales, Australia. Hindwing. Length 6 mm. Width 2.6 mm. Anal area ample but not expanding beyond the contour of the rest of the wing.
Types : Holotype tegmen, Specimen No. 54b, in Mr. Pincombe's Collection found by him at Warner's Bay, May 6th, 1923. Heautotype hindwing, Specimen No. 56 in the same collection, found at the same place and time in the same piece of rock not far from the tegmen. Both specimens are on a rather dark grey chert and only moderately well preserved, but with careful lighting all the veins can be made out.
(After TILLYARD, 1926)
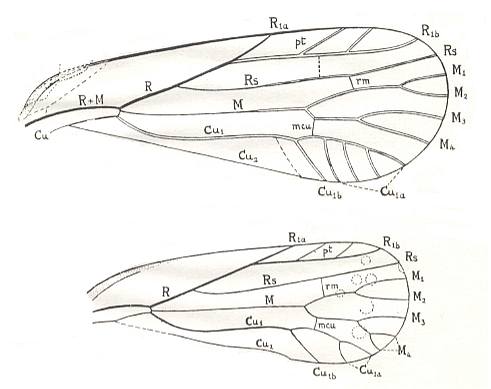
Figure 19b :
Top image : Orthoscytina mitchelli Till. Family Scytinopteridae, Order Homoptera. Upper Permian of Belmont, New South Wales, Australia. Tegmen. Length 10 mm. Greatest width 3.4 mm near apex.
Bottom image : Orthoscytina quinquemedia Till. Family Scytinopteridae, Order Homoptera. Upper Permian of Warner's Bay. New South Wales, Australia. Tegmen. Length 8 mm. Greatest width 2.6 mm near apex. The small circles probably indicate air or gas bubbles present during fossilisation.
(After TILLYARD, 1926)
. . . Prosbolopsidae), further [do belong to the subtype hemelytropterygia] the fossil Prosbolidae (see next two Figures) :
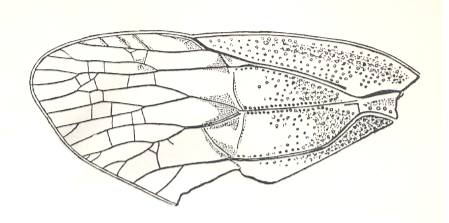
Figure 19c : Prosbole hirsuta Koken. Family Prosbolidae. Order Homoptera. Tegmen (hemelytron). Length about 4.6 cm. Upper Permian, Kama River, Russia. (After HANDLIRSCH, in TILLYARD, 1921)
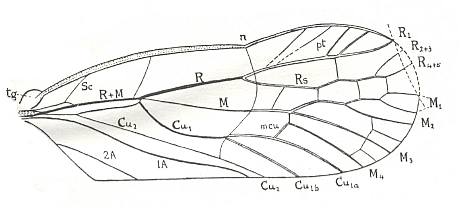
Figure 19d : Permoglyphis belmontensis Till. Family Prosbolidae, Order Homoptera. Upper Permian of Belmont, New South Wales, Australia. Tegmen, length 9.4 mm., greatest breadth 3.6 mm. n - nodus, tg - tegula.
From the nodus (n), a faint, curved, transverse line divides the tegmen as far as Cu2 into a somewhat roughened, granulate basal portion and a very smooth distal portion.
(After TILLYARD, 1926)
[although, in fact, as a result of the incipient division of the tegmen into a coriaceous and a membranous part, the Prosbolidae do foreshadow heteropterygia, or may already truly representing this type. And indeed, we take them to represent the subtype proheteropterygia of the type heteropterygia.]
. . . and [also belonging to the hemelytropterygian subtype], probably, certain booklice (Psocoptera), namely the extinct Sphaeropsocidae, see next Figure,
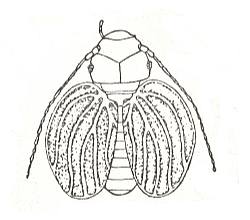
Figure 20 : Sphaeropsocus kuenowi Mast., Sphaeropsocidae, Order Psocoptera. Baltic amber.
(After ENDERLEIN from MARTINOV, in ROHDENDORF, 1949)
. . . and, finally, some bugs (Hemiptera) (Helotrephidae, Pleidae).
To the listed representatives of the present subtype (hemelytropterygia) we can add another recent but primitive Australian cercopid and a specialized representative of the group centered around the Scytinopteridae from the Triassic of Australia :
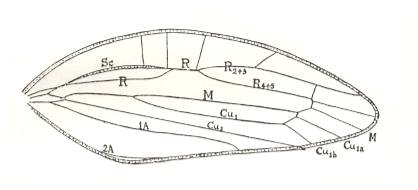
Figure 20a : Venation of tegmen of Philagra sp., Cercopidae, Order Homoptera. Recent. From Mount Tambourine, Queensland, Australia. (After TILLYARD, 1919)
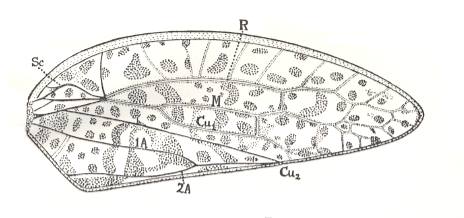
Figure 20b : Tegmen of Ipsvicia jonesi Till., Ipsviciidae, Order Homoptera. Upper Triassic, Ipswich, Queensland, Australia. Length of tegmen 14.2 mm, greatest width 5.6 mm. Veins of the clavus distinct, those of the rest of the tegmen faint, becoming very indistinct distally, so that their terminal branchlets cannot be made out with certainty (This is not due to faulty preservation, but is the actual condition of the venation in the insect, as in the case of Philagra (see Figure 20a ) and other Fulgoroids, in which the membrane of the tegmen has become much thickened). (After TILLYARD, 1919)
The establishment and meaning of this subtype is, in a certain degree, provisional. Things are namely such that in the original forms of hemelytropterygia an active role during flight of the forewings is characteristic. It is perfectly possible that stiffening and transformation of the forewings in the majority of these insects is merely a process of development of apterygia, i.e. reduction of the flight-function, and thus totally not the working-out of one or another special type of flight and wings. Let us recall that of shield-wingedness the loss of flight-movements by the forewings is characteristic.
Before we move on to the text of Rohdendorf describing the next subtype of elytropterygia, viz., eu-elytropterygia, it is worthwhile to dwell a little longer upon the fossil (permian-triassic) family Scytinoptyeridae and the recent Cercopidae (their wings belonging to the present subtype, hemelytropterygia, of elytropterygia), and also a little on the fossil (permian) families Archescytinidae (as to their wings belonging to the type of neuropterygia and Prosbolidae (as to their wings belonging to the subtype proheteropterygia of the type of heteropterygia (all four families belonging to the Order Homoptera).
Although of tough consistency, the forewings of the representatives of the family Archescytinidae nevertheless should be taken as membranous. They have no true shielding function. The forewings of Scytinopteridae and of Cercopidae, on the other hand, are completely hardened, i.e. from base to apex. The representatives of the family Prosbolidae, finally, have their forewings more or less divided into a chitinized basal half and a membranous distal half (which tendency is completed in the Heteroptera). As far conjectured, the representatives of all fossil homopterous families just mentioned hold their wings (at rest) roof-like over the abdomen, like the recent Cercopidae (and, to my knowledge, most recent Homoptera, in contrast to the Heteroptera).
According to BECKER-MIGDISOVA, 1948, the forewings (tegmina) of the representatives of the family Scytinopteridae (Permian, Triassic) apparently have just a shielding function. They are completely sclerotized (the distal part, as well as the proximal part, is covered by rough tubercles and warts. Often, as a result of the strong sclerotization, the venation is unclear). So indeed in the tegmen of the representatives of this family there is no division into a proximal hardened and a distal (more or less) membranous part, meaning that the wings in this family do not belong to the type heteropterygia, but, indeed, to the subtype hemelytropterygia of the type elytropterygia. The recent Cercopidae have still preserved the basic plan of venation and other characteristics of the tegmina (forewings). But individuals of the same species might significanly vary as to venation and coloring of their tegmina.
In our noëtic theory of organic evolution -- as presented mainly and provisionally on Fifth Part of Website -- we hold that all individual variations having taken place in the morphology of the individuals of the same organismic species are due to (local and individual) factors residing exclusively in the Explicate Order (which Order is precisely and exclusively the domain of materiality, individuality, and of spatial localities), while all species-constant characters (morphological, physiological, as well as behavioral) are developed and determined in the Implicate Order (in which no individuals, individual cases or factors do exist), and, after projection, materially appear in the Explicate Order. Further, we take it that all organismic species are such that they have not evolved (whether in the Implicate or Explicate Order) from each other in any way whatsoever, in other words we take the organismic world to be entirely polyphyletic, with the species (not the individual) as the unit-entity. As to insects, we further assume (as just a hypothesis) that, although embedded in, and influenced by, a functional context, the venation of the wings of insects generally is non-functional. Therefore, when in the Implicate Order (see the theoretical discussion in Part II of the present series of documents) a given original immaterial form develops into a strategy (in order to be able to exist materially in the Explicate Order), striving thereby, however, to preserve its original identity, the result will be, the preservation of the non-functional characters originally making up the content of such a form, while new functional characters are being added to the original core. If then the p a t t e r n of wing-venation of an insect is originally non-functional (except with respect to more or less evenly strengthening the wing-membrane), then it will be expected to persist despite the changes involved in the transformation (in the Implicate Order) of the original immaterial form into a fully-fledged strategy (still being immaterial, in the form of some sort of description). But of course, it may happen in some cases that the original venational scheme, as such representing at least a part of the content of the original immaterial form, is, because of functional factors, partly, or even completely, 'overwritten', overwritten that is, by functional characters, not belonging to the content of the original immaterial form but to the adventive elements of the strategy, or (overwritten) by characters belonging to neither. There are also cases in which the hindwings have been reduced to mere rudiments and the forewings, having lost their original flight-function, are just shields. In such cases we may expect there to be no or, at least, less of a control (i.e. damping) of influencing factors in the Explicater Order that may change the venation of such wings in the next generations. So here great individual variability is to be expected.
Indeed, there exist two main factors causing a partly or completely 'overwrite' of the original venational pattern (or of other morphological features in the representatives of a given organismic species, [features] expressing the original immaterial form, now materialized) : (1) the material (i.e. physical) factors in the Explicate Order, resulting in the well-known individual variation within existing organismic species, and thus also the individual variation of the wing-venation in such species, and (2) those adventive functional and species-constant characters that do suppress part (or the whole) of the original venation. The action of these factors thus results to obscure the original immaterial form insofar as it was represented in the non-functional wing-venation (or/and in other non-functional characters of the organismic species concerned).
In the forewings (tegmina) of the representatives of the homopterous families Archescytinidae (lower permian), Scytinopteridae (upper permian, upper triassic), Isviciidae (upper triassic), Prosbolidae (upper permian), and the recent Cercopidae, we see a certain venational configuration which is not only persistent throughout the Permian and the Triassic up to the present time, but is also preserved when the wings change type, i.e. we see that same venational configuration to be present in them whether the wings happen to be of the neuropterygian type (Archescytinidae), of the elytropterygian type (Scytinopteridae, Cercopidae), or of the heteropterygian type, and which configuration can be depicted as follows :
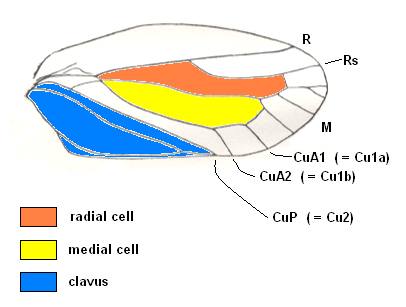
Figure 20bb : Persistent and characteristic venational configuration as we see it in the tegmina of the lower permian Archescytinidae, of the upper Permian (and upper Triassic) Scytinopteridae, of the upper permian Prosbolidae, (to a lesser extent in the tegmina) of the upper Triassic Ipsviciidae, and in the tegmina of the recent Cercopidae (all these families belonging to the Order Homoptera). This characteristic and constant venational configuration consists of : (1) a broad costal field, (2) a long radial cell, (3) a long medial cell, (4) the basal section of CuA [= Cu1] up-arching towards the origin of the free Media and either touching it and then leaving it again, or coalescing with it for a short distance, or being connected with it by a short cross-vein, or merely getting close to it for a 'moment', and (5) finally the presence of a characteristic clavus (anal area), distinctly separated from the rest of the tegmen by a straight concave vein, CuP [= Cu2].
Indeed, we see this venational configuration to be present in the forewings (tegmina) of the upper permian Scytinopteridae :
Figure 20c : Scytinoptera obliquo-ovata Mart., Scytinopteridae, Order Homoptera. Upper Permian of the Archangelsk Region, Sojana river, Letopala, Russia. Tegmen. Length 8.9 mm, width 3.9 mm. (After BECKER-MIGDISOVA, 1948)
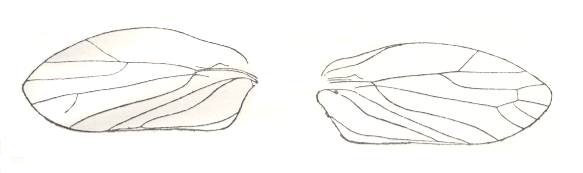
Figure 20cc : Scytinoptera reducta Mart., Scytinopteridae, Order Homoptera. Tegmina. Length about 7 mm. Upper Permian of the Archangelsk Region, Sojana river, Russia. Left : locality Sheimo-Gora. Right : locality Letopala. (After BECKER-MIGDISOVA, 1948)
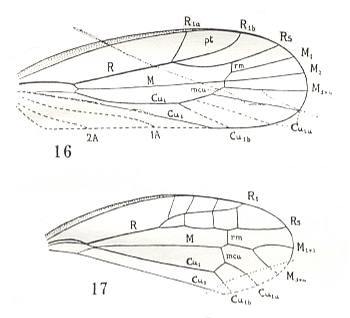
Figure 20ccc :
16 : Tegmen of Actinoscytina belmontensis Till. Scytinopteridae. Order Homoptera. Upper Permian of Belmont, New South Wales, Australia. Length 7 mm. Greatest breadth 2.4 mm.
17 : Tegmen of Permoscarta trivenulata Till. Scytinopteridae. Order Homoptera. Upper Permian of Warner's Bay. New South Wales, Australia. Length 6 mm. Greatest breadth 2.1 mm.
(After TILLYARD, 1926)
. . . in (some) recent Cercopidae (Cercopoidea, frog-hoppers, cuckoo-spit insects) :
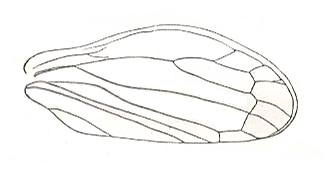
Figure 20d : Tegmen of Lepyroniella petrovi Gregor., Cercopidae, Order Homoptera. Recent, Abchasia (Western Caucasus [southern slope] ). This species has lost its hindwings, i.e. all what is left of them is a membranous rudiment of negligible size. It is known that the venation of the tegmina in this species varies greatly. However, the general venational plan remains intact in most individuals.
(After BECKER-MIGDISOVA, 1948)
We also encounter it in the tegmina of all representatives of the upper permian family Prosbolidae, which we do not hold to belong to the present wing-type [elytropterygia] but to heteropterygia (next four Figures) :

Figure 20dd : Prosbole hirsuta Koken. Family Prosbolidae. Order Homoptera. Tegmen (hemelytron). Length about 4.6 cm. Upper Permian, Kama River, Russia. (After HANDLIRSCH, in TILLYARD, 1921)
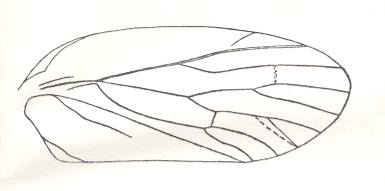
Figure 20e : Permocicada idelensis M. Zal. Family Prosbolidae, Order Homoptera. Tegmen (forewing). Upper Permian of the Archangelsk Region, Sojana river, Iva-Gora, Russia. Length of tegmen of species 9.45-12.0 mm. (After BECKER-MIGDISOVA, 1940)
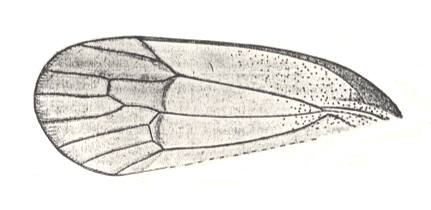
Figure 20f : Tegmen of Sojanoneura marginata Mart. Family Prosbolidae, Order Homoptera. Upper Permian of Tichije Gori, Ural, Russia. Length of tegmen 15 mm. Clavus not preserved. (After MARTYNOV, 1931)
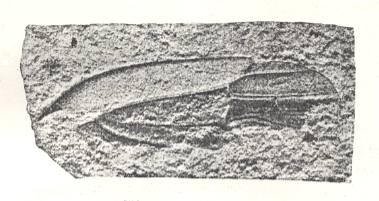
Figure 20ff : Fossil of tegmen of Sojanoneura marginata Mart. Family Prosbolidae, Order Homoptera. Upper Permian of Tichije Gori, Ural, Russia. Maximal length of tegmen 12.5 mm. Other specimen [than that of Figure 20f]. (After MARTYNOV, 1931)
. . . and we meet this venational configuration again in all the lower permian Archescytinidae (wing-type : neuropterygia) :
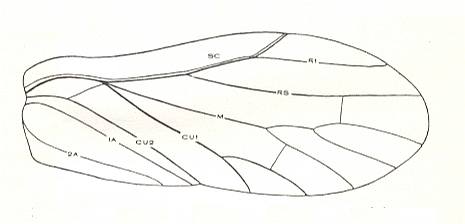
Figure 20g : Tegmen of Permopsylla permiana Carp. Family Archescytinidae, Order Homoptera. Lower Permian of Kansas (USA). Length of tegmen 4 mm, width 1.8 mm. (After CARPENTER, 1931)
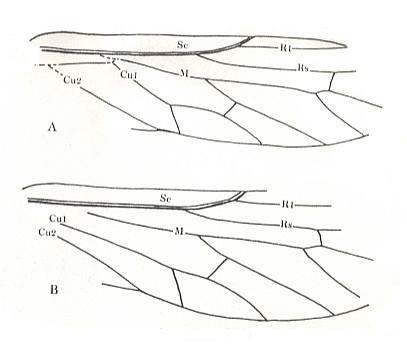
Figure 20h : Permoscytina kansasensis Carp. Family Archescytinidae, Order Homoptera. Lower Permian of Kansas (USA). Drawing of fore- and hindwing. Specimen No. 3881ab. Length of tegmen of species 11-12 mm, width 3.5 mm. (After CARPENTER, 1938)
. . . and also in the upper permian archescytinid Cicadopsylla :
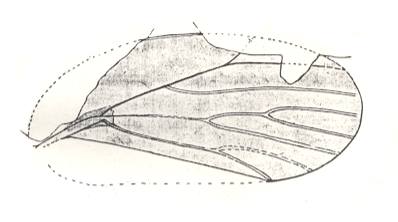
Figure 20i : Tegmen (without clavus) of Cicadopsylla permiana Mart. The missing clavus and basal costal field is shown dotted in. Family Archescytinidae, Order Homoptera. Upper Permian of Tichije-Gory, Ural, Russia. Total length 13.5 mm. Breadth 6.2 mm.
(After MARTYNOV, 1931)
. . . and again in mesozoic Scytinopteridae :
Figure 20j : Tegmen of Mesoscytina australis Till. The missing clavus is shown dotted in. Family Scytinopteridae, Order Homoptera. Upper Triassic, Ipswich, Queensland, Australia. Total length 9.4 mm. Breadth at apical end of clavus 3.5 mm.
(After TILLYARD, 1919)
As has been said, with respect to (functional) wing-type, the Archescytindae belong to neuropterygia, the Scytinopteridae and Cercopidae to elytropterygia (present type), while the Prosbolidae do, as to their wings, belong to heteropterygia, whereas taxonomically they all belong to the insect Order Homoptera.
Theoretical evaluation of the above presented persistency of a particular venational configuration in insects. Extension of the noëtic theory of evolution.
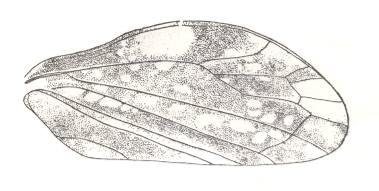
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is present, although there is no connection between CuA and M. |
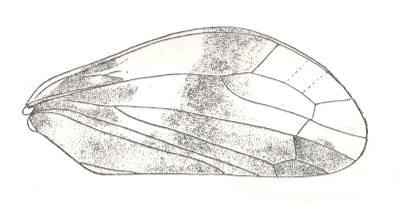
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is present. CuA coalesced for some distance with M. Basal piece of CuA absent. |

|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is present. CuA touching M. Basal piece of CuA present. |
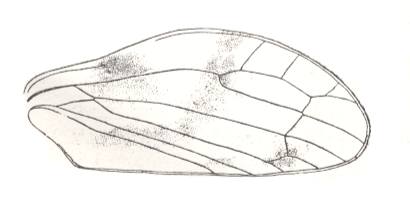
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is present. CuA connected with M by a short cross-vein. |
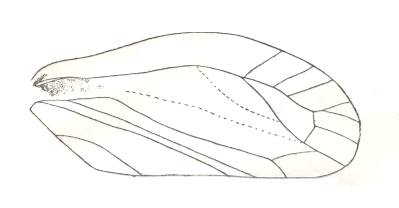
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is destroyed as a result of the reduction of M (only a trace of it is left). |
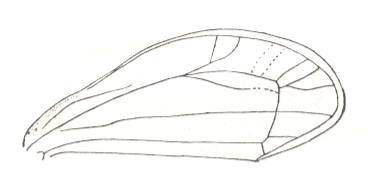
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb only partially intact. Clavus absent. |
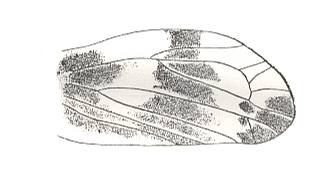
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. |
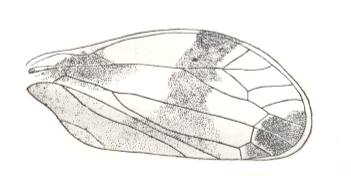
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is present. CuA coalesced with M for a considerable distance. Basal piece of CuA still present. |
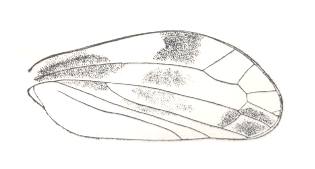
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is largely intact. |
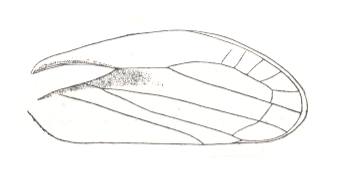
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is largely absent. |

|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb is present. CuA not connected with M. |
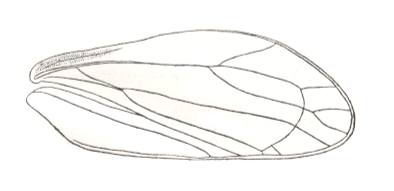
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb rudimentary. |
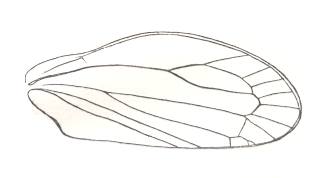
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb rudimentary. |
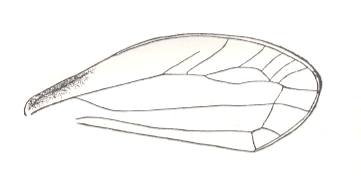
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb rudimentary. |
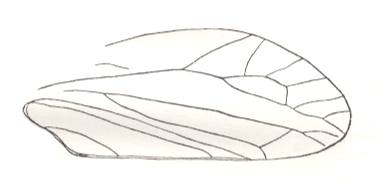
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb rudimentary. |
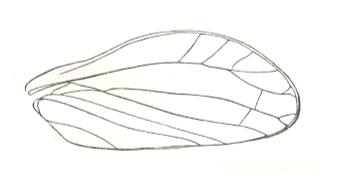
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb rudimentary. |
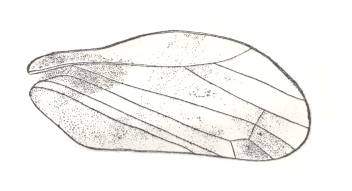
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb rudimentary. |
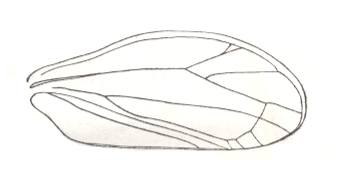
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb rudimentary. |
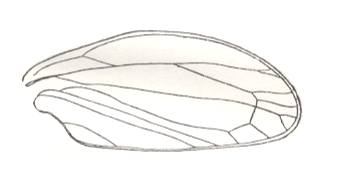
|
Lepyroniella petrovi, Family Cercopidae. Order Homoptera. The venational structure highlighted in Figure 20bb present. CuA not connected with M. |
What we see in these tegmina of Lepyroniella petrovi Gregor is that the venational structure highlighted in Figure 20bb is, despite great venational variation in this species, still present, or at least largely so, in almost all these tegmina. It proves to be a persistent feature, resisting the action of contingent (environmental) factors in the Explicate Order. And this persistent feature expresses part of the qualitative content of the original immaterial form that has, while keeping up its identity, developed into the particular strategy, in the Explicate Order represented by the species Lepyroniella petrovi. But this persistent feature is not only the material expression of a part of the mentioned original immaterial form, but is commonly possessed by a large number of other original immaterial forms as well, as is evident in the wide distribution of the venational structure (Figure 20bb) among insects of the Order Homoptera.
Summary of present extension of noëtic theory of evolution so far developed
Reality (i.e. that which exists in one way or another) is assumed to contain two interconnected ontological (i.e. as to the way of being) domains, viz., the material Explicate (unfolded along time and space) Order and the immaterial Implicate (enfolded) Order. While the entities present in the former Order do exist there materially (implying physical individuals, contingency, place and time), the entities of the latter Order are entirely immaterial (excluding individuals, contingency, place and time).
Material and immaterial forms differ from each other not necessarily as to their qualitative content, but as to their ontological composition (which determines their way of being). A form to be immaterial means that the ontological substrate, prime matter, is absent in it, meaning that its qualitative content is not ontologically carried by anything. A form to be material, on the other hand, means that its qualitative content (its form proper) is carried by prime matter, its ontological substrate. Prime matter only has a substrate function, it has no qualitative content whatsoever. Therefore, the addition of prime matter to an immaterial form does not add or subtract anything to or from the form's qualitative content. It only makes possible the existence of various physical here-and-now individuals all possessing precisely the same qualitative content, i.e. it makes possible the repetition of a same given form (a same given qualitative content) in place and time, and therewith introducing possible contingencies. This is true Greek philosophy (that of Aristotle), but in contrast to it we hold the material form, i.e. the materialized form, to be the most perfect way of being (while the Greeks attributed this to the immaterial pure form). And materialized forms do only exist in the Explicate Order. Or, in other words, existing in the Explicate Order is existing in the most complete way, because it is materially existing. When a given immaterial form is projected from the Implicate Order into the Explicate Order it is unfolded along the space and time dimensions of that Order. In organisms this means that a given immaterial strategy (a description) appears in the Explicate Order as a multitude of here-and-now individuals of a particular organismic species. In our theory we equate the immaterial condition of a given qualitative content with a description of that content. Being a description = being immaterial (i.e. without prime matter). Not a description made by someone or something, but just a description.
Because all immaterial forms or patterns intrinsically aspire after their ontological completion by becoming material, i.e. by coming to exist in the Explicate Order, the Implicate Order is as to its very nature ontologically geared to the Explicate Order, geared, that is, to express its forms materially, and, as a result of this, contains noëtically represented (i.e. in the form of a description) non-contingent existential conditions for these forms to exist materially in the Explicate Order.
Every immaterial form whatsoever, present in the Implicate Order, which cannot materially exist without having first undergone appropriate transformation, which (form) is, in other words, unstable, is, as to its very qualitative identity (i.e. as to what it is), such, that its subsequent course to noëtic stability leads it to a particular noëtically represented non-contingent existential condition (and, with respect to this given particular form, only to this particular condition), i.e. makes it reactive with that condition. The product, emerging from this noëtic reaction between (1) particular immaterial form and (2) corresponding noëtically-represented existential condition, is a particular strategy (describing how to exist in the Explicate Order). And only now the original immaterial form has achieved stability. This strategy is then in the Explicate Order expressed as a material organismic species provided with a set of special adaptations to the material counterpart of the noëtically-represented existential condition, i.e. (expressed) as an organismic species fully integrated in its proper environment. And so, every organismic species represents its own specific strategy, a strategy to exist in its own environment (in the most narrow sense). In the morphology of the individuals of such a species the qualitative content or identity of the corresponding original immaterial form may be seen materially expressed in the species's non-functional characters. The succession of appearance of organismic species and types one after the other in geologic time in the Explicate Order is experienced by us (in fact theorized by current evolutionary science) as a transformation of existing species into other species as a result of either the migration of the former into new environments, or the long-term influence on existing species of a changed environment.
4. Eu-elytropterygia
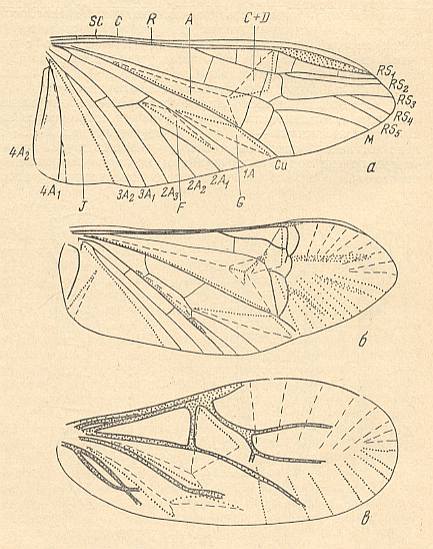
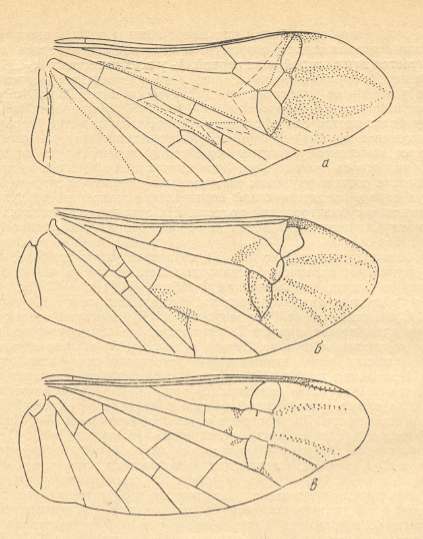
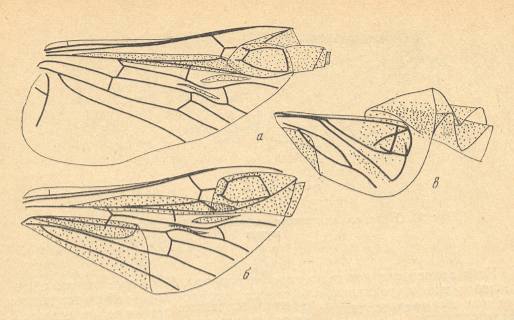
Most progressive is, as the probable derivative of blattopterygia, the chief subtype of shield-wingedness, expressed in the Order Coleoptera (beetles), eu-elytropterygia, of which extreme stiffening of the forewings with a strongly changed venation, is characteristic [see Figures 1-10 , especially Figure 4 ]. The hindwings, see Figure 10, acquire well-expressed traits of aerodynamic specialization -- they become pointed, strongly costalized, often recalling us, as to their structure, cases of extreme costalization in the type of twofold-wingedness (dipterygia). This correspondency sometimes ends up with extreme similarity. See next Figure.
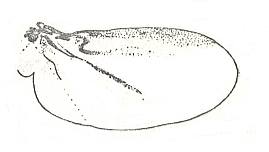
Among the diverse examples of eu-elytropterygia we may mention the existing further courses of differentiation of this type, consisting in the loss, for the forewings, of the necessity to lift, and setting free in this way the hindwings hidden underneath them (some Scarabaeidae -- Cetoniini and Scarabaeini, the genus Gymnopleurus), or [consisting] in the reduction of the size of the forewings, the elytra, as in the Staphylinidae (rove beetles),

. . . or, finally, in the loss of the necessity of folding the hindwings (a whole series of families of beetles, some Meloidae, Rhipiphoridae, Lymexylidae, Petriidae, and others). All these transformations of eu-elytropterygia, undoubtedly point to the working-out of [at least] traits of the most perfect type -- dipterygia, most perfect functionally (flight by a s i n g l e pair of wings), as also with respect to morphological similarity (the structure of the costalized hindwings).
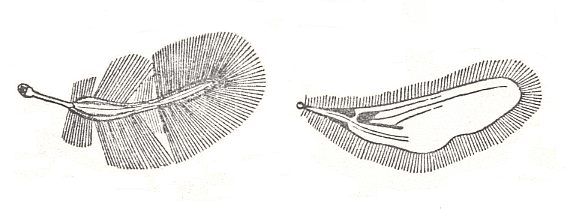
5. Metelytropterygia
Another kind of extreme form of shield-wingedness is illustrated by the order Dermaptera, earwigs, -- ultra-shield-wingedness, i.e. metelytropterygia (met-elytro-pterygia). Of this subtype shortening of the elytra down to a small size, to almost quadratic blades -- caps, holding the multiple-folded along longitudinal and many transverse folds in the form of a small compact packet very broad fan-shaped hindwings, is characteristic. See next Figure.
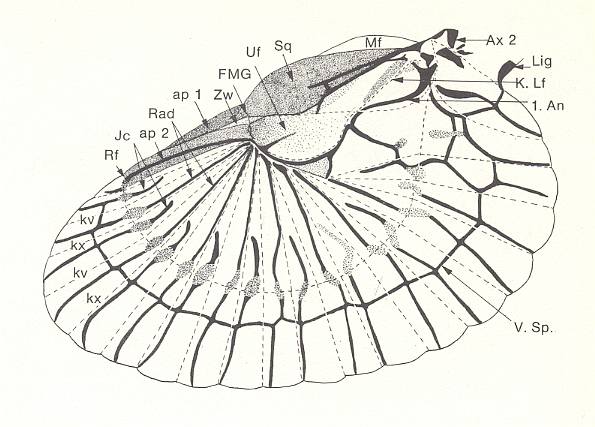
Figure 24 : Left hindwing of the earwig Forficula auricularia L., Forficulidae, Order Dermaptera. The wing is spread out, and the sclerotized structures have been kept dark. Folds are drawn as dashed lines. The wing-blade is divided into convex (kx) and concave (kv) radial folds, that are pushed fan-like together when the wing is folded. The in this way formed compact fan is then further, along the ring-fold RF, running perpendicular to the radial folds, turned up with the distal part. Where the ring-fold crosses the drawn-black Radial veins (Rad) and the intercalary veins (lc), the veins look "damaged" (indicated by punctuation). At the level of the wing-medium-joint (FMG) a further folding turns the wing down against the ulnar field (UF), and thus the twice-folded wing-packet is brought under the stiff elytron (Sq).
An - first Anal vein. ap1 + ap2 - external and internal apical field. Ax 2 - second axillary sclerite. K. Lf - concave longitudinal fold in the ulnar field (in flight it serves as some sort of flight-fuse. There is yet a second fuse). Lig - wing-ligament. Mf - marginal field. V.sp. - Vena spura. Zw. - intermediate field.
(After NACHTIGALL, 1968)

The origin of metelytropterygia of the earwigs is not clear. Perhaps it is a further stage of the protelytropterygia of the permian Protelytroptera (see Figure 16 ).
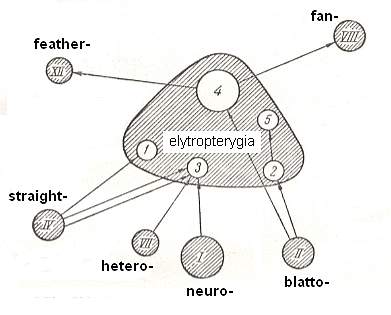
This concludes the exposition of shield-wingedness. The next document will deal with straight-wingedness (orthopterygia).
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for further study of the types of flight-devices in insects, Part V, Orthopterygia.