

e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam ) After having -- in the previous document -- dealt with the large superfamily Fungivoridea, we will now discuss the remaining superfamilies of the infraorder Bibionomorpha.
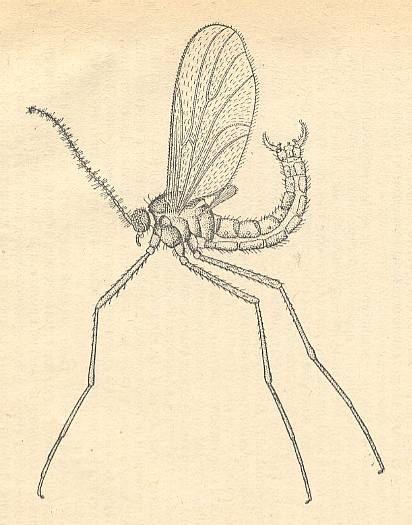
The superfamily Bolitophilidea --- which in the recent fauna is represented by a few tens of species (some 32) of two genera, Bolitophila and Messala of the one family Bolitophilidae, and of which the two mentioned genera are already found in the Baltic amber of the Upper Eocene --- is idiosyncratic although also visibly close to the extensive superfamily Fungivoridea. The features of the Bolitophilidea are still very insufficiently studied. Such features are (1) the little refinement of the wings (weak development of costalization [Figure 2 ], and a non-refined wing base [that is, basiala, see Figure 3 ] ), (2) the presence of legs of the thin type, (3) the presence of long antennae, and, finally (4), in the larvae the absence of the prostheca or bristly surface of the mandibles [See Figure 2B of Part VI ].
The features of feeding and way of life are little known. It is said that these diptera are very similar to the Fungivoridae, but precise data are practically absent. Probably this superfamily is a direct descendant of the primitive [that is, the first] forms of the Fungivoridea and Bibionidea. The refinement of the wing-base [that is, the basiala] and, connected with that, the development of costalized wings with a high wing-beat frequency did not show much progress (the lifting power was only slightly increased). [As the reader will recall, the "basiala", if developed at all, is that part of the wing-base which lies nearest to the thorax. One can reckon the basiala to begin immediately after the sclerites of the joint that connects the wing to the thorax, and about ending at the level of the humeral cross vein ( = the small proximal cross-vein between the Costa and the Subcosta ). The basiala contains the basal pieces of the major longitudinal veins, and various other structures.].
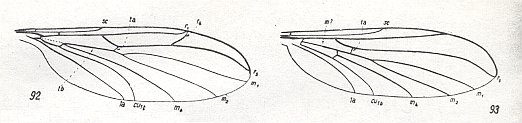
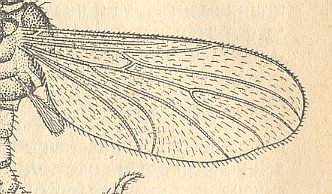
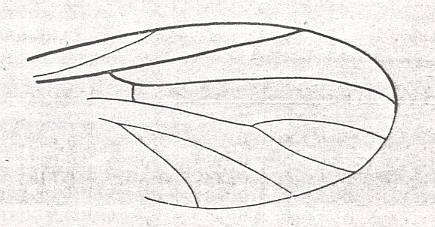
Figure 2 : Bolitophilidea.
92 -- Wing of Bolitophila hybrida MEIG. (Bolitophilidae)
93 -- Wing of Arachnocampa luminosa EDW. (Bolitophilidae, according to HENNIG)
( 92 : after HENNIG, 1954, 93 : from HENNIG, 1954, after TONNOIR & EDWARDS, 1927)
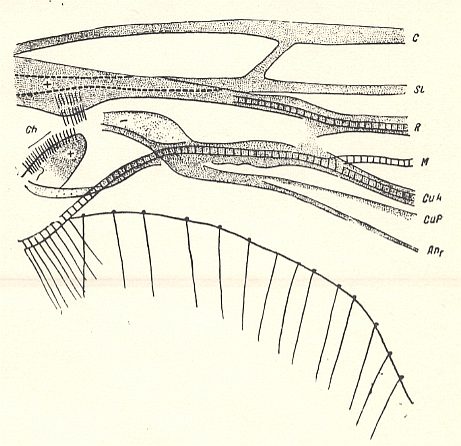
Figure 3 : Bolitophilidea.
Basiala of the wing of Bolitophila maculipennis WALK. (Bolitophilidae). Diagrammatic. The veins indicated from top to bottom are : C = Costa, SC = Subcosta, R = Radius, M = Media, CuA = Anterior Cubitus, CuP = posterior Cubitus, An1 = first Anal vein.
Ch = chaetarium [ = patches of bristles having a mechanical function].
+ means : lying on a convex ( = arched upwards) spot or ridge.
- means : lying on a concave ( = scooped out) spot or groove.
(After ROHDENDORF, 1946)
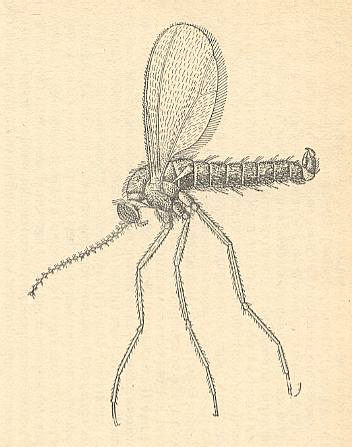
The legs were lengthened and acquired the character of the thin type (their becoming more prehensile went together with a weakening of the muscles of the femora [upper segment of legs] ). The larvae continued to have well-developed antennae (making it probable that the larva, or at least its head, remained very mobile and omnivorous), see next Figure.
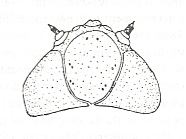
Figure 4 : Bolitophilidea.
Bolitophila pseudohybrida LANDROCK (Bolitophilidae). Head of larva. Top view.
(From ROHDENDORF, 1964, after HENNIG, 1948-1952)
All these features of the Bolitophilidae show the idiosyncrasy of this group, that is, their being different from the Fungivoridea. It is still difficult to single out the essence of the conflicts in the development of this group. Probably the basic conflict for all bibionomorphs -- incompleteness of the larval food -- was solved by the Bolitophilidae already early on, and the winged phase did not develop better aerodynamical features and strong legs. But this conclusion cannot as yet be confirmed by precise observations and investigations and therefore remains just a conjecture.
The wings of the Bolitophilidea belong to the ancient lifting venationally-attenuated (fungivoroid) type, namely to its 7th subtype, the bolitophiloid subtype , described in Part VI.
The superfamily Cecidomyiidea [ = Itonididea] or gall-midges (in the broader sense) consists of three families :
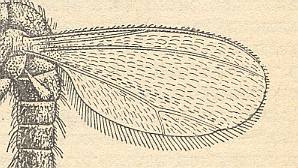
The next Figure depicts the basiala of the wing of a representative of the Cecidomyiidea :
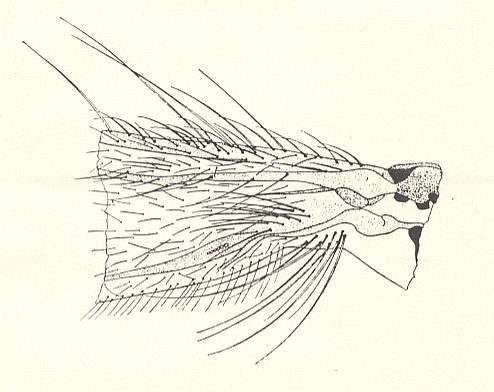
The superfamily Cecidomyiidea of which there are about 3500 species of three families, that is, about half of the number of recent species of the whole infraorder (Bibionomorpha), is undoubtedly a derivative of certain mesozoic representatives of the Fungivoridea, namely of the family Pleciomimidae, see next Figure.
The most ancient representatives of the superfamily are only known from the Baltic amber of the upper Eocene (Tertiary) and belong to a series of recent genera. The features of the Cecidomyiidea are studied relatively well. The conflicts, determining the historical development of these diptera, originated as a result of the larvae living in tissues of plants, and [these conflicts] consisted in the presence of abundant, but hard to assimilate, food and the difficulty of taking it in with gnawing mouthparts. The solution of this conflict took place in the direction of the development of extra-intestinal digestion (reduction of the mouthparts, the complexification of the set of ferments of the saliva) in the larvae, and further in the direction of diminishing the absolute size of the body of the insect, and the development of aphagia [ = not taking food] in the winged phase. The refinement of feeding of the larva and the development of [sophisticated] sensory organs [antennae] in the winged phase turn out to be the determining features. The further history of this group of Bibionomorphs is complex : in certain cases changes took place in the organization of the larvae. They, for instance, changed into predators, living in the open on plants, of aphids, or they developed the ability to parthenogenetically reproduce themselves (paedogenesis). This further development of the gall-midges is expressed in the taxonomic system of this superfamily, which includes three rather sharply distinct families.
The family Lestremiidae (see Figure 5 above ) is relatively poor in species and is characterized by (1) larvae which develop in rotting wood, (2) a rich venation (see Figure 5a above ) of relatively large wings, (3) long first tarsal segments, and by (4) the absence of projections on the antennal segments.
These features bear witness to the presence of conflicts in the history of this family, connected with (1) the development of the larvae in accumulations of vegetable remains, (2) detecting these substrates which are distributed in the soil [for example the forest-floor], and (3) with the requirements for spreading and colonizing. All this caused the retention of running legs (with well-developed tarsi [ = feet] ), and of poorly costalized wings. Newly developing features included only the organizational changes of the larvae.
The chief family of this superfamily, the Cecidomyiidae [ = Itonididae], the gall-midges proper (see Figure 6 above ), which is richest in species, is characterized by (1) strongly expressed univorous [ = specialized in feeding on one single type of food only] larvae, which live in diverse tissues of living plants, by (2) reduced venation (see Figure 6a above ) of the softened pseudo-feather- (see below) and often shortened wings , finally, by (3) the development of complex projections on the antennal segments and of weak tarsi with a shortened first segment.
All this bears witness to the conflict that appeared in connection with living in spatially restricted local substrates, that is, living in the organs of certain species of living plants, and, consequently, the necessity for the winged phase to find them, mainly by flight. The next Figure depicts some larvae of Cecidomyiidae.
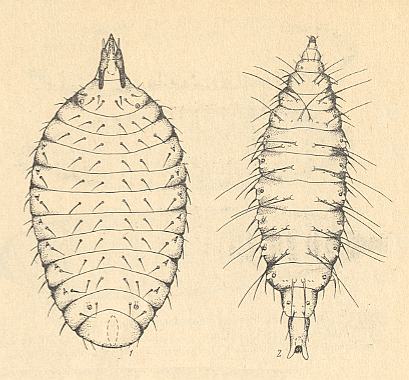
Finally, the last family, the Heteropezidae, which is poor in species, is characterized by the development of the remarkable phenomenon of paedogenesis (reproduction accomplished by the larva), by the reduction of the venation of the wings which acquired, as did the representatives of the previous family, traits of pseudo-feather-wingedness (see below), and, finally, by the decrease of the number of tarsal segments. These features neatly bear witness to the presence of exclusively favorable conditions of the life-activities of the larvae, together with difficulties and insufficiency in feeding of the winged insects : This conflict indeed determined the origin of the Heteropezidae [the conflict being solved by paedogenesis].
In comparing the historical development of all three families of gall-midges, we can draw conclusions about the different character and different evolutionary directions of these groups. The refinement of larval feeding and of the sensory organs of the winged insect -- all this characterizes the evolution of the group as a whole. The sensory organs of the imago [adult insect] and the larval feeding did change little in the representatives of the family Lestremiidae, which can rightly be seen as the least specialized in the whole superfamily. The genuine gall-midges, Cecidomyiidae, distinguish themselves by diverse refinements of the features of larval feeding alongside the [further] development of sense-organs (antennae), and the working-out of pseudo-feather-wingedness [see below]. This group is significantly more specialized than the previous group. Finally, the representatives of the last family, the Heteropezidae, are characterized by the most peculiar refinement of individual development, together with the reduction of the flight-apparatus and the weakening of the legs and the sense organs of the winged phase. This group is the most specialized of the superfamily.
In concluding all this, it is necessary to mention the processes of the diminution of the absolute size of the body which took place in the history of the gall-midges. In itself the diminution of the size of the insect cannot form the basis for asserting the regressive character of the historical development, which assertions are often made when discussing these phenomena. Considering the history and changes of the existential conditions of the superfamily of gall-midges we can clearly notice the usefulness of the diminution of body-size which allows them to colonize [infect] the most diverse confined vegetable substrates. It allows to solve the main biological conflict, namely the insufficiency of food, by means of using precisely those substrates which are inaccessible to the overwhelming majority of other diptera as a result of their spatial attenuation.
Next we produce an account of the Cecidomyiidea as given by OLDROYD, 1964. As far as we can see he treats Cecidomyiidea as a single family (together with the Cecidomyiidae including the Lestremiidae and the Heteropezidae).
This family has gone a long way on the line of evolution of its own, ending up as the unique family Cecidomyiidae, the gall-midges. In almost every aspect of their biology the gall-midges manage to do things differently from any other flies.
Adult gall-midges are tiny, fragile flies, with very few of the wing-veins that are the great stand-by of the systematist, and with antennae that are a miracle of elaboration on the most minute scale, but possibly more of a confusion than a help in the classification of the family. The biggest handicap to understanding this family, however, is that most of the larvae live in the tissues of growing plants, which they provoke into making a swelling known as a gall.
The larvae are among the most featureless of all maggots.
Not all larvae of Cecidomyiidae live in galls, however. A few still follow the ancestral habit of feeding in rotting vegetable matter, and in dung. Some are feeders in fungus, especially fungoid growths covering other plants or woodwork, in moulds, mosses and liverwort. Featureless larvae of a pink color should be suspected of being Cecidomyiidae. They are said to suck the vegetable juices, and also to take any microscopic animals they may encounter.
Some Cecidomyid larvae are truly carnivorous : for example the predatory Aphidolestes pierces the body of aphids and sucks them dry, in the same way as the larvae of some hover-flies of the family Syrphidae.
The transition to the predominant gall-making Cecidomyiidae is perhaps through certain genera that attack the roots of plants, and which may be present in large numbers, enough to kill the plant. This is an obvious link with the similar habit of the larvae of Bibionidae.
One further distinction of the Cecidomyiidae is that species in a few different genera are able to reproduce by paedogenesis [ Here we must think of one of the families of the superfamily Cecidomyiidea, the Heteropezidae ]. This word comes from the Greek for a child, and means that an animal becomes sexually mature, and produces offspring, without itself ever reaching adult form. The reproductive cells are of course set apart from the somatic, or body-building, cells at an early embryonic stage, and they usually remain dormant until the body has become adult. These exceptional larvae have a few large eggs, in number up to about thirty-five, which can be seen through the thin cuticle. These soon become larvae, and litterally devour their parent from inside. They in their turn suffer the same fate [that is, they produce eggs, etc.], so that for several generations the adult, with its different ways and its different problems, is entirely eliminated. For the time being the midge becomes like the aphid, rapidly reproducing through its asexual generations in the summer, and never leaving its birthplace. In this way, from five very large eggs that were originally laid under loose bark by an ovipositing female may arise a great mass of larvae. Such masses of larvae of Miastor may commonly be found under the bark of cut logs.
After a time, the daughter larvae abruptly cease to produce eggs of their own, but go on to pupate in the usual way. All the larvae that arise from one original egg are necessarily of the same sex, but both male and female larvae are paedogenetic, and so normal sexual reproduction can be resumed by the mass [that is, this mass, paedogenetically produced, contains groups of females and groups of males].
There is described a gall-midge, Tekomyia populi from Germany, that is paedogenetic as a pupa, producing its daughter larvae without completing its metamorphosis into an adult fly. The reproductive stage of Tekomyia was called a 'hemipupa' by its discoverer, and another hemipupa, that of Henria psalliotae, is described and figured by another researcher. This latest example is a Cecidomyiid that infests mushroom compost in England, and is potentially a pest, particularly if this method of reproduction allows it to multiply more rapidly.
We will now discuss the functional wing-type -- the pseudo-feather-winged type -- which is possessed by two families of the Cecidomyiidea. The essence and distribution of this type is taken (with additions when needed) from ROHDENDORF, 1951.
Pseudo-feather-winged (itonidoid) type
Representatives of the type
Wings of the pseudo-feather-winged (itonidoid) type are characteristic for the extensive family of gall-midges-proper Itonididae [ = Cecidomyiidae] and for the related family Heteropezidae. See next Figures.

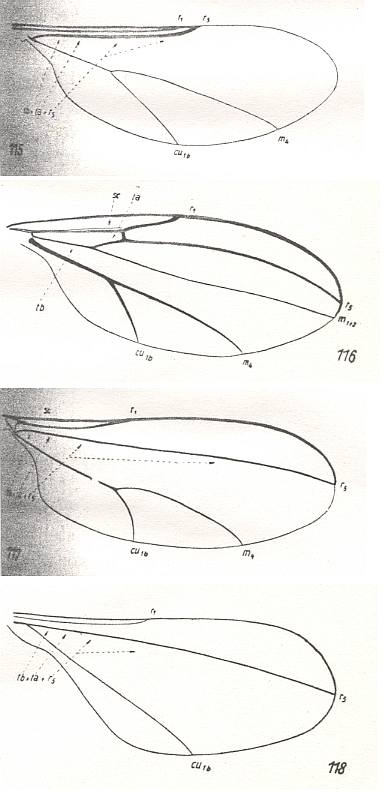
Size of wings
Shape of the wings
Wings always moderately elongate, often short, 2-2.5, rarely 3 times longer than wide. The fore-margin moderately convex, often almost rectilinear. Hind-margin more convex. Apex small, little distinct. Anal lobe weakly expressed, in the form of a convex projection of the wing. Basiala, see Figure 7 above , little distinct, the proximal boundary of the anal lobe [which would then mark the distal boundary of the basiala] not sharply expressed. The alula is absent. Wing-scale only present as a germ. The wing-tip is often very obtuse.
Skeleton of the wing
How the insects hold their wings when not flying is little known to me [Rohdendorf]. The wing-venation is strongly reduced. In most cases virtually without a trace of costalization. The veins are rectilinear, thin, distributed over the whole wing-blade, widely separated from each other.
The costal vein runs up to the wing-tip but not beyond it. The hind-margin is, consequently, without a marginal vein (the assertion of the presence of the costal vein along the hind-margin of the wing in gall-midges, common in the literature, is certainly wrong ! ).
The subcostal vein is almost completely reduced.
Two simple [ = unbranched] radial veins [or, as one might say, branches].
Medial veins present as three or one branch, sometimes totally absent. The two anterior branches are the weakest, forming the characteristic fork as seen in the fungivoroid wings. These veins are often reduced.
The posterior medial vein is always unified with the cubital vein as it were its branch. These veins are significantly more rarely reduced. The basal part of the medial vein is stronger. In the case of reduction of medial branches it often forms, as it were, the continuation of the cross-vein rm to the wing-base.
Cubital vein almost rectilinear, curved at its end.
Anal veins reduced.
The basiala is very peculiar and possesses two well-expressed convex elements and a central concave one. Sometimes at the end of the 'handle' of the radial vein we find the indication of a fragma in the form of a transverse projection or of some sort of [small] separating wall. The 'handle' [ = knob-like proximal end] of the radial vein is strongly broadened and firm. The chaetaria are present as clearly distinct plates-with-bristles on the upper surface of the basiala, [that is,] on the handles of the radial and second anal vein. [ For the various structures of the basiala in general, see in Part II where we deal with the functional interpretation and evolutionary evaluation of the features of the wings of the order Diptera, especially the Figures 1a and 1b ].
Coverings of the wing
The whole wing-membrane is covered by many rather densely packed microtrichia which are very short (3-4 mu). Almost always there is a covering of many fairly long macrotrichia which cover the whole wing-blade fairly uniformly. The macrotrichia are arcuately curved, not particularly thin, spinelets, of which the length varies from 60 to 120 mu. Flat spinelets are absent. The hind-margin of the wing and the wing-scale with long macrotrichia.
The nerve system of the wing is not studied.
Functional characteristic
The way of life of these utterly small diptera is, in general terms, well known. The larvae of the great majority of the representatives of the family Itonididae live in the tissues of living plants. As a rule they are narrowly monophagous. Some forms are predators on the surface of plants, eating aphids and ticks. Some other species of the mentioned family, and also almost all species of the family Heteropezidae, live in the larval phase in rotting wood and under the bark of trees. The majority of the winged insects is aphagous with a relatively short life. The flight of the itonidids undoubtedly has a completely determined fairly important meaning in their life-activities, making it posssible for the monophagous insect to find its food plant. Other abilities for moving around are apparently of secondary significance.
In evaluating the flight features of these insects we must first of all take into account their minute or most minute sizes, which determine characteristic features in the development of this locomotory function. The low body-weight of the itonidids ( The weight of two representatives of the genus Contarinia is known to be as low as 0.0007 grams) and the structure of their wings, clearly point to their flight apparatus to belong to the feather-winged type, namely to the peculiar pseudo-feather-winged subtype (pseudo-ptilo-pterygia) (ROHDENDORF, 1949). The pseudo-feather-wingedness of the gall-midges bears witness to special features of flight of these insects, which [flight] is not fast or particularly steerable. Passively being carried by air-currents probably is of great significance for the gall-midges. To this the low load bears witness and also all of the structure of their body (slender body, the long thin prehensile legs, and the development of hairs on many parts of the body and its appendages). It is certain that during flight the actual load exerted on the wings of the insect is still lower, because the projective surface-area of the whole body without the wings is sufficiently large and significantly changes the magnitude of the general load exerted on the insect as a result of dividing the load over other appendages of the body. Exact observations of the flight of these insects have not yet been made.
History and transformations of the type.
The available paleontological documents almost only clarify the upper Eocene fauna preserved in the Baltic amber which includes many species of the families Itonididae [ = Cecidomyiidae] and Heteropezidae. To understand the ways of formation of the present wing-type this material has little to offer. It just indicates the close relationship obtaining between the eocene and recent forms. The transformation of the pseudo-feather-winged type in fact only consists of the presence of the different stages of reduction of the wing-venation which hardly allows to divide it into individual subtypes. First of all we must notice the richest venation [as compared with that of other members of the type] of the representatives of the groups Campylomyzinae and Porricondylini. These forms we can naturally consider to be the most primitive forms and the starting-points for the remaining forms. The chief mass of other gall-midges possesses a more reduced wing-venation (see Figure 11, above ), and here belongs the majority of the representatives of the family Itonididae. Finally, the species of the family Heteropezidae and some of the family Itonididae (Lasiopterini) have the most reduced wing-venation and form the extreme most specialized group of the itonidoid type.
The connections of this type with other types are clear. The pseudo-feather (itonidoid) wings originated from the ancient lifting venationally-attenuated (fungivoroid) wing-type , namely from its two subtypes, the pleciomimoid subtype and the lycorioid subtype (all described in Part VI). These processes of the formation of the type have a clearly regressive character and consist in the reduction of the wing-venation which looses the features of costalization, and which was significantly weakened, [consist further] in the development of a cover of macrotrichia, and, what is especially important, [consist in] the accompanying decrease of body-size. This latter process -- decrease of body-size -- was the decisive fact in the formation of the itonidoid type of wings, and at the same time [the decisive fact] in the regressive character of the evolution of these organs. As had already been said (Part VI), where we considered the pathways of evolution of the fungivoroid wings, a regressive character of changes in one or another single organ-system does not necessarily mean an overall regression of evolution of the group as a whole. This is especially clearly shown by the example of the evolution of the gall-midges, of which the flowering and development was determined by progressive changes in the organization of their developmental phases [larvae], namely the working-out of extra-intestinal digestion in their larvae, accompanied by various changes of the structure of their mouthparts, the refinement of metamorphosis, etc. At this background or framework of progressive changes, the regressive changes of the wings and flight form just a one-sided specialization of a single organ-system of which the function was changed in connection with changes in the living-conditions.
( End of the exposition of the functional wing-type as it is present in (certain) representatives of the superfamily Cecidomyiidea.)
In the next document we will continue with the infraorder Bibionomorpha. And that means we will deal with the next superfamily of it, the Bibionidea.
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part VIII.
Back to Aristotelian metaphysics Part I
Back to Aristotelian metaphysics Part II
Back to Aristotelian metaphysics Part III
Back to Aristotelian metaphysics Part IIIa
Back to Aristotelian metaphysics Part IV
Back to Aristotelian metaphysics Part V