

e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam ) Philosophical Intermezzo
The reader should be aware that the present series of documents he is now consulting is not meant to be, or to become, a textbook of entomology. Neither it is meant to compile all the known facts about the diets and habits of insects. Many books and articles have been written about insects and their ways of life, and the reader will easily find them in scientific bookstores or university libraries. There he or she can consult expositions by professionals. And although I have spend a number of years to study insects (among other things recent Tachinidae [the larvae of which are entomo-parasites] and a study of the literature on fossil insects, including books and articles in Russian [because especially many fossil insects are found and described in Russia and in the territories of the former Soviet Union] ), and having thus acquired a fairly extensive knowledge about insects and their evolution, I am not a professional in these areas. As should be clear from the content of this website as a whole, I may call myself a professional in philosophy, especially in ontology.
(1) Prime matter, (2) substantial form, and (3) a set of non-essential determinations, are the chief three ontological components of a material thing.
The intention of the present series of documents on insect evolution is to consolidate and put to the test our new conception of Substance and of the proposed ontological structure of Reality as a whole (Implicate and Explicate Orders).
With respect to our new, or, expressed better : supplemented, conception of S u b s t a n c e (in the metaphysical sense) we can say that it, in a way, still remains A r i s t o t e l i a n in spirit, in spite of the fact that we have brought up the Implicate Order (first proposed by David BOHM) and integrated it in our new ontological theory.
As can be read off from the foregoing documents, we had to concede the existence of a purely noëtic ( = thought-like) domain in addition to our familiar space-time domain. A number of properties attributed by BOHM to his Implicate Order turned out to fit well in our concept of this noëtic domain. We had to acknowledge this domain in order to account for the living state of matter. The undeniable holistic features of the organic world are already foreshadowed by the crystalline state of matter, which was already extensively studied on this website. Already here we needed the existence of the Implicate Order (as we have developed the concept of it further). But much more so we needed it to account for the features of individual development, organic morphogenesis. But then the conception of the Implicate Order, so far developed, turned out to lack features that would account for organic evolution, especially for the evolutionary origin and development of the many 'smart' adaptations that we find in organisms of all sorts. Indeed, we can confidently maintain that such adaptations (including transformations of instincts) and most other features of organic evolution defy any general explanatory biological and physical theory. That is to say, all such general theories are embarrassed or utterly perplexed when it comes to organic evolution. If the reader wants to know why exactly, and in what way, they are so perplexed, he or she should consult the book (one out of the many of them saying this) The Collapse of Chaos, written by COHEN and STEWART, and first published in 1994. But be aware of the ongoing confusion in this book between ontology (here in the sense of w h a t is, or might be, actually the case in Reality, whether we can know it or not) and epistemology (which is about how we come to know, especially in science, and whether there might be things that we can never know exactly and must therefore be contented with analogies or gross approximations).
In order to o n t o l o g i c a l l y, or if one would like to call it, m e t a p h y s i c a l l y, account for organic evolution ( which 'accounting for' is not equivalent to a scientific explanation, but to giving things a broader [ than just physical ] context ! ) we had to introduce our conception of the Implicate Order and especially our idea of n o ë t i c r e a c t i o n s taking place in that Order, and also a new conception of Substance, new with respect to the classical Aristotelian conception of it ( Of course, a new conception of Substance has already been tried in First Part of Website , but now we can evaluate this attempt as being just a first stage in the development of such a new conception ).
We had made a begin with such a new conception of Substance, such that it is compatible with all the known facts of organic evolution, in the beginning of Part I of the present series of documents. About it we can now add the following :
Because there is a constant 'interaction' (or, perhaps better, interconnection) between the Explicate and Implicate Order, namely by the constant p r o j e c t i o n s (unfoldments) and i n j e c t i o n s (enfoldments) of formal contents from one Order to the other, we cannot interpret the Implicate Order and its content as a transcendent domain, transcendent with respect to the space-time world (Explicate order). Despite the fact that the contents of the Implicate Order are immaterial, this Order must be held as being i m m a n e n t with respect to the Explicate Order.
And if we hold that the very s a m e essence ( = the 'what-is-it?' ) or f o r m (in the metaphysical sense) of any given true Substance is present in the Explicate Order (here visible as distributed among individuals) as well as in the Implicate Order (here 'visible' as one particular noëtic entity), then we can agree with Aristotle that the essence or f o r m of any given s u b s t a n c e (in the metaphysical sense), that is, the form ( = substantial form) of any given thing, is i m m a n e n t in that thing, that is, it does not belong to some separated transcendent domain, which in turn means that we, with Aristotle, do not agree with Plato (who held that the essence or form of every being or thing in the material world is also -- but now in a pure form -- present, as a so-called "Idea", in some transcendent domain).
Our extension of the Aristotelian conception of Substance consists in our recognizing the fact that there are some substances that intrinsically can and will d e v e l o p, and that means adding or subtracting elements to its form or essence. Indeed, i n o r g a n i c substances (in the metaphysical sense), such as crystals, can change as a result of changed external conditions : it can either result in the substitution of one non-essential attribute by another (the external shape of the growing crystal has changed as a result of contact with the walls of the container), or it can result in an essentially different substance altogether (a Sillimanite crystal has transformed into an Andalusite crystal as a result of a change in pressure and temperature). But while in o r g a n i c substances (also in the metaphysical sense), such as individual organisms (or even species), we also recognize their ability to change either non-essentially or essentially (which latter means the disintegration of the organic substance into a great many other more elementary substances (atom, molecules)), we, in addition to such organic substances, also recognize as representing genuine organic substances (in the metaphysical sense) those entities that are known as higher-ranking organic taxa. And it is precisely these substances -- the higher-ranking taxa -- to which we ascribe the intrinsic ability to e v o l v e, whereby qualitative moments (formal contents) are either added to the essence or form of the substance (and then thus intrinsically b e l o n g to it) or subtracted from it (and thus intrinsically not belonging to the essence or form of the substance anymore), without, however, changing the 'personal' identity of such a substance, that is, the same substance can, and will, evolve, as we are describing it with respect to insect taxa such as ' Tipulidae', 'Chironomidae', etc.
The Aristotelian S u b s t a n c e (in the metaphysical sense) is said by him to consist of prime matter and (substantial) form. Prime matter is informed by the substantial form or essence. The matter-form 'composite' can in its turn be 'informed' by non-essential determinations or affections ('accidents'). Prime matter is considered by him as the ultimate substrate of change, which here then is essential change consisting in the substitution of the present substantial form by another (while the matter-form composite is the substrate for non-essential changes).
Well, as has been said, this aristotelian conception of Substance is more or less changed and supplemented by us, forced, as we could say, by the facts of evolution. In Aristotle's time there did not exist any idea or theory that would only approximately come close to the modern conception of organic evolution. But I am convinced, that if Aristotle were to have knowledge and insight about the facts of organic evolution as extensively as we nowadays do, or as we do since the days of Darwin, he would certainly agree at least with the general lines of our proposed extension of his concept of Substance.
So let us now concisely (that is, as less as possible repeat things we already laid down in Part I of the present series) interpret the Aristotelian S u b s t a n c e in terms of our theory of the Explicate and Implicate Orders :
Let us characterize these three components.
A non-essential form (that is, one from the set of non-essential determinations) is a content that is added to the prime matter -- substantial form composite, i.e. it is an extrinsic further determination of this composite. And "extrinsic" here means that the added content does not (neither is it going to) per se belong to the content of the composite, but, when added, only per accidens belongs to it. So this per accidens content it is not necessarily implied by the content of the composite, it will not automatically be generated by it.
The composite, consisting of prime matter and substantial form, carries the set of per accidens added contents : It is further determined by these contents. It is that which ' lies under ' these determinations -- it is truly substance.
The substantial form is the pure what-is-it? of the material thing (itself consisting of prime matter + substantial form + per accidens determinations). It is the intrinsic or per se formal content of the thing. We can also say that it is the essence of the thing. This substantial form is present in the Explicate Order -- where it is the essence or formal content of a material thing -- but at the same time it is, not as a duplicate, but as this same substantial form, present in the Implicate Order -- where it exists as a noëtic entity.
Prime matter has of itself no (qualitative) content. It is just the ultimate substrate that can carry a substantial form, that is, can be informed by one or another such form.
Through (that is, thanks to) prime matter the thing is extended -- unfolded -- along space and time dimensions, that is, in virtue of prime matter the thing is a physical thing, and as such it is residing in the Explicate Order. Moreover, through prime matter the thing can be further determined by extrinsic contents (per accidens determinations). Finally, through prime matter the substantial form manifests itself as existing individuals (existing individual things), that is, in virtue of prime matter the substantial form is instantiated. Only in this way, that is, as instantiated, the substantial form is present in the Explicate Order.
So a thing being physical, individual, and involving the per accidens, ensues from its informing prime matter. In other words, it is the substantial form being carried by prime matter that causes it to manifest itself not only in the Implicate Order but also in the Explicate Order.
In the Implicate Order there are no individuals, no per accidens connections, and no space and time dimensions, that is, there are no physical things or processes. It consists of en-folded (injected) contents (en-folded along space and time dimensions) which (contents) as such (that is, as residing in the Implicate Order) are noëtic entities. The just mentioned en-foldment along space and time dimensions here means that the thing is, as it were, folded back along these dimensions, resulting in the thing becoming time- and spaceless. Upon un-folding (along space and time dimensions) a given content appears (projects) in the Explicate Order as one or more individual physical things or processes (where a process is a succession of physical things).
In this way every genuine substance (in the metaphysical sense) stands with one foot in the Explicate Order and with the other in the Implicate Order. However, this metaphor should not tempt us to assume that the noëtic content as it resides in the Implicate Order is some sort of duplicate of the formal content of a physical thing residing in the Explicate Order. It is one and the same content, but in the Explicate Order it cannot exist on its own, but is always carried by prime matter which in turn determines its (multiple) instantiation (resulting in individuals or individual cases) and is responsible for all kinds of per accidens determination. This means that the substantial form is not residing in some transcendent domain (as Plato maintained), but is residing in the thing (as Aristotle held). But 'looking' at the thing from within the Implicate Order we only see its substantial form.
And, as we have argued, also any (higher-ranking) biological taxon is a substance. In the Explicate Order we see this substance as repeatedly instantiated, and thus the only thing we in fact see is a number of more or less similar individuals (organisms) in space and time, whereas in the Implicate Order we see this substance as a single definite noëtic content or entity. And it is this entity that might noëtically react with other such entities present in the Implicate Order. Such noëtic reactions are considered to be analogous to chemical reactions. And like silver does not chemically react with water, there are noëtic entities that are such that they do not noëtically react with each other. But like the metal sodium does chemically react with water, or, as a further example, salt (NaCl) does chemically react in solution with silver nitrate (AgNO3) resulting in a precipitation of silver chloride (AgCl), many noëtical entities, when present in the Implicate Order, will react with each other resulting in the (noëtic) generation of noëtic reaction products. Upon projection of these products into the Explicate Order, that is, upon unfoldong along space and time dimensions, we see, in the Explicate Order, some material biological process going on, resulting in the generation of some definite material biological entity or pattern. And in the present context such a material process is then an e v o l u t i o n a r y process, such as the historical development of some adaptation or of some new taxon. And precisely all this will form the metaphysical background of our investigation of insect evolution. This background does not constitute a scientific explanation of organic evolution but merely provides some rationale for its baffling phenomena. In fact it says that there are 'things behind the scenes', that is, noëtic entities and their reactions, which we (as observers), however, only encounter, in the Explicate Order, as -- little understood -- physical, chemical, and biological processes between individual material entities in space and time.
End of philosophical intermezzo.
We continue with the Infraorder Tipulomorpha of which we have so far considered the Superfamily Tipulidea (previous document). Here we will consider the next superfamily, the Chironomidea.
This, with respect to number of species, second largest superfamily of the Tipulomorpha, the Chironomidea, consists of three, rather different families :
The superfamily Chironomidea as a whole is characterized by strongly costalized wings (which means that the stronger veins have shifted towards the anterior margin of the wing, that is, to the costal vein), usually elongated, sometimes strongly broadened. The wings can be reckoned to belong to the Traction-Costalized type (described and distinguished by ROHDENDORF, 1951). The muscular apparatus is strongly developed. The legs never belong to the Bristly subtype of the Thin type (described also by ROHDENDORF, 1951). They are often adapted to gripping or are thin sensory devices.
An especially important feature is the organization of the larvae, which, as a rule, lost their immediate connection with atmospheric air and breathe by means of tracheal gills.
These two basic features of the Chironomidea, namely, on the one hand, the larvae quitting the surface film of the water basin and breathing through gills, and on the other, the significant strengthening of the flight apparatus, which here boils down to the development of flying with a high wing-beat frequency, realized by the costalized wings helped by the strong muscular system, are most significant for understanding the conflicts which have determined the origin of this superfamily of Tipulomorphs (ROHDENDORF, 1964). [In spite of the development of a strong flight apparatus, as remarked by ROHDENDORF, we must realize that all Chironomidea are small fragile insects. They are easily blown away by wind, so they cannot normally go out and buzz around to find egg-laying sites. The main reason, I think, for having their flight apparatus strengthened is for them to be able to execute their swarming habit. With respect to the Simuliidae, being, it is true, also small flies but nevertheless more robustly built, things could be a bit different.].
The ability of the larvae to immediately breathe in a watery medium, without contact with the atmosphere, points to a further enlargement, accomplished by the descendants of the ancient Tipulomorphs, of the ecological range of living in the aquatic basin. Especially the acquisition of this ability leads to the feasibility of living in rapidly flowing water (as we see in the black-fly larvae) and further in bottom conditions (as we see in the larvae of most non-biting midges). The original aquatic meta- or amphipneustic larvae (that is, larvae possessing either only one pair of functional hind spiracles or, in addition to them, some other pairs at a number of middle body-segments) of the first Tipulomorphs had remained connected with the surface film of water, and that means connected with small near-bank sections of a water-basin, and could not inhabit the fast-flowing stream because they met impediments to making contact with the air in such environments. In populating flowing water by the ancestors of the Chironomidea the transition to the bottom of the water-basin and the development of breathing by gills turned out to be the solution of the conflict that had appeared as a consequence of the expansion of their ecological range. Not less important are also the biocoenotic changes in the first Chironomidea that had left the surface layers of the water-basin for its bottom : we here mean the important protective advantages of a hidden way of life that undoubtedly was acquired by the ancestors of the Chironomidea who moved away from the exposed zones of the water-basin in which [zones] they were most vulnerable to enemies (predators of insects -- dragonflies, beetles, fishes, and birds).
The described most important feature of the larval stage of the first forms of Chironomidea, consisting of the acquirement of the ability to live at the bottom of a water-basin at the same time lets us undestand the origin of a better, that is, a stronger, flight : the development of strong costalized wings. The localized and limited nature of sites appropriate for the habitation of the larval phase (originally, different sorts of streams and brooks), together with the condition of an insufficient amount of caloric food in such environments (relatively poorly populated rapidly flowing water-basins), stimulated an increased activity of the winged phase, that had to spread [its species over the region] and eat. Consequently, the further development of the flight apparatus turned out to be yet another constitutive feature for the solution of the conflict which [solution] created this superfamily together with the development of breathing by means of gills ( ROHDENDORF, 1964).
The historical development of the different families of the Chironomidea is little known. We can only indicate certain most general features of the them. The features of individual development, especially the feeding, respiration, and habitat of the larval phase, and further the feeding, organs of locomotion, and the integration of the body of the winged phase, all these aspects of the organization determine the idiosyncrasy of the individual families of the Chironomidea, which are in fact very different groups. Instead of a meticulous analysis we will, to begin with, restrict ourselves to the most important and basic features of the families.
The most diverse -- that is, having the largest number of species -- family Chironomidae ( = Tendipedidae) is characterized by having very active larvae, many of them living at the bottom of water-basins with most diverse hydrological regimes, that is from small cold snowy waters to the most southern lakes and to marine coasts. With respect to the content of oxygen in the waters colonized by chironomid larvae there are great differences -- from mountain streams saturated with oxygen to stagnant waters replete with organic refuse containing only traces of oxygen. Very remarkable are the depths which can be populated by larvae of Chironomidae. Cases are known of population of depths of 325 m by larvae of these flies. It is probably right to maintain that the larvae of Chironomidae are the only insects which are able to populate such deep zones of water-basins.
INTERMEZZO ABOUT THE EVOLUTIONARY INTENSIFICATION OF A GIVEN ORGANIC FUNCTION.
The described sexual dimorphism together with the reduction of mouthparts in the imago ( = adult insect) is an example of the intensification of a given function, which can be seen as a general, and, as it seems, necessary, trend in evolution.
If this concerns the function of just one or another single morphological structure, then the primitive condition consists in the repetition (in the body of a given organism) of this structure, that is, we find in the organism a chain of copies of that structure, as we see it for instance in the antennae, that is the olfactory organs, of Diptera : The primitive condition here is the presence of a large number of more or less similar antennal segments as we see it in, for instance, crane-flies. In higher flies this number is strongly reduced, leaving only three segments. And, moreover, these differ strongly from each other, most certainly as a result of 'division of labor' (or, for that matter, the other way around, that is, the structural differences of the antennal segments resulted in a division of labor). Probably the olfactory function has been totally concentrated in the third antennal segment of higher flies.
A bit more abstract -- but nevertheless still an instance of intensification of a function -- is the case of the above mentioned sexual dimorphism together with the reduction of mouthparts in adult chironomids. Let us explain.
Generally the life of an insect goes through a number of larval stages (instars) and ends up with the imaginal (that is, adult) stage (and there are even [recent] insects -- the mayflies -- that have two consecutive winged phases in turn following upon the last larval [wingless] instar). In many insects these larval stages are very similar to the adult stage with respect to morphology as well as to the way of life, especially the feeding habits. This can, for instance, be seen in grasshoppers and relatives. So here we can compare the whole series of stages (all larval and imaginal stages taken together) with a chain of more or less identical segments such as we saw in the crane-fly's antennae. There is little 'division of labor' between the individual members. The only functions of the imago that are not present in the larva are that of reproduction and often that of geographically spreading the species.
However, in many insects there is a radical difference between larva and adult, namely in all insects that undergo a complete metamorphosis during individual development, like we see in beetles, two-winged flies, bees, wasps, and butterflies : holometabolic insects. Here there is a more advanced 'division of labor' of the insect's functions between larva and adult. The larva serves, among other things, to build up reserves of nutritive materials (later to be used by the adult), while the adult serves to reproduce, that is, to let eggs mature and find a mate, and it also serves to spread the species it represents over wide areas.
But very often, that is, as a result of the structure and habits of many species, the larval food, acquired during the larval stage, is not enough for the coming adult : either its quantity is insufficient or it does not have the appropriate quality for the eggs in the female adult to ripen or for the adult to be active at all. In these cases the adult also feeds : it sucks blood, or it takes in nectar from flowers, or is a predator. In such cases many functions are more or less evenly distributed among the larval and adult stages, except reproduction and spreading.
But in many insect species a stronger division of labor sets in : There, larval food is enough and is also of the right quality to let ripen the eggs in the female. And mating places are in the immediate vicinity of those sites where the eggs have to be laid, resulting in the fact that only short distances have to be traversed by the winged phase. All this makes feeding of the adult insect superfluous, eventually resulting in the evolutionary reduction of its mouthparts. This is a particular stage or degree of so-called des-imaginization, that is, the reduction -- and, sometimes, the complete disappearance -- of the adult stage (indeed, there are species that reproduce as larvae !). So here we again have a case of the reduction of the number of 'segments' and the concentration of the function in one of them (and of another function in the other remaining segments, if they remain at all). So we here have the function of feeding intensified, that is restricted to, and improved in, the larval phase, while the function of reproducing and spreading is now the sole function of the adult (that is, it has lost most other functions not connected with reproduction and spreading) and is improved by, among other things, sexual dimorphism.
( end of Intermezzo )
Continuing with the characteristics, as given by ROHDENDORF, 1964, of the family Chironomidae, their wings are long and fairly narrow and relatively (as compared with the other families of the group) moderately costalized and as such belonging to the narrow subtype of the traction-costalized type (described and distinguished by ROHDENDORF, 1951). The legs of the members of this family are very peculiar. They carry special spines or combs at the posterior end of the tibia ('shinbones') evidently having a supporting function. The forelegs differ strongly from the other legs, they are very long and, as it seems, do not take part in supporting the body. They seem to be peculiar tactile organs, developed in both sexes especially in the males. The insects always hold their forelegs lifted from the ground (pendulous) while stretching them out forward.
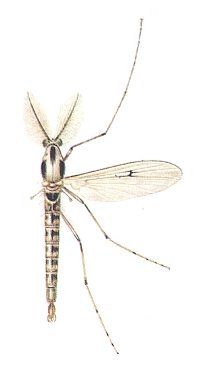
Figure 1 : Chironomus plumosus (Chironomidae), common midge, 10-12 mm.
(After ZAHRADNIK, 1977, in Thieme's insektengids (insect guide))
As we have said, the des-imaginization of Chironomidae is clearly expressed in their body features.
Following OLDROYD, 1964, with respect to Chironomidae, we could add the following : It used to be thought that all Chironomidae had aquatic larvae, but this is not so. There are many terrestrial larvae, living in moss, humus, and compost-like materials, dung, rotting wood, and in soil at the roots of grasses and other plants. The non-biting midges with terrestrial larvae do not form a natural group within the family Chironomidae, but are isolated genera, and even species, that have taken to the land independently. Like the aquatic larvae, they have no open spiracles, and sometimes two closely allied species may be one aquatic and the other terrestrial.
[On the basis of this account of OLDROYD's it seems very likely that the primary way of life of the Chironomidae, and therefore of their ancestral species, is aquatic, and that later some species ventured into terrestrial environments. Therefore we will concentrate on the aquatic forms]
The water-living larvae are like small worms, rather plump, with a well-developed head that cannot be withdrawn into the body. The rest of the body is generally smooth and cylindrical, except for a two-fold proleg (false leg, or pseudopod) on the prothorax [that is the anterior part of the thorax], and a cluster of appendages at the rear of the body : paired prolegs, and often two sets of gills. There are tracheal gills, which increase the diffusion of oxygen into the closed tracheal system, and blood-gills, which are believed mainly to adjust the balance of salts in the blood in relation to the surrounding water. And, as have been said earlier, as a final refinement of adaptation some chironomid larvae carry haemoglobin in their blood.
For the species Chironomus plumosus -- a common species -- it is reported that the principal method of feeding is the filtering of planktonic organisms and particles.
Although the aquatic larvae of non-biting midges are very much alike in their appearance, they have cultivated a wide variety of habitats. Some remain in their fixed cases all their lives, others have movable cases, and some live free in the mud. A few adventurous types attach themselves to the larvae of mayflies and other aquatic insects and live as 'commensals', which is a polite term for eating the crumbs that fall from the rich man's table. Symbiocladius is a true parasite of mayfly nymphs, weaving a silken sac under the wing-pads of the nymph, making a small hole in its skin, and sucking its blood. Some Endochironomus live with water-snails, species of Limnaea and Physa, and may possibly parasitize them, and Chironomus limnaei is said to complete its whole development within the body of snails of the genus Limnaea. Others, like Chironomus varus, merely attach themselves to the shell. Larvae of a few chironomid midges mine into the stems of water-plants. Finally, the entire subfamily Tanypodinae are carnivorous as larvae, using their efficient mandibles to pierce and devour the larvae of other insects.
As we might expect in a family that is so highly adapted to living in water, Chironomidae have colonized waters that are still or torrential, stagnant or highly aerated, warm or icy. The subfamily Diamesinae in particular like the cold waters of the Arctic as well as those of the high mountains, and may be found breeding in water that is running off a glacier, with the adult flies settling on the snow and ice above.
The second largest family of the superfamily Chironomidea, the Ceratopogonidae ( = Heleidae), biting midges, is still little studied, although undoubtedly being of great interest. See next Figure, A and B :
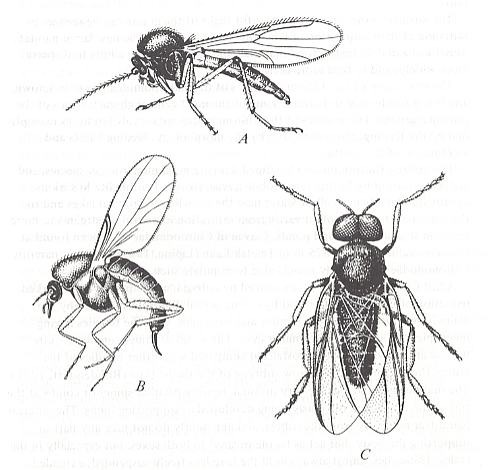
Figure 1a :
A -- Palpomyia flavipes Meigen (Ceratopogonidae, female), general view, length 3 mm.
B -- Leptoconops bezzii Noé (Ceratopogonidae, female), general view, length 1.2 mm.
C -- Simulium noelleri Friederichs (Simuliidae, male), general view, length 4 mm.
( A and B after Goetghebuer, C after Rubtsov, 1956.)
The larva of one of the species of Ceratopogonidae is depicted in the next Figure.
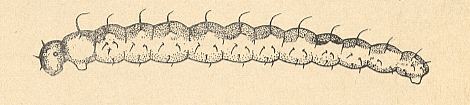
Figure 2 : Forcipomyia sp. (Ceratopogonidae), larva, side-view.
(After KRIVOSJEINA, 1969.)
Very interesting is the fact of the colonization by the representatives of one particular group of biting midges, the Forcipomyiinae, of terrestrial substrates, which sharply contrasts these insects not only from [most of the] the rest of the Chironomidea but also from the majority of the Tipulomorpha, and accordingly coming close to another superfamily, namely the Psychodidea. It must be noted that the transition of the [first] Forcipomyiinae to a terrestrial habitat is almost certainly secondary [meaning that being adapted to a terrestrial environment of this particular group of Ceratopogonidae is not reflecting the original habitat of the Ceratopogonidae. This original habitat of the family must be the aquatic environment, because their larvae are apneustic, that is, they have no open spiracles, and can get their oxygen only by diffusion from surrounding water or a watery medium. Only later on a particular population of some one species of the family has ventured into a terrestrial environment and has subsequently adapted to it, resulting in a new species (alongside the original species consisting of those populations that remained in the aquatic habitat). This new species became the ancestral species of the subfamily Forcipomyiinae which members are terrestrial.]. The sharp difference contrasting the Ceratopogonidae (biting midges) with the Chironomidae (non-biting midges) boils down to the presence in the winged form of the former of well-developed mouthparts which indicates the importance of imaginal feeding of these insects contrasting them with the aphagia of the Chironomidae. The status of being imaginal [adult] predators as original, that is basic, primitive, or first, is evident, as well as the habit of blood-sucking from vertebrates being derived from their originally being entomophagous animals, that is, predators of insects. The importance for the vital function of the biting midges of adult feeding -- that is, the important role it plays in the life of any member of the family -- is clearly expressed by the organization of their adult bodies : strong prehensile legs, costalized strong wings, and the integration of the body. All these features clearly indicate a totally different pathway of the historical development of the Ceratopogonidae and points to ways to discover the conflicts which had determined their origin. However, our poor faunistic and morphological knowledge of the biting midges prevents us to present the very essence of these conflicts. We can only remark that the peculiar subfamily Leptoconopinae (see Figure 1a B) is certainly an offshoot from one of the groups of biting midges, which, as it seems, has originated as a result of the development of extra-intestinal digestion of the larva (which lost its head capsule and has been transformed into an acephalous "muscoid" larva), together with the development of ultra-costalized lifting wings and blood-sucking from vertebrates. The final resolution of the problem concerning the pathways and determining conditions of the historical development of the biting midges can only be accomplished only after the investigation of their taxonomic system and of the living-conditions of these insects. ( ROHDENDORF, 1964).
We continue the description of the habits of the Ceratopogonidae (still concentrating on those habits that are original, that is, not derivative, for the family), now based on the exposition of OLDROYD, 1964.
Ceratopogonid flies are very tiny indeed : those whose wing-length reaches 2.5 mm, or 1/10 inch, are big ones, and some scarcely attain a length of 1 mm. Only the females have a piercing proboscis. Only three genera are known to suck the blood of warm-blooded animals : Culicoides, Lasiohelea, and Leptoconops. Most genera suck the juices of flowers, or feed upon other small insects near there own size, including non-biting midges and small mayflies.
Some flies of the large genus Forcipomyia do not crudely and greedily suck dry their insect victim, but cling to the wing, and delicately pierce one of the wing-veins. They may be found attached to adult Neuroptera -- alder-flies, lace-wing flies, and so on -- and to Lepidoptera (butterflies, moths). Flies of the genus Pterobosca cling to the wings of bigger crane-flies and dragon-flies and may have their legs specially adapted for hanging on like an aerial hobo. Perhaps the trick of feeding from the wings rather than the body of its victim shows prudence (with respect to preventing itself from being attacked) as well as refinement of taste.
The little flies of our family sometimes mass in large numbers on flowers. These swarms consist entirely of pollen-loving females, and they must play an important part in the cross-fertilization of spring flowers.
Remarkable is the fact that Culicoides anophelis chases mosquitoes that have gorged themselves on blood, human or animal, and robs them of a little of it by piercing the abdomen : a literal example of the biter bit.
The larvae of Ceratopogonidae are apneustic. They thus have the appearance of a family with aquatic ancestors, whose terrestrial descendants are colonizing media that are really alien to them. The truly aquatic larvae are long and worm-like, and move along by wriggling like tiny eels. They live mostly round the margins of ponds and streams. They do not confine themselves to fresh water, but feed also in brackish, or even salt, more especially that of pools and lakes inland, where the salinity has risen through evaporation.
The 'terrestrial' group of biting midges, as we have seen, is really an offshoot of the aquatic ones, and its larvae live in soggy places, in decaying leaves, manure -- i.e. a mixture of dung and vegetable matter -- under loose bark, in rotting fungi, in fact in all the standard compost-like materials. That this is a return to a [remote] ancestral habitat [maybe of the first Diptera] is shown by the fact that these larvae have no spiracles. Although they have modified their shape, becoming more flattened, and varied in form to suit their individual habitat, they have not managed to recover the open spiracles that their ancestors lost when they took to the water.
Some larvae of this group have it both ways, by living in the small accumulations of stagnant water that accumulates in the cavities of plants such as in the axils of the epiphytic Bromeliads of tropical America. These cavities become very foul with organic decay, and provide food for a variety of saprophagous larvae, which in turn are preyed upon by carnivores.
Ceratopogonidae are a family that it is difficult to generalize about, principally because they are so versatile, and make the most of this half-world between the land and the water. Thus the single genus Forcipomyia contains species that live in compost-like materials, in ants' nests, in rotten wood, in damp moss and algae, and in the Bromeliads that we have just mentioned. SAUNDERS suggests that the uniform factor in the larval habitats of Forcipomyia is "some dark enclosed cavity where the atmosphere approaches saturation, and where moulds and fungi are abundant".
See Figure 1a C, but also the next Figure :
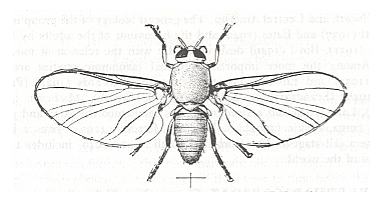
Figure 3 : Simulium venustum, female, about x 9, N.America.
(From RICHARDS & DAVIES, Imms' General Textbook of Entomology, 10th edition, 1977.)
The larva of one of the species of Simuliidae is depicted in the next Figure.
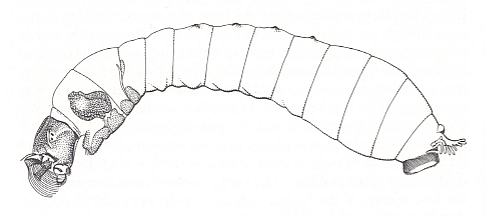
Figure 4 : The aquatic larva of Simulium damnosum, with its mouth-brushes and posterior sucker, a static larva filtering its food from moving water.
(After OLDROYD, 1964)
The third, with respect to number of species, very important family of black-flies, Simuliidae, is characterized by totally different features which sharply contrast these insects with the other Chironomidea. The (1) strongly expressed rheophilic nature of the larvae, which live a sedentary life in the strong water current, and which have developed the ability to feed on microorganisms (microphagia) (collection of minute food particles from the water by means of special rotational devices, transformed appendages of the head), together with (2) the intense integration of the body of the winged phase, (3) its habit of sucking blood from vertebrates, (4) the development of broad peculiar costalized wings ( fan- or flabellate-two-wingedness or strepsidipterigia [all terms being equivalent], described by ROHDENDORF, 1949, p. 151 ) -- all these features bear witness to the character of conflicts having determined te origin of the Black-flies. The first forms of Chironomidea (to which superfamily the family Simuliidae belongs) which [apparently] lived in fast flowing water developed the ability to live in very fast moving waters. Colonizing the rapids of a swift stream turned out to be possible thanks to the development of an immobile (sedentary) way of life by way of (1) building tubes, (2) anchoring to the substrate, and (3) developing microphagia. Such a solution of the conflict, although guaranteeing the larva to live in open, not heavily populated, more protected conditions of the stream, at the same time turned out to be the cause of obtaining insufficient [quantities of] food and other difficulties. The insufficiency of larval food resulted in the [evolutionary] intensification of adult feeding, first of all by way of significant refinement of locomotion, by integration of the body, by the development of large broad wings, concentration of the sense organs of the head, by [the development of] prehensile strong legs, and, finally, most importantly, by blood-sucking from vertebrate animals. This pathway of the history of the Simuliidae indicates the significance of the changes of the different aspects of the organization of these insects and makes possible to evaluate the properties and character of their historical development. Basic was the development of protective features of the larva and its feeding. In the sequel this determined the refinement of the whole body (its integration) of the winged insect, its feeding and locomotion. One must assume that all processes in the historical development of the black-flies proceeded at a relatively slow rate -- this group is a relatively ancient one.

Figure 4a : Simulium equinum (Simuliidae), 2.2--3.5 mm
(After ZAHRADNIK, 1977, in Thieme's insektengids (insect guide))
With respect to the ecological features of the Simuliidae we, consulting OLDROYD, 1964, can add the following :
Functional type and subtypes of the wings of the representatives of the s u p e r f a m i l y Chironomidea [ = Tendipedidea].
( The description of functional types and subtypes of wings is taken -- with additions and changes -- from ROHDENDORF, 1951.)
The wings of the representatives of the superfamily Chironomidea all belong to the
Traction costalized (tendipedoid) wing type.
Description of the type (and its subtypes) as such :
Representatives of the type.
To the traction costalized (tendipedoid) type belong the many forms of the large superfamily Chironomidea [ = Tendipedidea], which includes three separate families Chironomidae [ = Tendipedidae] (non-biting midges), Ceratopogonidae [ = Heleidae] (biting midges, gnats), and Simuliidae (black-flies or buffalo-gnats).
Wings of some representatives of the type are depicted here :
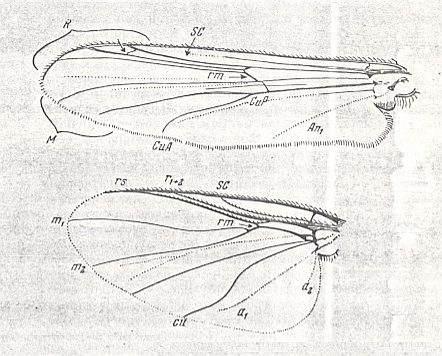
Wings of the traction costalized (tendipedoid) type.
Top : Pelopia sp. (Chironomidae).
Bottom : Simulium sericatum MEIG. (Simuliidae).
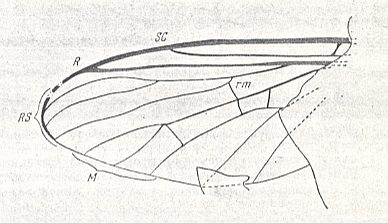
For the abbreviations signifying the identities of veins, see previous document .
(From ROHDENDORF, 1951, after HENDEL)
Sizes of wings
Shape of the wings
As to their shape, the wings of this type are divided among thee subtypes, partially corresponding to the three families.
The wings of the first subtype, to which belong the [wings of the] representatives of the family Chironomidae (see Figure above, top image ) and many Ceratopogonidae, are very elongated, about four times as long as their width, and having their margins almost parallel. The anterior margin of the wing is entirely straight up to the wing-tip. The apex [ = wing-tip] of the wing is very small, about one fifth of the length of the wing. The hind margin is moderately convex in its apical half and almost straight in its proximal half. Anal lobe in the form of a marked projection, qua shape approaching that of a quadrant of a circle.
The wings of some Ceratopogonidae and a few Chironomidae (for example Corynoneurinae), forming the second subtype within the tendipedoid type, are short, only 2-3 times longer than their width. The anterior margin is straight only along three fifths to one half of the wing-length, and with it the apex is very large. The hind margin of the wing is markedly convex. Anal lobe in the form of a moderate convex projection, far from having the shape of a quadrant of a circle.
Still broader are the wings of the representatives of the third subtype, that is, in the Simuliidae (see Figure above, bottom image ). Their wings have a length which is only 13/4 - 2 times larger than their width. The fore margin of the wing is straight up to 1/4 - 1/5 of the length of the wing. The apex is not large. The hind margin is strongly convex, and with it the anal lobe is in the form of a marked projection.
The basiala is well-individualized in the whole type and is characterized by the development of a very strong and firm phragma which actually separates the basiala from the wing-blade. The alula is clearly marked off in the first and last subtype and only in the Ceratopogonidae not clear. The alula has the shape of a convex lobe, usually provided with hairs. In the Chironomidae there is a small wing-scale. When the insect is not flying its wings are held one over the other along its body.
The skeleton of the wing
The venation of the wings of the tendipedoid type are characterized by very far progressed processes of costalization, which [processes] appear in the form of reductions, shifts, and coalescence of veins. The costal vein always runs only along the anterior wing margin, rarely reaching the wing-tip. Sometimes it even does not reach the middle of the anterior margin. The subcostal vein usually is weakly expressed and delicate, lying between two strong and thick veins, the costal and radial vein. Sometims the subcostal vein disappears completely. Rarely it is developed as a distinct vein (Simuliidae). The radial veins have markedly come close to each other, and include no more than three separate longitudinal branches, rarely supplemented by a fourth [branch, forming a] short fork on the middle radial branch. Surprisingly more often we will see a reduction of the number of radial veins down to two (the majority of Simuliidae and Ceratopogonidae) or even down to one (for example the genus Brachypogon from the Ceratopogonidae). The medial veins are always significantly thinner and more delicate, counting three (Ceratopogonidae and Simuliidae) or two (Chironomidae) branches. Cubital and anal veins also very delicate, often badly marked off. Of cross-veins there are only the usual main ones ( rm [between radius and media], mcu [between media and cubitus], and the humeral cross-vein [between costa and subcosta] ), where the medio-cubital is often not clear and shifted in the direction of the wing-base. The last medial branch usually sharply separated from the two anterior branches, where its base has somtimes completely disappeared (Chironomidae).
The processes of costalization reach their extreme expression in Ceratopogonidae (for example the genus Leptoconops) or in Chironomidae (the group Corynoneurinae), in which the wings in fact are transformed into free lobes of the membrane at its base strengthened by a firm thick vein which is the product of coalescence of the radial and costal veins. They lively let us remember the wings of those totally diffent insects which are parasites, namely the minute Chalcididae of the insect order Hymenoptera [sawflies, wasps, bees, and ants] !
Coverings of the wing
The wings of this type are always covered with delicate short hairs (microtrichia). Sometimes on the membrane has developed a larger (Ceratopogonidae) or smaller (some Chironomidae) number of spinelets, which are, however, not transformed into scales. Sometimes the spinelets appear only on the veins, they are then short and strong (Simuliidae). In the basiala, namely on the proximal part of the radial trunk in it, there exist two groups of sensory organs looking like short, and thick, small spinelets or hairs. More in detail these sense organs and the nerve system of the wing are not studied.
Functional features
Up to now the flight [features] of insects having a traction costalized type of wings is not studied accurately, but all of their structure allows us to assume an especially high wing-beat frequency. Biologically, flight is of important significance to the representatives of almost all three subtypes. The first subtype (majority of Chironomidae and some Ceratopogonidae) includes wings of insects which are able to perform prolonged air 'dances', swarming in masses, sometimes high in the air (tens of meters). The females of these midges fly toilsomely, having a relatively heavy abdomen filled with eggs. The flight of insects possessing wings of the second subtype (Ceratopogonidae and some Chironomidae) is little known. We should mention the presence among these insects (Ceratopogonidae) of blood-suckers, and with this not only from vertebrates but also from other insects being themselves able flyers like dragonflies. The high quality of flight in Ceratopogonidae therefore turns out to be biologically a truly essential acquisition in their evolution. Completely clear is the significance of refined flight in the third subtype, the wings of black-flies, Simuliidae, active blood-suckers in mammals and birds. The amount of blood sucked by a black-fly sometimes doubles the weight of its body. Therefore the necessity of possessing a strong flight apparatus is evident. For the black-flies a good flight is also important for hunting their victim which has the ability to move quickly. In describing examples of the great biological significance of the flight function in Diptera having wings of the traction costalized (tendipedoid) type, it is necesary to note cases of the reduction of wings, the development of apterygia [ = wingless condition], especially in females, which have originated from the first subtype-as-a-base. Such are certain Chironomidae, for example Corynoneurinae, Clunioninae. Especially remarkable in this respect are certain marine insects, namely species of the genus Pontomyia, in which the reduction of flight ability also affects the males, where the females are transformed into peculiar worm-like organisms devoid of appendages, which never go ashore.
History of the type and its transformations.
The first representatives of the tendipedoid type are known in the form of remains in jurassic sediments of Karatau [southern Kazachstan], and belong, presumably, to the first subtype whose representatives are closest to the original forms. The dixoid type [see below, at the Dixidea, Figure HERE ] is undoubtedly the source of origin of the tendipedoid type, more precisely the more primitive forms of the Dixoid type, not possessing such broad wings with rich venation. The refinement of the dixoid type consists in the increase of costalization -- that is, the forwardly-directed shift of the whole system of radial veins, the weakening of the posterior half of the wing by way of either complete reduction of veins, or their weakening -- and the improvement of mechanical structures in the basiala, that is the developing of an especially firm phragma and of various lobes at the hind margin of the wing-base. The partition of the tendipedoid type was already described above in the exposition of the functional features. Here we must only add that while the wings of the type's first subtype (we could call it the "narrow-winged tendipedoid subtype") stand most closely to the original forms of the type, the two other subtypes were already significantly [evolutionarily] moved away from the original forms.
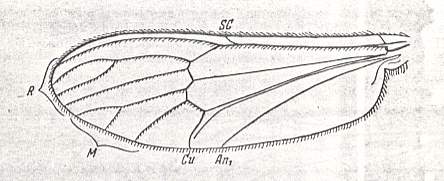
The superfamily Dixidea, see next Figure, comprising in the recent fauna just one family, the Dixidae (about 100 species, distributed among six genera and subgenera), and today undoubtedly having the nature of a well-expressed fairly specialized phylogenetic relict group. The most ancient representatives of this superfamily were discovered in the middle Jurassic fauna of Karatau [southern Kazachstan] (the family Dixamimidae) [ As regards the geological age of the fossil sites of Karatau (southern Kazachstan) (Michailovka and Galkino) a mid-jurassic age is always given, but it must almost certainly be : Upper Jurassic (Malm). See SHAROV, 1968, p. 11, and ROHDENDORF, 1957, p. 67-68, and HENNIG, 1969, p. 76.].
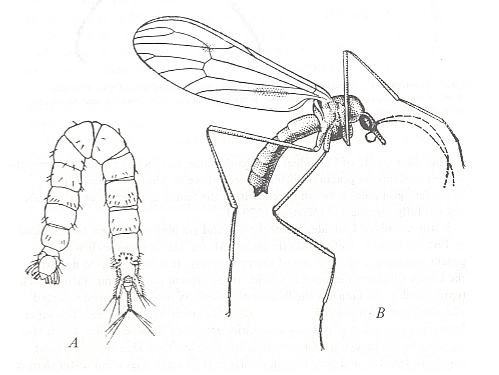
Figure 5 : Dixidae
A -- Dixa sp. (Dixidae), general view of living larva (note the characteristic curved body).
B -- Dixa maculata Meigen, female, general view.
(After LINDNER, 1930)
This group -- Dixidea -- of Tipulomorphs is certainly most closely related to the first forms of the superfamily Culicidea (to be treated next) : up to more or less recent (1964) times many authors even characterized the superfamily Dixidea as just being a subfamily of the family Culicidae [mosquitoes], basing themselves on the similarity of the larvae of these flies, whithout taking into account the peculiar features of the winged insects. Such disparity of the organization and classification of larvae and winged phases is often to be seen in the system of Diptera, especially among the ancient infraorders, bearing witness to such a character of the historical development in which the processes of specialization and adaptive changes have proceeded unequally in the larvae and in the corresponding adults.
We will now supplement these data obtained from ROHDENDORF by those obtained by OLDROYD, 1964 : Dixidae are non-biting flies rather like small crane-flies, but distinguished by not having a V-shaped groove or suture of the thorax. There are only two genera, Dixa which is world-wide and has about 200 species, and Neodixa which is confined to New Zealand. The larvae are cylindrical, with a pair of spiny prolegs on the first two abdominal segments. These are another group of larvae that are only just aquatic, crawling about on vegetation or rocks at the water's edge, and often having the body curved into the shape of an upside-down letter U (see Figure 5 ), still kept wet by surface tension, but actually above water-level except at the two extremities. This is a curious adaptation, since it would seem more logical to have the ends exposed with their open spiracles to the air, and the middle wetted to avoid desiccation.
Dixa larvae collect small organisms in the water, using mouth-brushes. The eggs are laid in a gelatinous mass, rather as in Chironomidae, but on a solid sub-stratum and not floating in the water. The pupae of Dixidae are rather like those of mosquitoes.
The representatives of the family Dixidae all have wings that belong to the functional
Traction broad (dixoid) wing type.
Description (taken from ROHDENDORF, 1951, with changes) of the type as such :
Representatives of the type
The single recent family of the peculiar relict insects, the midges Dixidae, have wings whose structure is such that justifies placing them into a separate functional type, the traction broad (dixoid) type. To this type also belong some fossil jurassic forms. See next Figures.
Wing of Dixa sp. (Dixidae). (from recent fauna).
Specimen nr. 2647. Length of wing 4.5 mm.
This wing belongs to the traction broad (dixoid) type.
(After ROHDENDORF, 1951)
Wing of Dixamima villosa ROHD. (Dixamimidae). Jurassic of Karatau [southern Kazachstan], PIN No 334/167.
This wing also belongs to the traction broad (dixoid) type.
(After ROHDENDORF, 1951)
Size of wings
The shape of the wings
The wings of this type are elongated, about three times longer than wide. Fore-margin straight up to the apical fourth [the latter] consisting of a rounded apex. The hind-margin is strongly convex. The anal lobe is well expressed. The basiala is sharply individuated, with a clearly developed phragma. The alula is in a germinal state, in the form of a lightly projecting broad lobe. Wing-scale unclear, also in a germinal state. When the insect does not fly it holds its wings backwardly spread out.
Skeleton of the wing
The wing-venation is characterized by an expressed costalization, determined by a shift of the subcostal vein ( running up only to the middle of the wing margin ! ) and the first radial vein towards the wing's fore-margin. Very characteristic is the position of the end pieces of the radial branches which run parallel to the apical fore-margin, curving a little backwards, and end up on the margin nearly at the very wing-tip. The number of radial (four) and medial (three) veins is the same as in the wings of the culicoid type (See next superfamily). Of cross-veins there exist only the main ones (rm and mcu). Costal vein firm an thick only along the wing's fore-margin, along the hind-margin it is very thin, almost vanishing. The venation of the basiala is characterized by the development of a sharp projection from the basal part of the rather wide radial vein, the phragma. The general character of the venation of the wing-blade brings this type close to the specialized tipuloid wings, namely to those where a reduction of veins had taken place (some Limoniidae and, partially, Ptychopteridae [ = Liriopeidae] ). See for the tipuloid wings, that is, the primitive traction (tipuloid) type, Part II, previous document, HERE . Characteristic is the absence of the medial cross-veins and of the closed cell in the center of the wing-blade.
Coverings of the wing
The wing carries, in addition to hairs-microtrichia, on the majority of the longitudinal veins series of thin and short spinelets which have not been transformed into scales (as in Culicidae). The hind-margin of the wing and the alula with longer spinelets. Scales are completely absent on the wings, which [feature] sharply distinguishes this type from the culicoid type. Sensoria and nerve system of the wing are not investigated.
Functional features
The flight of Dixidean midges is not accurately investigated. Probably it is weak and is biologically not connected with obtaining food. These midges live not far from water-basins, among bushes and trees, performing the known airy 'dances', alternately ascending and descending in the air, like the many other and most different groups of Nematocera.
The history of the type and its transformations.
Undoubtedly, the first representatives of this type appeared already in jurassic times. This is indicated by the discovery of peculiar jurassic insects from Karatau [southern Kazachstan], the Dixamimidae, see Figure above , possessing characteristic wings, coming closest of all to the dixoid type. The differences of the wings of Dixamima from the true, recent, Dixa boil down to the large absolute size of the insect and at the same time the smaller size of the wings. In the evolution of the dixoid type undoubtedly a decrease of size of the insect took place, which also was one of the factors determining the formation of the type. The source of the dixoid type were special forms of tipuloid wings (namely from the architipuloid subtype) in which the basiala started to refine as a result of the intensification of the wing-beat, the increase of its frequency, together with the development of costalization. [It is perhaps more probable that the evolutionary increase of wing-beat frequency in the development of the dixoid type became possible only after the basiala had been refined (and the degree of costalization had reached a certain level).] [See for tipuloid wings, that is, wings of the primitive traction (tipuloid) type, and subtypes Part II, previous document, HERE ]
See next Figures.
Figure 6 : Culicidea
A -- Cryophila lapponica EDWARDS (Chaoboridae), general view of larva from above.
B -- Mochlonyx cinctipes COQUILLET (Chaoboridae), female, general view from above.
(A, after MONCHADSKI, 1936, B, after CURRAN, 1934)
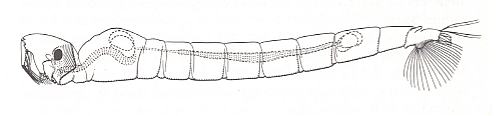
Figure 7 : Culicidea
The 'phantom larva' of the midge Chaoborus ( Corethra) (Chaoboridae). An aquatic larva living permanently in [more or less] deep water. Note the pairs of hydrostatic air-sacs fore and aft, the carnivorous mouthparts, and the posterior blood-gills, which are used not for respiration but to manipulate salt-balance.
(After OLDROYD, 1964)
As usual, we start our discussion of evolutionarily important features using ROHDENDORF's exposition. It is about the superfamily in general, but separately touches upon the two constituent families as well.
[Let us summarize our paraphrasing of ROHDENDORF's exposition of the origin of the superfamily Culicidea, resulting in its two families.
The first Tipulomorphs were (supposed to be) the result of the larvae of one or more species of ancient Diptera having gone over from a terrestrial (probably semi-liquid) to an aquatic habitat (and later on in the history of the Tipulomorphs some returned to more terrestrial habitats, such as in the soil at the roots of plants). Generally the aquatic sites, suitable for the larvae to live in, are easy to find by a female that is about to lay her eggs. This constituted an advantage with respect to the earlier semi-liquid habitats which are more isolated in their occurrence. But the aquatic habitat contained little food suitable for the larvae as they were structured at the time, and from this initial situation a solution was developed, in fact two different solutions resulting in two different lines of evolution : In one line the development of the blood-sucking habit by the winged phase remedied the shortage of larval food -- the Culicidae were born. In the other line the larvae developed independence of atmospheric air, making it possible to inhabit the whole vertical range of the water basin. In this way the stage was set to solve the food shortage problem by becoming predators and thus becoming able to acquire high caloric food -- the Chaoboridae were born.
Of course all this is rather speculative and vague, but it serves to direct our attention to the supposed fact that it is ecology that drives evolution.]
In the history of the true Culicidae, which must be considered to be the immediate descendents of the first Culicidea, we see examples of analogous solutions of conflicts concerning feeding and respiration, namely the development of a carnivorous way of life of the larvae of the subfamily Megarhininae, which is accompanied by phytophagia (nectarophagia) of the winged phase, or [as another example] the development of the peculiar way of larval repiration through the tissues of higher plants in mosquitoes of the genus Mansonia, and so on. [ ROHDENDORF, 1964 ]. [So within the further development of the one family Culicidae a fanning out took place to more or less new and different ecological niches, while the majority of the members of the family sticked to the original ecological niche.]
We now add some data concerning the ecology of the family Culicidae (an after that the ecology of the family Chaoboridae) taken from OLDROYD, 1964.
Adult mosquitoes, Culicidae, are typically nematocerous flies [so also the Chaoboridae], slender, fragile, with antennae, abdomen, and wings all narrow and elongate. They are distinguished from nearly all other related families by having the veins of the wings festooned with scales, and from all other families by the combination of scales with a long projecting proboscis. Scales are modified hairs, and many of the hairs of the rest of the body are similarly modified, so that the abdomen often has a striking pattern of bands or spots of color. Bands of scales can often be seen, too, on the legs, and can be used in the identification of particular species. In 1959 Stone, Knight, and Starcke catalogued 2426 species of mosquitoes throughout the world.
From the viewpoint of natural history, as well as of structure, mosquitoes fall into three subfamilies [the attributed ranks of and within the group of mosquito-like flies often differ in different authors], Anophelinae, Culicinae, and Megarhinidae (Toxorhynchitinae) [OLDROYD takes them to be the three tribes of the subfamily Culicinae (as contrasted with the subfamily Chaoborinae) ]. As in other [nematocerous] families we have met so far, only females suck blood, and this habit is believed at first to have been universal in the family [or superfamily sensu ROHDENDORF, and even including the Dixidae ]. Certain members of even the Culicidae have later abandoned bloodsucking, and feed only from flowers. This is true of the whole subfamily Megarhininae, and individual species in the other two subfamilies have also ceased to bite [and, as we know, the Chaoboridae and also the Dixidae do not suck blood]. As we will see again with the horse-flies, those that have given up their blood-meal have, as it were, stagnated, making little evolutionary advance, and declining in numbers. The bloodsuckers on the other hand, have found in the search for blood an evolutionary challenge, and have multiplied and flourished in their recent evolution.
Male mosquitoes, and those females that are able occasionally or permanently to lay eggs without a blood-meal, are assumed to take nectar from flowers. Some females may bite cold-blooded vertebrates, or other insects. Ocasionally, males have been noticed among a mass of female mosquitoes congregating on a person, or on the walls of stables and other farm buildings. Some of these have been proved to be gynandromprphs : that is, the body is a mosaic of male and female cells, more particularly male on one side and female on the other, with one biting mandible and maxilla instead of a pair of each. Such flies seem to be partly female in instincts as well as in structure. Yet, apart from this explanation, males and non-biting females may be attracted on occasion by sweat, dung, mucus, and all the other media that attract flies.
One remarkable eccentricity must be mentioned [OLDROYD, p. 84]. Mosquitoes of the genus Harpagomyia (now confusingly called Malaya), widely distributed from Java to tropical Africa, have discovered that certain Crematogaster ants, which run up and down tree-trunks, are carrying honey-dew which they have taken from aphids. A mosquito hovers about an inch from the trunk and suddenly alights in front of an ant, not touching it, but vibrating the wings in an apparently hypnotic way and waiting until the ant opens its jaws. The proboscis of the mosquito is pushed into the ant's mouth and the honey-dew is filched from it.
This is not, as it might seem, an occasional report that could be a traveller's tale. It has been filmed in detail, and is apparently the normal method by which all the mosquitoes of this genus feed. The proboscis is suitably modified, and probably takes no other food. That the ant should give up its booty in this docile way is at first surprising, but it must be remembered that ants are very highly evolved social insects whose life involves reciprocal relationships with many other insects. In this instance the ant has only just taken the honey-dew from an aphid, and it is a pleasant irony that its reflexes should be cleverly exploited by the mosquito.
We think of mosquito-larvae as aquatic creatures. After all, we know that ponds, creeks, swamps, water-courses of all kinds breed mosquitoes, and so do temporary pools and puddles. Both larvae and pupae are familiar as 'wrigglers', swimming actively about in deep, clear water. Yet they are air-breathing insects, and only imperfectly able to exist away from the free air : much less so, in fact, than most other larvae of Nematocera of the 'watery' group. They are really creatures of the surface film, enjoying the advantage of breathing atmospheric air, combined with the protection, resulting from the presence of the water, against desiccation, and the constant supply of microscopic food from the water. The nearest equivalent lies in those Psychodids [which will be treated in the next section about the superfamily Psychodidea] that live in a surface film over stones and in shallow water, and we may see in this a hint that mosquitoes have evolved in a direction of their own, exploiting fully the peculiar advantages of the water-air boundary.
The surface of the water must be clean, at least over a reasonable proportion of its area, or else the larva cannot reach the air without getting its siphons blocked. Since the ability to keep a tube open through the surface film depends on the surface tension, anything which lowers this is a menace to the larvae. In theory at least, any substance in solution reduces the surface tension below that of pure water. Hence mosquito-larvae prefer the cleanest water for breathing purposes, though they may find more to eat in water that is stagnant [This is clearly an example of an internal conflict between feeding and respiration solved by the development of acquisition of food by the winged phase : blood.].
There is probably a complete sequence from the 'standard' microphagous mosquito-larvae, through feeding on cast skins and dead insects, to actively attacking other aquatic creatures, including other mosquito-larvae. The best-known predaceous larvae are those of Megarhinus ( Toxorhinehites ), where we note that flesh-feeding has been transferred to the larva from the adult, which does not suck blood.
Now some data about the family Chaoboridae, also taken from OLDROYD (pp. 75).
Chaoboridae are mosquito-like flies (see Figure 6 ) with hairs and scales. They have a short proboscis, but do not bite, and the male flies have plumose antennae. The adult flies sometimes occur in immense swarms, and are particularly notorious over the lakes of the African Rift Valley.
Collectors of pond-life [including myself (JB)] are familiar with the 'phantom larva' of Chaoborus (see Figure 7 ), a sausage-shaped, transparent larva with two glistening, kidney-shaped air-sacs. This carnivorous larva lies horizontally in the water, slowly descending, until its prey comes near. It is completely aquatic, has no open spiracles, breathes entirely by absorption, and rarely comes to the surface except at night. By day it may lie in the mud at the bottom.
The pupae of Chaoboridae swim actively, like those of mosquitoes.
All representatives of the superfamily Culicidea have wings of the
Traction scaly (culicoid) wing type.
Description (taken from ROHDENDORF, 1951, with changes) of the type as such :
Representatives of the type
Wings of the traction scaly (culicoid) type are possessed by the representatives of the two closely related families of the superfamily Culicidea, viz., the Culicidae (mosquitoes) and the Chaoboridae. See next Figure.
Wing of Culex pipiens L. (Culicidae).
(From ROHDENDORF, 1951, after HENDEL)
Size of the wings
The shape of the wings
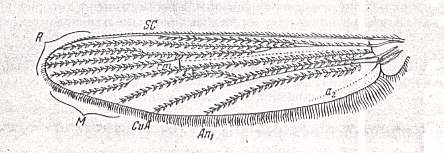
The wings of this type are always strongly elongated, 4 - 41/2 times longer than their width. Fore-margin straight almost up to the very wing-tip. The curve of the margin begins very close to the end of the wing. The apex of the wing is [therefore] short. The hind-margin of the wing is uniformly convex, and with it the widest part of the wing-blade lies markedly distally from its middle. The basiala is clearly distinguished, see next Figure.
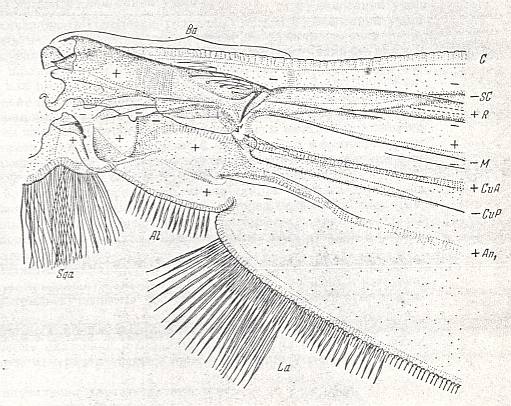
Figure 1g : Theobaldia alaskaensis LUND. ( Culicidae).
Basiala and base of wing-blade of right wing of male. Top view.
Specimen nr. 2570. Length of whole wing 6.5 mm., of basiala 0.75 mm. Macrotrichia and scales not drawn.
For abbreviations see Figure 1a in previous document .
(After ROHDENDORF, 1951)
The anal lobe is clearly expressed, sometimes in the form of an almost rectangular rounded projection. The alula is well developed, in the form of a broad rounded projection. The [two, on each wing one,] wing-scales are developed, having the shape of rounded projections, along the margin provided with long spinelets. Thoracic scales are absent. When the insect is not flying it holds its wings one upon the other over the abdomen. Sometimes the wings are held obliquely backwards.
Skeleton of the wing
The wing-venation of the representatives of this type is rich, showing a moderately expressed costalization : The long subcostal vein and the first radial branch run parallel to each other and to the wing-margin, but not coming very close to the latter. At the same time the costalization consists in a clear suppling of the [area at the wing's] hind-margin where the veins are more or less broadly separated from one another leaving the anal lobe almost free of veins. All radial veins (numbering four) and the anterior medial vein run parallel, where the radial veins end up at the fore-margin and at the wing-tip, while the medial veins end up at the hind-margin. There are three medial branches. Of the cross-veins in the wing-blade there are only the two main ones, the radio-medial [rm] and the medio-cubital [mcu], where the medio-cubital has been transformed into the basal trunk of the last medial vein, which is usually observed in recent Diptera. The costal vein runs all around the wing. The basiala carries a sharply expressed phragma, which is the transverse outgrowth delimiting the basiala from the wing-blade and which unifies the 'handles' [ = swollen proximal ends of longitudinal veins] of the veins : the proximal end of the radial vein is very broad, completely covering the [corresponding part of the] subcostal vein.
Coverings of the wing
In addition to hairs, microtrichia, which are distributed over the whole wing-surface, all longitudinal veins carry series of scales. The margin of the wing carries differently-built scales. The fore-margin carries smaller adjoining spinelets-scales that gradually become smaller when going along the margin from base to tip. Beyond the wing-tip the marginal spinelets suddenly increase in length and keep on increasing up to the projection of the anal lobe along which the spinelets reach their maximum length. Along the margin of the wing, especially along the anal lobe, there are three sorts of spinelets (see Figure above ) : The longest are the lanceolate spinelets-scales, then shorter spinelets placed between the adjacent long ones, and, finally, the shortest hairs, densely bordering the wing-edge [and lying] among the bases of the spinelets. The spinelets that lie along the margin of the alula differ totally from those fringing the anal lobe. They are thinner and shorter. On the wing one can easily distinguish special sense organs lying in transverse rows of minute bumps with spinelets, in the form of seemingly transverse stripes in the basiala, namely on the anterior surface of the broadened part of the radial vein.
Functional characteristic
The blood-sucking mosquitoes (Culicidae) have a fairly fast and well-steerable flight. They are capable of flying fairly great distances (some species of the genus Anopheles up to 3 km). The biological significance of flight is very great for the females, which are actively and prolongedly seeking out and 'hunting down' their victims which are vertebrates. The males perform characteristic mass flights, swarming in large aggregations. Characteristic to the flight of mosquitoes (Culicidae) probably is a large store of energy : the mosquito must perform long flights, and, in addition, after having taken a blood-meal significantly increasing its weight (more than two times), must be able to fly to a sheltered place. Undoubtedly the flight of the mosquito is fully refined. This is being testified by its wing-beat frequency, that varies between 248 to 307 beats per second, which until now appears to be the highest value known in all insects. Indeed, it is probably characteristic for mosquitoes to have a high wing-beat frequency which makes possible to develop a strong lifting-force and a refined steerability. The, as it seems, insufficient mechanical specialization of the wing-venation which has little changed and only insignificantly costalized is amply compensated for by the most remarkable covering [of the wings], the abundance of spinelets and hairs, which apparently create useful aerodynamic effects during the fast wing-beat. The presence of spinelets at the wing's hind margin undoubtedly take over the effect of the absent, better, little developed, posterior membrane of the wing. Another important refinement is the structure of the basiala, undoubtedly possessing a high degree of firmness and at the same time flexibility.
[How to deal functionally and ecologically with the other family (Chaoboridae) having this type of wing, will not be easy. They are non-biting. The larvae of them are -- in contrast to those of the true mosquitoes (Culicidae) -- aquatic predators. They presumably have gathered enough protein-containing food for letting to ripe the eggs in the female, making a blood-meal superfluous.]
History of the type and its transformations
Fossils can very little illuminate the history of this type. They are only known from tertiary deposits. All these fossil remains belong to genera also living today. Recent Culicidea are very uniform in their wing structure. It is, however, certain that such uniformity of the wings is only a consequence of their being still insufficiently studied. Actually there are differences of the evolutionary pathways of the wings of the different midges or mosquitoes of this type, which is evident by the fact of their existing in a large number of forms and by the presence of essential differences in their way of life. The first forms that had led to culicoid wings belonged to the tipuloid type, namely to little costalized forms of them, and moreover those forms in which a firm basiala with a strong phragma started to develop. Me (Rohdendorf) thinks that among recent insects such wings are possessed by the representatives of the family Nemopalpidae, not known to me concretely ( They, as relict forms (family), belong to the superfamily Psychodidea, and are known from countries of the southern hemisphere). Such an ancestral 'proculicoid' type did not only form the basis of [the formation of] the true culicoid wings but also of the costalized wings of the dixoid type.
This concludes the exposition about the superfamily Culicidea.
In the next document we will continue with the exposition about the superfamilies of the Tipulomorpha.
Will be supplemented if necessary . . .
e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part IV.
Back to Aristotelian metaphysics Part I
Back to Aristotelian metaphysics Part II
Back to Aristotelian metaphysics Part III
Back to Aristotelian metaphysics Part IIIa
Back to Aristotelian metaphysics Part IV
Back to Aristotelian metaphysics Part V