

e-mail :
 ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam ) After having discussed the dipterous infraorder Bibionomorpha (= Nematocera Oligoneura) in an introductory fashion (by paraphrasing a text in ROHDENDORF 1946), and having inserted much about the habitats and feeding habits of Dipterous larvae (by paraphrasing a text in KRIVOSJEINA 1969), it is now time to start the main discussion concerning the evolution of and within the infraorder Bibionomorpha.
Features of the historical development of the chief superfamilies of the infraorder Bibionomorpha.
The different groups in these Diptera had a very different history : In order to get a clear picture of the pathways of evolutionary development of them it is necessary to consider the individual superfamilies of the infraorder more closely. We will follow ROHDENDORF 1964 and clear up and add things where necessary.
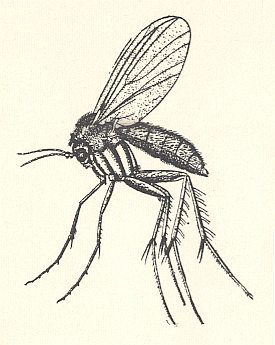
Figure 1 of previous document and the next Figure very clearly depict the general appearance of fungus-gnats in the broad sense (Fungivoridea ( = Mycetophilidea)).
The superfamily Fungivoridea [ = Mycetophilidea] consists of ten families :
Allactoneuridae
Ditomyiidae
Ceroplatidae
Diadocidiidae
Macroceridae
Manotidae
Lygistorrhinidae
Mycetobiidae
Fungivoridae [ = Mycetophilidae]
Sciaridae [ = Lycoriidae]
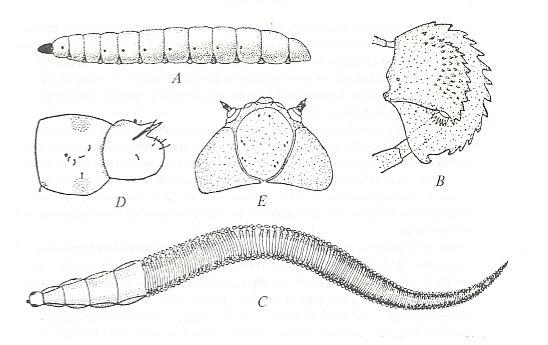
Figure 2 : Fungivoridea and Bolitophilidea. Larval structure.
A -- Brachypeza radiata JENKINSON ( Fungivoridae), larva, side view.
B -- The same, its upper jaw (mandible).
C -- Macrocera anglica EDWARDS ( Macroceridae), larva, top view.
D -- Ditomyia fasciata MEIGEN ( Ditomyiidae), larva, posterior end of body, side view.
E -- Bolitophila pseudohybrida LANDROCK ( Bolitophilidae [belonging to the superfamily Bolitophilidea] ), larva, head from above.
(From ROHDENDORF, 1964, after HENNIG, 1948-1952, enlarged.)
The superfamily Fungivoridea is one of the most ancient groups, known from Triassic times, and widely distributed in the recent epoch over the world, including 10 families with more than 3200 species. This superfamily is as a whole clearly characterized by the fact that the adult phase is very active (development of powerful running legs [see Figure 1 ] and broad peculiar wings (see Figure 3 ) with a mechanized basiala [see Figure 4 ] and wing-venation).
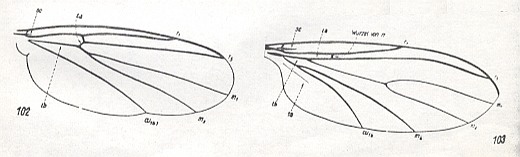
Figure 3 : Wings of Fungivoridea.
102 -- Zygomyia notata STANN. ( Fungivoridae).
103 -- Lycoria bicolor MEIG. ( Sciaridae).
(After HENNIG, 1954 )
The superfamily is further characterized by larvae with a powerful head and characteristic saw-like jaws (see Figure 1 B ). They feed on plant tissues (rotting or living) and do not possess long antennae.
The family Fungivoridae [ = Mycetophilidae], also called fungus-gnats (see Figures above), which is the largest family of the superfamily, and known from the Paleogene [early Tertiary] (but originated, probably, not later than the boundary between Jurassic and Cretaceous), is characterized by the narrow connections of these insects with higher fungi in whose fruiting bodies their larvae live. The (1) insulated distribution of this nutritive substrate in time and in space, (2) the climatic features (winters of the temperate zone, which [zone] is the principal region of the [larval] development of the family), (3) the nature of the habitat (abundance of plant residues of the upper layer of the forest-floor, all this determined in the clearest form the above desribed conflicts that evoked the formation of the quickly running and actively digging long-living winged stadia of fungus-gnats. These processes in their turn evoked new conflicts. First of all, living of the winged form in vegetable remains for a long time, often hibernating in these conditions, forced to reduce or strongly slowed down the refinement of flight features. Secondly, complete food [containing all the necessary ingredients] of the larva was more and more strongly called for, that is, the necessity to accumulate fat reserves [for overcoming the winter]. This became possible by the transition of larval feeding to the most caloric parts of the fruiting bodies of the fungi, namely their spore-carrying tissue. Such a process we indeed observe in many of the youngest groups of the family.
The second in number of recent species is the family of soil-dwelling midges Sciaridae [ Lycoriidae]. And they are characterized by different features. In these fungivoridea the new developing tendencies in their historical development consisted in the refinement of larval feeding, creating the ability to eat rotting leaves of trees, leaves, fallen on the ground, which determined the most important role played by these diptera in soil-formation. An immediate connection of these diptera with fungi evidently was of no great significance in their history. Only the swallowing of the rotting vegetable mass together with the saprophytous flora (bacteria?) the contact with lower plants [fungi] was realized. The refinement of the winged phase of the Sciaridae consisted in the improvement of the flight apparatus (moderate costalization of the wings). The legs were little strengthened and the body did not acquire a stream-lined shape. Feeding probably was of little importance to the winged phase. In part, aphagia was developed, and the lifetime of the winged insect was shortened. Such a kind of solution of the conflict, having made this group of diptera, allows this group to be evaluated as being progressive.
The other families of the superfamily Fungivoridea include a significantly lower number of species, and the majority of these families are relict groups. Knowledge of their features is still very incomplete and does not allow to definitely ascertain the nature of the conflicts which determined their development. Earlier I [ROHDENDORF] have given some existing peculiar traits of many relict families of this group (ROHDENDORF, 1946, [see Part V ] ). It should be added that the new data, that I came to know, concerning the development of Macroceridae [ = Macroceratidae] and Ditomyiidae, even more support my assumptions about the remarkability of the historical development of these relict groups -- poor in species -- of Fungivoridea. The structure of the larvae of Macrocera, see next Figure,
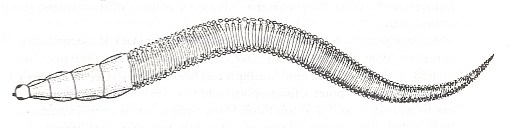
Figure 4 : Larva of Macrocera anglica EDWARDS ( Macroceridae). Top view.
(From ROHDENDORF, 1964, after HENNIG, 1948-1952, enlarged)
is in such a degree peculiar that it clearly bears witness to the noteworthiness of the living conditions of this form. Something similar may be said also of the representatives of the family Ditomyiidae, also distinguished by the remarkable organization of their larvae, see next Figure,

Figure 5 : Larva of Ditomyia fasciata MEIGEN ( Ditomyiidae). Posterior end of body, side view.
[For the interpretation of this drawing it is probably best to consult HENNIG's work from where this drawing came.]
(From ROHDENDORF, 1964, after HENNIG, W., 1948-1952, enlarged)
while the winged phase is characterized by very ancient features.
What now follows is an account of the way of life of the representatives of the superfamily Fungivoridea as we have found it in OLDROYD, 1964, The Natural History of Flies, pp. 41.
As the reader will recall, the superfamily Fungivoridea [ = Mycetophilidea] consists of ten families :
Allactoneuridae
Ditomyiidae
Ceroplatidae
Diadocidiidae
Macroceridae
Manotidae
Lygistorrhinidae
Mycetobiidae
Fungivoridae [ = Mycetophilidae]
Sciaridae [ = Lycoriidae]
Some of these families are (also) more or less separately considered in OLDROYD.
The land-midges of the superfamily Mycetophiloidea [ Fungivoridea] are flies of the compost-heap, flitting about generally in shady places, always to be found where there is gently decaying vegetation, dung, rotten wood, or a growth of mould or fungus. That neglected piece of woodland with fallen and fungus-covered trees are the haunts of this group of flies.
The adult flies are all harmless creatures, and have no equipment for biting. They have long antennae like a string of minute beads, and the wing-veins are few, with hardly any cross-veins. In spite of the fact that they look so featureless and all alike, about 2000 species of Mycetophilidae [ = Fungivoridae, fungus-gnats] and over 300 of Sciaridae [ = Lycoriidae, soil-midges] are known to exist. Most of these insects (of the two families) have been discovered in cool, temperate regions of both hemispheres. It is likely that they are less common in hot countries, but it is certain that many of such small and fragile flies must exist in shady, steamy areas of the tropical rain-forest, as well as in the temperate forests that occur on the higher parts of tropical mountain ranges.
There are unrivalled opportunities for evolution offered to a larva by a compost-like medium. One of the many advantages of an almost uniform temperature, often above that of the surroundings, and an unlimited supply of vegetable food, is : a short larval life, and two or three weeks is generally enough, at any rate in the warm months of the year. This means that such flies can pass through perhaps six, eight, or even ten generations between spring and autumn, whereas many aquatic larvae may be delayed by low water-temperature, and many soil-living larvae by the difficulty of finding enough suitable food. The advantage in speed of [genetic] mutation, [natural] selection, and evolution is obvious.
Larvae of Mycetophilidae and Sciaridae are essentially terrestrial, and usually have eight pairs of open spiracles, one pair on the thorax and seven on the abdomen. They are slender, worm-like creatures, only a few millimetres long, with a small but distinct dark head, and often showing the spiracles as a row of tiny spots like portholes along the body. See next Figure.
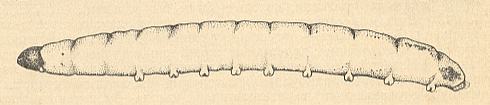
Figure 5a : Larva of Rhymosia sp. ( Mycetophilidae). Lateral view.
(After KRIVOSJEINA, 1969)
They are typically vegetable-feeders, but some of them prefer to live in growing fungi, which they tunnel until the firm mass of the fungus begins to liquefy and rot sets in.

Figure 5b : A male adult of the New Zealand glow-worm, Arachnocampa luminosa (Fungivoridea-Ceroplatidae). This is a rare photograph of an elusive fly, but its appearance is typical of land-midges all over the world.
(After OLDROYD, 1964)
Similar 'glow-worms' -- of course quite unrelated to the true glow-worms, which are beetles -- have been reported from caves in Tasmania and New South Wales (Australia). It is thought that the glow attracts insects into contact with a mass of hanging threads forming part of the web of the larva : these threads are sticky, and have droplets that perhaps are poisonous, containing oxalic acid, but which are not themselves luminous, though they appear so in the flashlight photographs. The prey is mostly chironomid midges breeding in pools in the caves and ravines concerned.
We will now describe the functional type and subtypes of the wings of the representatives of the superfamily Fungivoridea (taken -- with changes -- from ROHDENDORF, 1951 ). Not all members of this superfamily should necessarily have wings of precisely this type, and some representatives of groups outside the Fungivoridea could have wings that belong to this type.
Ancient lifting venationally-attenuated (fungivoroid) wing type.
Description
Representatives of the type
Wings of the ancient lifting venationally-attenuated (fungivoroid) type are possessed by almost all representatives of some superfamilies of the Nematocera -- Fungivoridea, Bolitophilidea, Bibionidea.
We give some Figures, starting with recent relict representatives.
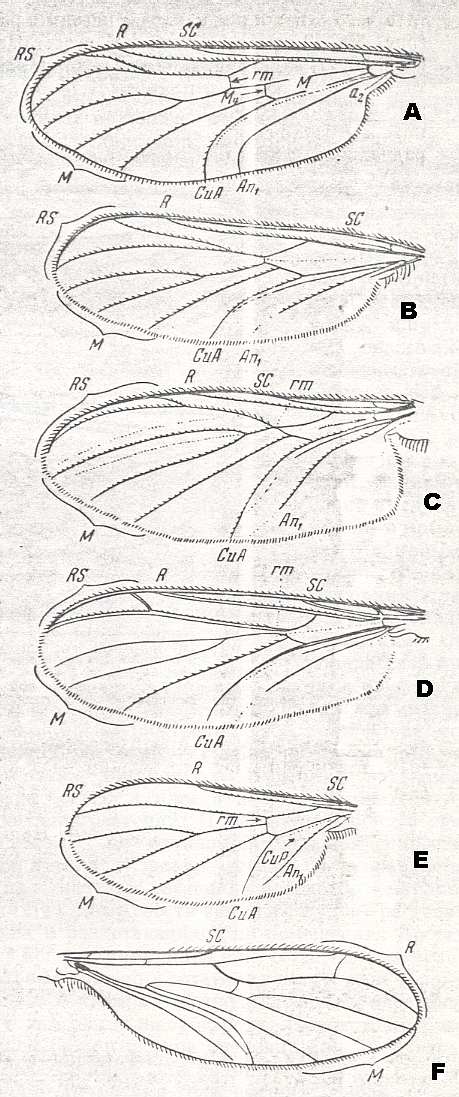
Figure 6 ( A, B, C, D, E, and F ): Wings of recent relict representatives of the ancient lifting venationally-attenuated (fungivoroid) type.
A -- Bibionidea. Hesperinus imbecillus LW. ( Hesperinidae). Length 8.5 mm.
B -- Fungivoridea. Ditomyia fasciata MEIG. ( Ditomyiidae). Length 5-6 mm.
C -- Fungivoridea. Macrocera sp. ( Macroceratidae [ = Macroceridae). Length 4-8 mm.
D -- Fungivoridea. Ceroplatus sp. ( Ceroplatidae). Length 10-12 mm.
E -- Fungivoridea. Diadocidia sp. ( Diadocidiidae). Length about 4 mm.
F -- Bolitophilidea. Bolitophila sp. ( Bolitophilidae). Length 4.5 mm. Specimen No. 2885.
Abbreviations as usual.
(From ROHDENDORF, 1951. A-E after HENDEL, F - after ROHDENDORF, 1951)
The next figure depicts some recent non-relict representatives of the type.
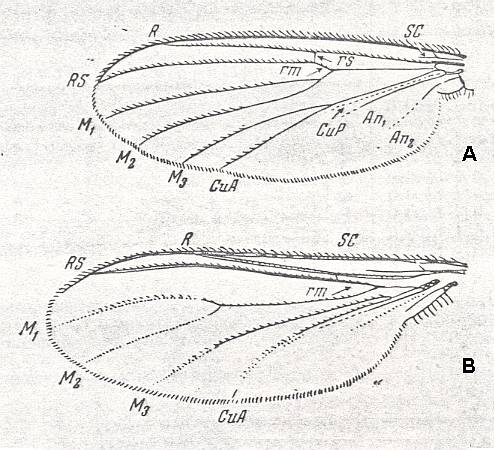
Figure 7 ( A and B ): Fungivoridea. Wings of recent widely distributed representatives of the ancient lifting venationally-attenuated (fungivoroid) type.
A -- Fungivora sp. ( Fungivoridae [ = Mycetophilidae] ). Length 5 mm.
B -- Lycoria sp. ( Lycoriidae [ = Sciaridae] ). Length 2 mm.
Abbreviations as usual.
(From ROHDENDORF, 1951, after HENDEL)
The next Figure depicts some mesozoic representatives of the type.
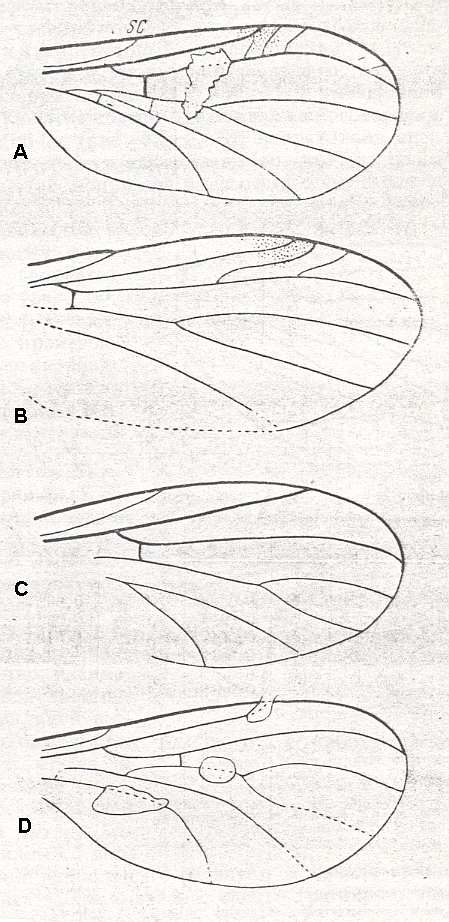
Figure 8 ( A, B, C and D ): Fungivoridea. Wings of fossil representatives of the ancient lifting venationally-attenuated (fungivoroid) type.
A -- Polyneurisca atavina ROHD. ( Pleciofungivoridae), Jurassic of Karatau [southern Kazachstan]. PIN, No. 340/167. length 1.75 mm.
B -- Eopachyneura trisectoralis ROHD. ( Pleciofungivoridae), Jurassic of Karatau [southern Kazachstan]. PIN, No. 2452/673. length 3 mm.
C -- Pleciomima secunda ROHD. ( Pleciomimidae), Jurassic of Karatau [southern Kazachstan]. PIN, No. 287/2465. length 2.25 mm.
D -- Antefungivora prima ROHD. ( Pleciomimidae), Jurassic of Karatau [southern Kazachstan]. PIN, No. 2452/316. length 1.5 mm.
(After ROHDENDORF, 1951)
The next Figures depict a number of schematically drawn basialae [ = proximal parts] of the wings of a number of Fungivoridea (Fungivoridae, Ceroplatidae, Ditomyiidae).
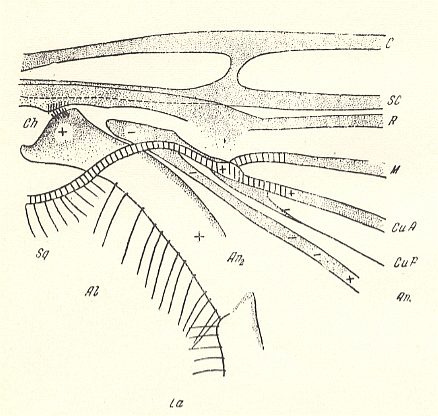
Figure 9 : Fungivoridea. Basiala of wing of Boletina trilineata MEIG. ( Fungivoridae). Schematic.
The veins indicated from top to bottom are : C = Costa, SC = Subcosta, R = Radius, M = Media, CuA = Anterior Cubitus, CuP = posterior Cubitus, An1 = first Anal vein.
La = anal lobe (lobus analis), Al = winglet (alula), Sq = wing-scale (squama alaris). Ch = chaetarium ( = patches of spinelets [forming two brushes] and having [probably] a mechanical function).
+ means : lying on a convex ( = arched upwards) spot or ridge.
- means : lying on a concave ( = scooped out) spot or groove.
(After ROHDENDORF, 1946 )
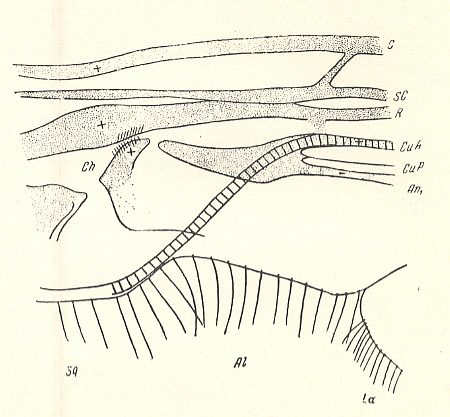
Figure 10 : Fungivoridea. Basiala of wing of Zelmira semirufa MEIG. ( Ceroplatidae). Schematic.
La = anal lobe (lobus analis), Al = winglet (alula), Sq = wing-scale (squama alaris). Ch = chaetarium.
(After ROHDENDORF, 1946 )
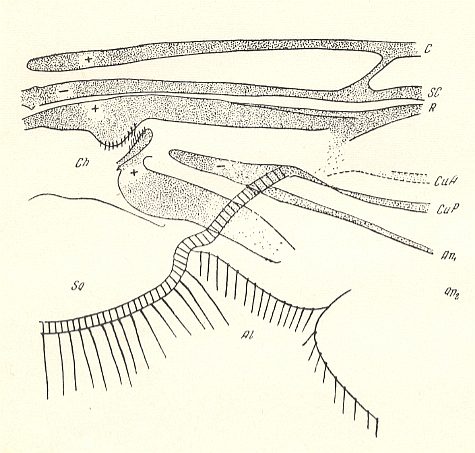
Figure 11 : Fungivoridea. Basiala of wing of Asindulum flavum MEIG. ( Ceroplatidae). Schematic.
Abbreviations as in the previous Figures.
(After ROHDENDORF, 1946 )
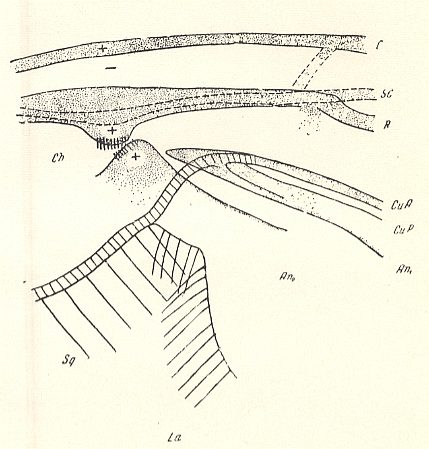
Figure 12 : Fungivoridea. Basiala of wing of Symmerus annulatus MEIG. ( Ditomyiidae). Schematic.
Abbreviations as in the previous Figures.
(After ROHDENDORF, 1946 )
Size of the wings
Shape of the wings
The wings are moderately elongated, usually 2.5 times longer than their width, sometimes shortened (only two times longer than wide) or elongated (up to 3.25 - 3.5 times longer than wide -- Bolitophilidae).
Fore-margin straight in the proximal half or in two thirds of it. The apex is, consequently, relatively large, but not especially sharply distinguished [from the rest of the wing]. Hind-margin uniformly and fairly strongly convex. Anal lobe well developed in the form of an obtusely angled projection. The basiala is, as a rule, sharply individuated by the proximal boundary of the anal lobe and by a fracture or broadening of the end of the basal trunk-piece of the radial vein (See for example Figure 11 ). More rarely the basiala is weakly expressed (as in Bolitophilidae, see Figure 3 of Part VII ). The alula usually present in the form of a weakly convex insert. Wing-scale present as a germ.
Skeleton of the wing
When the insect is not flying the wings are held obliquely backward. Sometimes the ability is developed to fold the wings along the abdomen. The venation is characterized by processes of reduction, affecting almost all vein-systems, especially the radial and medial systems (See Figure 6 and Figure 7 ). Shifting or thickening of veins is relatively uncommon. Costalization basically is realized as a result of processes of reduction, and only partly as a result of strengthened -- by some degree of thickening -- elements of the venation, or as a result of a shift of them.
The costal vein is present at the fore-margin only. It does not run beyond the wing-apex.
The subcostal vein is moderately developed, never longer than half of the wing, often reduced, short, and weak (Fungivorinae [ = Mycetophilinae], Lycoriidae [ = Sciaridae] and some others).
The radial system of veins is the most developed and strong system, consisting of a firm and usually long (often reaching almost 3/4 of the length of the wing ! ) anterior branch, and also of one or two (more rarely more ! ) firm posterior branches.
Of the medial veins there are, as a rule, three branches, rarely reduced to two, or even to one (genus Azana ). The two anterior medial branches form a fork that is very characteristic of this wing-type, that is, a fork formed by two weak veins and lying in the middle of the wing-blade [ = at the level of its median line]. Sometimes there has taken place a reduction of the common trunk of the medial system, as a result of which the [remaining] veins [of this system] look as if they are branches of the radial system, such as in Ceroplatidae, see next Figure,
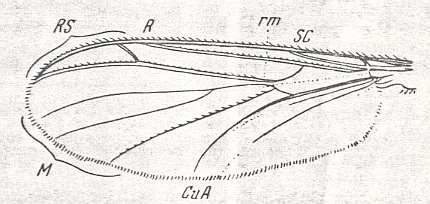
Figure 13 : Wing of Ceroplatus sp. (Ceroplatidae [ Fungivoridea] ). Length 10-12 mm. Reduction of medial trunk.
(Also dipicted in Figure 6 above).
Abbreviations as usual.
(From ROHDENDORF, 1951, after HENDEL)
in Mycetobiidae, in Macroceratidae (see Figure 6C ), in Ditomyiidae (see Figure 6B ), and others. The third medial branch coalesces with the medio-cubital cross-vein and assumes the position of [as it were] the anterior branch of the cubital vein (see Figure 6 B,C,D ). Also transformations of the radio-medial cross-vein are common, which rarely preserves its original position between the posterior radial and anterior medial veins as in Bolitophilidae, see next Figure,
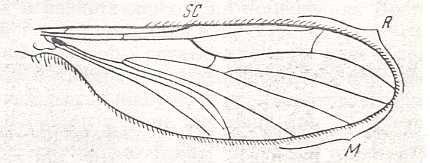
Figure 14 : Wing of Bolitophila sp. (Bolitophilidae [ Bolitophilidea] ). Length 4.5 mm. Original position of the radio-medial cross-vein (rm).
(Also dipicted in Figure 6 above).
Abbreviations as usual.
(After ROHDENDORF, 1951)
in Ditomyiidae, Allactoneuridae, and some others. Usually rm [radio-medial cross-vein] either disappears completely, as a result of the coalescence of radial and medial veins (Ceroplatidae and Macroceratidae, see Figure 6C ), or it transforms into a longitudinal vein and then looks like being the immediate base of the posterior radial branch ( Fungivoridae, Lycoriidae, and others, see Figure 7 above ).
Functional characteristic
The general traits of the way of life of the insects having wings of the present type [ancient lifting venationally-attenuated (fungivoroid) wing type] are known. These minute diptera are closely connected with the soil principally of woodland, with accumulations of vegetable remains and of lower plants -- fungi, which are fed on by the larvae of the majority of forms of these midges. The winged forms have the ability to run fast and sometimes even to dig themselves in and hiding themselves amongst fragments of rotting plants. The fungivoroid flight as such is not investigated. It is probably not distinguished by high speed. However, many representatives of the type have an easy and steerable flight : We can see on indoor plants the behavior of lycoriid (sciarid) midges lively running on the surface of earth (soil) in pots with plants, often also freely flying up and about in the room. The difficulty of catching the midges in their natural habitat among rotting vegetable remains in the forest and among fragments of wood of tree-stumps, dry leaves and pine needles, is also well known. In such environments the insects are very quickly moving around, not only running on the substrate but also often flying off. Flight is of great biological significance to these insects, making it possible to find the sites necessary for reproduction which often are very locally distributed (fruiting bodies of higher fungi, rotting wood) and, in addition, to find hiding-places in which these insects hibernate. Indirect indications of the high quality of flight are the many cases of finding fungivoroid midges on window-glasses of rooms which are many hundreds of meters away from the sites of development of these insects. Together with these data, bearing witness to the great significance of flight to the life activities of the fungivoroids, we must also mention the existence of reduction of the flight ability among this group of diptera. Some forms of these insects are known to be without wings (a series of genera of Lycoriidae [ = Sciaridae], for example Caenosciara, Dasysciara, Peyrimhoffia and others, and of Fungivoridae [ = Mycetophilidae] the genera Pnyxia, Dahlica ).
Also, among the representatives of the family Fungivoridae species are known which possess very large muscular legs and relatively weakly developed wings (the genera Sceptonia, Zygomyia, Delopsis ). Probably these insects fly badly and not often. Until now, precise data on flight features of the fungivoroid midges are absent.
History and transformations of the type.
There exists many fossil documents revealing the history of fungivoroid wings. The diverse jurassic and tertiary species of a whole series of families of the Oligoneura [roughly : Bibionomorpha] illustrate the history and the differentiation of the type, which was present in the form of a series of subtypes already in jurassic times.
We shall now list and describe these subtypes.
1. First of all, the primitive fungivoroid subtype is characterized by the existence of many branches in the radial system of veins ending up at the edge of the wing (for example the genus Polyneurisca of the family Pleciofungivoridae, see Figure 8 A, B ). This subtype is still insufficiently known. In addition to the peculiar structure of the radial veins, we must note that the wings undoubtedly possessed a whole series of other features, as for instance, a little refined and insufficiently strengthened basiala [which usually is not discernible in fossils]. The primitive fungivoroid wings are a direct derivative of the eoptychopteroid subtype of tipuloid wings, and in their turn were the source of the bibionoid type and of the ancient pleciofungivoroid subtype of the fungivoroid wings (see next subtype). The mentioned eoptychopteroid wings belong to the fifth subtype of the primitive traction (tipuloid) wing type, see in Part II, HERE .
2. The pleciofungivoroid subtype is characterized by the existence of a strong well-developed anterior branch of the posterior radial vein [radial sector], by the presence of the common trunk of the medial veins, by the original position of the radio-medial cross-vein (rm), which often is shifted toward the wing-base. Wings of this subtype were possessed by many species of the jurassic family Pleciofungivoridae, see next Figures,
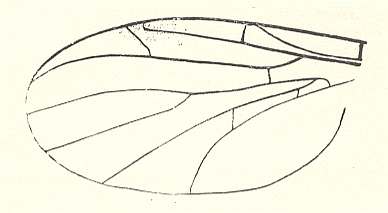
Figure 15 : Wing of Transversiplecia transversinervis ROHD. (Pleciofungivoridae). Length 3.5 mm. Well-developed fork of radial-sector. Original position of the radio-medial cross-vein (rm).
Jurassic of Karatau [southern Kazachstan]. Specimen PIN, No. 2452/358.
(After ROHDENDORF, 1946)
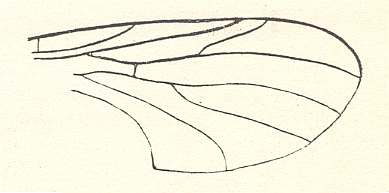
Figure 16 : Wing of Prohesperinus abdominalis ROHD. (Pleciofungivoridae). Length 3.25 mm. Well-developed fork of radial-sector. Original position of the radio-medial cross-vein (rm).
Jurassic of Karatau [southern Kazachstan]. Specimen PIN, No. 2452/580.
(After ROHDENDORF, 1946)
The subtype is barely represented in the recent fauna and only a representative of the single family Hesperinidae [Bibionidea] (See Figure 6A above ) is a residue of this once abundant subtype. The specialization in this relict form is interesting : the females lack wings.
3. The pleciofungivoroid wings were the starting-point of many other subtypes of the present type, of which, first of all we must mention the pleciomimoid subtype, which originated as a result of reduction of branches of the posterior radial vein [radial-sector], that is, the radial-sector lost its fork(s) and became one single vein, and [as a result] of the shortening of the anterior branch [radius], of the increase of the degree of costalization (by means of the weakening of the medial veins), and, finally, as a result of the decrease of the general size of the insects. In the jurassic fauna this subtype was represented by the many species of the Pleciomimidae, see Figure 8 D,C . The direction of specialization of the wing-venation in its generality reminds us of what we saw in the family Pleciofungivoridae, for example a shift towards the wing-base of the cross-vein rm and the weakening of the medial fork.
In the recent fauna pleciomimoid wings are only observed in some forms of the relict family Diadocidiidae,
see Figure 6 E .
4. Another derivative of pleciofungivoroid wings, the allactoneuroid subtype, was represented in jurassic times by the family Allactoneuridae. See next Figure.
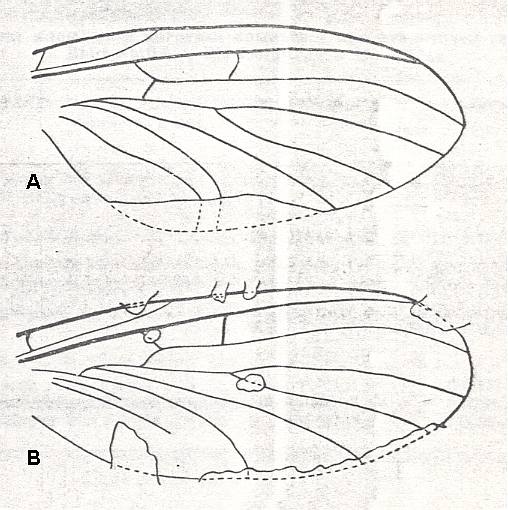
Figure 17 : Wings of the allactoneuroid subtype of the ancient lifting venationally-attenuated (fungivoroid) type.
A -- Mesosciophila venosa ROHD. (Allactoneuridae). Jurassic of Karatau [southern Kazachstan], Geol. Inst. SAGU, No. 26. Length 4.5 mm.
B - Mesosciophilodes angustipennis ROHD. (Allactoneuridae). Jurassic of Karatau [southern Kazachstan], PIN, No. 2452/172. Length 2.75 mm.
(From ROHDENDORF, 1951, after ROHDENDORF, 1946)
In the recent fauna this subtype is represented by the large family Fungivoridae, see Figure 7A, above , and also next Figure.
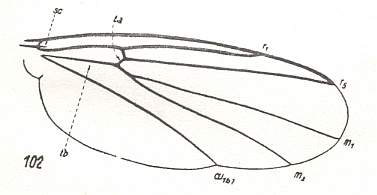
Figure 17a : Wing of Zygomyia notata STANN. (Fungivoridae), belonging to the allactoneuroid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(After HENNIG, 1954)
In HENNIG, 1954, we found a drawing of a wing of a recent representative of the family Allactoneuridae :
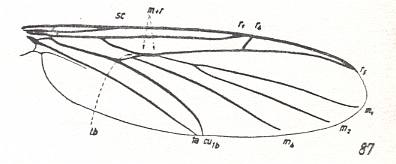
Figure 18 : Wing of Allactoneura nigrifemur ENDERL. (Allactoneuridae), belonging to the allactoneuroid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(From HENNIG, 1954, after EDWARDS, 1925)
This subtype is characterized by an increase of the degree of costalization of the wing, realized in a totally different way, namely by the elongation and strengthening of the anterior radial vein (the radius proper) together with a shift of the cross-vein rm to the wing-base and its transformation into [as it were] the basal [ = proximal] part of the posterior radial vein (the radial sector) (See Figure 7A, above ).
5. The last derivative of the pleciofungivoroid subtype we see in the wings of the tertiary and recent families Mycetobiidae (See next Figure), Ceroplatidae, Macroceratidae, and Ditomyiidae (See Figure 6 B,C, D, above ).
For the structure of the basiala of members of the Ceroplatidae see Figure 10 above and Figure 11 above .
For the structure of the basiala of a member of the Ditomyiidae see Figure 12 above .

Figure 19 : Wing of Mycetobia pallipes MEIG. (Mycetobiidae), belonging to the ceroplatoid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(From HENNIG, 1954, after FREEMAN, 1951)
The subtype they represent can be called the ceroplatoid subtype. The direction of changes of the wing-venation consisted in the realization of a peculiar form of costalization : The radial veins did not decrease in number. The middle branch became strong and simple [i.e. not branched] (See Figure 19 and Figure 6B ). The medial veins shifted towards the posterior radial vein, and, in the majority of cases the cross-vein rm disappeared as a result of the coalescence of medial and radial veins. The proximal part of the medial vein was reduced and in its place a large basal cell was formed.
6. The allactoneuroid wings (fourth subtype) formed the basis of the formation of the youngest, lycorioid specialized subtype, see Figure 7B, above , which is expressed in the representatives of the soil-dwelling midges, Lycoriidae [ = Sciaridae] (see also next Figure) and some of the gall-midges, namely the Lestremiidae.
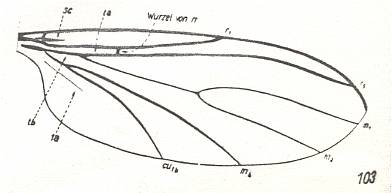
Figure 20 : Wing of Lycoria bicolor MEIG. ( Lycoriidae [ = Sciaridae] ), belonging to the lycorioid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(After HENNIG, 1954)
The subtype is characterized by far-progressed processes of costalization. In this subtype only two radial branches are preserved, and the cross-vein rm changes its original position, becoming the basal trunk of the posterior radial vein, and the direct continuation of the basal part of the media. This complex vein, as also the anterior radial branch [radius proper] and the costal vein, are strongly elongated and become the chief skeletal element of these peculiar costalized wings. All remaining veins of the lycorioid wings are weak, and the subcostal vein is almost completely reduced. The lycorioid subtype is only known from the Caenozoic upwards : The time of its formation must of course be set in cretaceous times.
7. The last subtype of the ancient lifting venationally-attenuated (fungivoroid) wing type, the bolitophiloid subtype, is found in the representatives of the relict family Bolitophilidae, see Figure 6 F, above and next Figure,
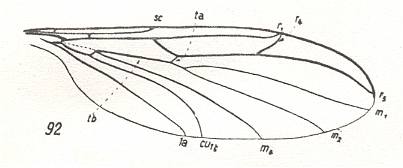
Figure 21 : Wing of Bolitophila hybrida MEIG. (Bolitophilidae) belonging to the bolitophiloid subtype of the ancient lifting venationally-attenuated (fungivoroid) type. Interpretation of wing-venation according to HENNIG, 1954.
(After HENNIG, 1954)
and is characterized by a series of peculiar features, bearing witness to a totally different way of origin. First of all the wings of this subtype characterize themselves by the indistinct basiala, in which the proximal parts of the cubital and anal veins are fairly well-distinguished and form an insufficiently compact concave element of the basiala. Together with this feature we can observe (1) a far-progressed reduction of the 'handle' (proximal end-piece) of the second anal vein [The anal chaetarium is, however (judging from ROHDENDORF's drawing of it -- see next link), strongly developed, and this could be the 'handle' of the second anal vein, the rest of the vein being disappeared], (2) the absence of the boundary between the alula and anal lobe, and (3), which is especially important, a strong lengthening of the wings. For the basiala of a representative of this subtype see Figure 3 of Part VII . In addition, this bolitophiloid subtype is characterized by a weakly developed costalization and by other values of surface-area and load of the wings. The latter is very small and is virtually certainly the lowest of all representatives of the order (Diptera) (going down to 0.006 gr/cm2 ).
The connections of the ancient lifting venationaly-attenuated (fungivoroid) wing type with other types are more or less clear. Its point of departure or source of origin were forms of the primitive traction (tipuloid) wings, namely the eoptychopteroid subtype thereof [5th subtype], which led, in a parallel way, to two subtypes of the fungivoroid wings, the primitive fungivoroid subtype and the bolitophiloid subtype. In its turn the primitive fungivoroid subtype was the source of origin of the ancient lifting venationally-dense (bibionoid) type [see for this type there where the superfamilies Bibionidea and Scatopsidea are dealt with] and of the remaining forms of the fungivoroid type. In addition, from the pleciomimoid subtype undoubtedly came forth the characteristic wings of the pseudo-feather-winged ( itonidoid ) type [The latter may also have come from scatopsidae-like wings] [For the pseudo-feather-winged type see there where the superfamily Cecidomyiidea is dealt with].
In evaluating the direction of development of the fungivoroid wing type we can note various changes of features of the wing-venation having the character of particular improvements of the quality of the wing. These include the improvement of costalization of the wing which took place in the history of the type on several occasions and in different ways in the different subtypes.
In the pleciofungivoroid and pleciomimoid subtypes costalization was realized as a result of a shift of the radio-medial cross-vein (rm) toward the base of the wing, but in the latter subtype in addition to it a reduction of the middle radial branches took place. One can notice an extreme shift of the radio-medial cross-vein, a change of its position and its transformation into [becoming] the proximal part of the posterior radial branch, together with the lengthening of those veins, in the allactoneuroid subtype, and, finally, a significant strengthening of this system of veins with at the same time a weakening of the posterior [systems of] veins in the lycorioid subtype. This subtype is certainly the most progressive one. The development of peculiar broad wings of the ceroplatoid subtype, and the elongated wings of the bolitophiloid subtype with a little refined basiala, can naturally be evaluated as examples of a moderately progressive or a narrowly-specialized direction in the evolution of the wings of this type [that is, the ancient lifting venationally-attenuated (fungivoroid) type].
The phenomena of a regressive evolutionary pathway in the fungivoroid wings are very interesting : Regression in this group of diptera expresses itself in the decrease of wing-size, their shortening, a deterioration of mechanical qualities (weakening of costalization) all the way down to the complete reduction of the whole organ, the complete loss of the wings, that is, to the development of winglessness. These processes take place in subtypes in which the general direction of [evolutionary] development is characterized in many cases by progressive features : so in the allactoneuroid and especially in the lycorioid subtype, being relatively very progressive, we see cases of the development of winglessness. This is especially remarkable in the example of the pleciomimoid subtype (almost completely extinct) which was the starting point for the characteristic regressive itonidoid wing type [ See for this type where the superfamily Cecidomyiidea [ = Itonididea] is discussed]. Undoubtedly such a kind of pathway of development of regressive forms on the basis of progressive ones took place in the history of the type. The paradoxical nature of such a position is only seemingly so. In considering cases of development of winglessness in some representatives of the fungivoroid wing type (for example the genus Pnyxia of the Fungivoridae [ = Mycetophilidae], or many Lycoriidae [ = Sciaridae] ), we easily notice the connection of this process with a change in living-conditions : These insects become more closely connected with the soil [such as the forest-floor], more exactly expressed, with every kind of concealed soil-sites -- hollows or clefts in the soil or even simply in caves (various species of Lycoriidae). In their turn, such kinds of changes of the living-conditions directly express changes in feeding [habits] of these insects. For example a species of the genus Pnyxia (a representative of the family Fungivoridae with reduced wings) has larvae living in bulbs of potatoes, but could also be found in the burrow of a mole, that is, in conditions very far from those of the usual larval sites of this family. Especially remarkable are the differences in the living-conditions of gall-midges ( Itonididae [ = Cecidomyiidae] ), which are undoubtedly derivatives of fungivoroid diptera, and of which the wings can rightly be evaluated as a regressive direction in the evolution of the pleciomimoid and lycorioid subtypes.
On the whole, this process of development of regressive wing-forms on the basis of clearly expressed progressive subtypes can be clarified only under the condition of the evaluation of the w h o l e evolutionary development of the given group of organisms. This can serve as a wonderful example of the limitedness of a purely comparative anatomical method of investigation of only a single system of organs and neatly shows how dangerous it is to draw general conclusions on the basis of such one-sided data. The over-all development of a certain group of organisms, which led to the progressive development of one or another system of organs and functions (in our cases of wings and flight), went in some cases further along a different way, along a way, which led to the development of other organs and functions (the digestive system and feeding), and where organs (wings) that earlier had their importance had become of mere secondary importance and their refinement harmful.
We have now reproduced ROHDENDORF's 1951 text dealing with the fungivoroid wing type (and have added explanations and some more figures). This means that we not only have extracted important data from his text, data about the structure of wings and the life-habits of their possessors, but have also doctrinairily largely followed ROHDENDORF, that is, we have here reproduced and followed his general thesis that holds that evolution is driven by ecology. And this implies that many evolutionary changes -- the so-called progressive ones -- must be seen as functional improvements and refinements with respect to the performance of a given organ system. In addition ROHDENDORF also recognizes regressive evolutionary processes, but rightly insists that in evaluating such processes as regressive we must take into account the whole evolutionary development of the given group of organisms. Indeed, when we do so we see that often the group as a whole, or certain subtaxa of this group (that is, precisely there where regressive processes have been observed), are not simply regressive, not regressive groups, not regressive organisms : In such cases, it is true, certain organs have degenerated, but they did so for the benefit of other organs, organs that changed progressively. All these processes are part of 'answers' or 'solutions' to problems which necessarily appear when the habitat of an animal population of a given species changes more or less permanently. And in many cases some regressive processes are necessarily part of the overall process of adaptation. As a third type of evolutionary process ROHDENDORF recognizes narrow specialization. Such a process is neither progressive, nor regressive. The result of such a process is a feature -- morphological or physiological -- (evolutionarily) developed in the organism, [a feature] that lets this organism to be precisely fit into a very special and subtle aspect or 'corner' of the overall habitat. In contrast to a progressive development, the narrow specialization makes the organism evolutionarily vulnerable. Small changes in the environment can cause extinction of this narrowly specialized species.
Because, as we have explained in Part II, the flight-regime has been evolutionarily developed such as to become compatible with how the insect experiences the environment on the basis of the specific morphological and physiological structure of its eyes and antennae, an evolutionary change in the flight-regime (made possible by corresponding changes of the wings) does not necessarily mean an improvement of flight-capabilities (higher speed, better steerability, etc.). True, it can, and often is, such an improvement (which for example was clearly the case in the origin of robber-flies), but in most cases -- especially where the original and derived insects have a more or less similar morphology, size, and way of life -- such a change in flight-regime (from original to derived) results in merely a different flight-regime, different that is, from the original one, not necessarily technically improved. Such a new flight-regime, whether merely different or improved, is an adaptation to the precise new habitat of the adult fly, and allows the latter to move about precisely in it. We must stress that with "precise new habitat" we do not mean a geographically local habitat, that is, not a spatially different location, but only a micro-physiognomically different location. This is thus a type of location or micro-landscape that can be present at many geographically different locations. Only by having acquired such an adaptation -- along with adaptations in other organ-systems -- the adult fly (the winged insect) can perform its overall function which consists in finding food (nectar, blood), in finding a mate, in geographically expanding the (new) species' range, and for the female in finding the appropriate substrate for the development of its larvae.
A given species of fly represents a definite qualitative content. This content is, as a result of the process of injection ( from the Explicate [ = physical] into the Implicate [ = noëtical, i.e. thought-like] Order), present in the Implicate Order as a noëtic entity, that is, as an immaterial thought-like enfolded entity. And as qualitative content of the given species of fly it defines, and thus determines and delimits, the respective qualitative contents of several potential ecological niches, where one of them has become actual. And because these potential and actual ecological niches are qualitatively defined and delimited by the qualitative content of the given species of fly they too can be injected into the Implicate Order and exist there as noëtic entities. And, further, according to our metaphysical theory (expounded in Part I-IV of the Dynamic Metaphysics [nomological] Series), with the transition of an original species of fly into a new species we have to do with a noëtic reaction taking place in the Implicate Order between the qualitative content of the original species -- as such a noëtic reactant -- and the qualitative content of one of its potential ecological niches -- this content also being a noëtic reactant -- resulting in a noëtic reaction-product. This noëtic reaction-product is the qualitative content of the new species of fly still existing as a noëtic entity in the Implicate Order (that is, not yet as a material entity existing in the Explicate Order). When conditions in the Explicate Order are right, this noëtic content will (as a copy) be projected into the Explicate Order ( It might also be assumed that the whole course (i.e. all stages) of the noëtic reaction as such is, together with its product, projected into the Explicate Order ). This means that the projected noëtic contents will be unfolded along space and time dimensions (they become physical), which we (in the Explicate Order) observe as the evolutionary development of certain morphological or physiological adaptations of the new species of fly to its new ecological niche, a development which generally takes much time. And one of these adaptations can be seen in transformations of the wing-structure, especially of its venation. The new wing-venation then is a co-product of the mentioned noëtic reaction. It is fully integrated with the rest of the fly's organization. It is part of the adaptation to the new ecological niche (in the narrowest sense), because it is the morphological expression of a new flight-regime enabling the fly to move about precisely in this new niche, that is, in conformity with this niche. And for the flight-regime to be in conformity with precisely the new niche means the existence of a qualitative compatibility between the way of flying and the nature of the environment precisely as perceived by the fly. And so evolution of flies, as driven by ecology, results -- among other things -- in sequences of transformations of wing-venation (often consisting in reductions and/or shifts of veins) as such [these sequences] not necessarily leading to an aerodynamically improved and refined flight, but merely to a different flight, that is a flight better adapted to certain subtle aspects of the new ecological niche. In such a way, I think, we must interpret the changes in wing-venation as discussed above in the context of the distribution of functional wing-types over taxa of Diptera.
End of the exposition and discussion of functional wing-types as they occur especially (but not exclusively) in the superfamily Fungivoridea (Diptera, Bibionomorpha).
We have now discussed the superfamily Fungivoridea, that is, some features of the general morphology and of the way of life of its representatives, and also the functional wing-types they possess.
e-mail :  ( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
( Please write in ' Subject ' entry : ' METAPHYSICS ', in order for me to be able to distinguish your mail from spam )
To continue click HERE for the further study of Organic Evolution, Part VII.
Back to Aristotelian metaphysics Part I
Back to Aristotelian metaphysics Part II
Back to Aristotelian metaphysics Part III
Back to Aristotelian metaphysics Part IIIa
Back to Aristotelian metaphysics Part IV
Back to Aristotelian metaphysics Part V