The range of molecular compounds, MC, is utterly rich, and the possibility-in-principle to generate them is almost everywhere to be found.
If unification between several already saturated-as-to-chief-valences identical molecules into higher organized independent particles of molecular size takes place, one speaks of "association", in the case of unequal molecules one speaks of "aggregation".
[Here, in this note, and in the main text, we are dealing with how to evaluate the phenomenon of "molecular compounds" in the context of Unimol. A molecular compound must be distinguished from a true chemical compound, an atomic compound, such : In true molecules, atomic compounds, the chemical parts, conceived in isolation, are atoms or chemical groups that still have left free chief (i.e. true) valences, unsaturated valences. But as soon as they are considered as having chemically taken up into a molecule, i.e. now conceived as parts of a molecule, all their valences are in fact saturated. In molecular compounds, on the other hand, this is not so. There, the chemical groups, the "molecules", making up the molecular compound (MC), the "super-molecule", are not only saturated as to their chief, i.e. true, valences before their unification (as in many chemical reactions) into the super-molecule, but remain so saturated when constituting the super-molecule, the molecular compound, so their type of bonding is quite different at least from a covalent bond.]
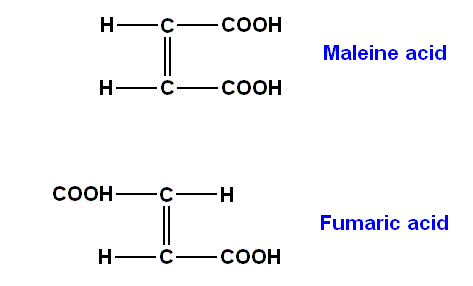
The formation of a molecular compound (MC) can chiefly be reduced to the dipolar or multipolar nature of the components (and thus to inter-molecular or adjunct-valence forces). In addition to inter-molecular super-molecule formation, there may be intra-molecular association (Compare maleine acid with the association of both COOH groups [both at the same side of the chain or "axis", both pointing in the same direction], and fumaric acid in which this is not so [both COOH groups pointing in opposite directions]. Consequence : strong difference in heat-of-evaporation.). Maleine acid and fumaric acid are isomerous (same composition, different structure) acids, in this case, respectively, cis- and trans-isomers :

A measure of the degree of [bond-]strength in MC is the "bond"- or better association-energy, which can be determined as heat-after-mixing of the reaction A + B ==> AB. The counter-value, and thus the separation-energy, may always statistically easily be produced again, so that most often already a light shaking already results in a breakdown.
Recently, one has pointed to yet another type of association, the so to say histological association. "In the domain of minerals, as also in the animal and plant kingdom, a whole series of solid two-component systems is known : The fibrous molecules of a substance A, mostly a polyelectrolyte, are ordered parallel to one another and fixed by chemical bonding. In this spatial network as matrix a second substance, B, is deposited such that its crystals with their longer parameter are aligned with the structural direction inherent and pre-existing in the matrix. An example of such a system is the pearl, made up of conchyoline and aragonite" (cited from a 1957 source). Here it is, of course, a clear case of non-living substance. [but it also occurs in living systems :] In the case of vertebrate bones this [non-living state] is already essentially different, because here there is a species of Ca-salt of semi-vital nature, possessing true transitions to genuine living substance. [So, apart from molecular compounds, intra-molecular associations, and hydration, there exist, in the organismic, also this described type of association.].