
The chemical compound Butane (C4H10) is a so-called saturated hydrocarbon, that is, its carbon atoms are totally saturated with hydrogen, and thus the molecule is without double or triple bonds in the carbon chain :

In fact it should be such that the difference is specific with respect to the subject, that is, that the extension of the concept (i.e. its range) is equal to that of the concept denoting the subject (Butane), or, said differently : the extension of the concept denoting the difference must be equal to the extension of the concept denoting the species, that is, the subject.
See next Figure.
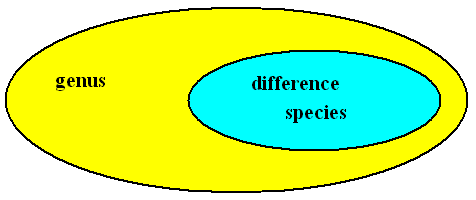
Figure above : The Difference 'contracts' the extention (range of applicability of the concept) of the Genus into that of the Species. Therefore the extention of the Difference must be equal to that of the Species.
Here (that is is our example concerning Butane), however, the term 'saturated ' is broader than the subject, because also, for instance, Propane is saturated (thus here the extention of the difference does not coincide with that of the species). The subject should then be 'hydrocarbon-with-only-simple-bonds-in-the-carbon-chain' instead of 'Butane' (In fact the term 'saturated' -- still in a chemical context -- is still broader, because 'saturated' can also be the case with respect to other chemical elements). But this subject does not stand for one individual being and also not for a species. It stands for a genus. One of its species is Butane (another species is Propane,etc.).
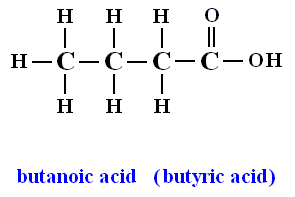
Figure above : Like Butane, Butanoic acid contains four linearly bonded carbon atoms in each of its molecules.
But as soon as we add to this qualification : 'completely saturated with hydrogen' (that is, with hydrogen only [excluding now butyric acid which, in addition to hydrogen also contains oxygen] ), then we have a complete and specific characterization of Butane :
containing four linearly bonded carbon atoms, completely saturated with hydrogen.
However, this characterization then is also equivalent (with respect to content) to the species, and is thus not a genuine difference anymore.
So we must stick with the difference 'containing four linearly bonded carbon atoms'.
The genus and the difference of (the species) Butane can now be finally indicated as follows :
Genus = saturated hydrocarbon (and not just hydrocarbon)
Difference = containing four linearly bonded carbon atoms
The definition of Butane accordingly reads :
( Butane = ) a saturated hydrocarbon containing four linearly bonded carbon atoms.
'Hydrocarbon' indicates that the compound consists of hydrogen and carbon only (distinguishing it from butyric acid [which also contains oxygen] ). The possibility of a branched carbon chain is still left open.
'Saturated ' indicates that no double or triple bonds occur between the carbon atoms.
'Containing four linearly bonded carbon atoms' excludes branched carbon chains, and determines the number of carbon atoms in the molecule.
As we can see, not every difference can, qua its extension, coincide with the species (that is to say, 'containing four linearly bonded carbon atoms' is not only the case in Butane, but also in Butyric acid). Sometimes it has a larger extension.
From all this the usefulness of considering also other examples than that of man is evident. Much can come to light as a result of testing such other examples.